Summary:
SS1P is an antimesothelin recombinant immunotoxin (RIT). Pancreatic ductal adenocarcinoma (PDAC) cell lines are resistant to SS1P, despite high mesothelin expression. The aim of this study is to examine whether combining SS1P and BH3-mimetic ABT-737 induces cell death in a panel of PDAC cell lines. ABT-737 binds and neutralizes several antiapoptotic BCL2 family proteins, but has a low affinity for the short-lived MCL1 and BCL2A1. SS1P inhibits protein synthesis, which has shown to downregulate MCL1. PDAC cell lines KLM-1, BxPc-3, and Panc 3.014 were resistant to SS1P or ABT-737 alone. Combining both compounds led to a significant increase in cell death. After 48 hours of treatment, cell death was observed in 92% of KLM-1, 55% of BxPc-3, and 23% of Panc 3.014 cells. Panc 3.014 had the highest number of mesothelin-binding sites (92 × 103), followed by KLM-1 (58 × 103) and BxPc-3 (3 × 103). ABT-737 had no effect on SS1P internalization, but enhanced SS1P-induced protein synthesis inhibition significantly in KLM-1, to a lesser extent in BxPc-3, and very little in Panc 3.014. SS1P alone or in combination with ABT-737 downregulated MCL1 in KLM-1 and BxPc-3, but not in Panc 3.014. Similar observations were made for BCL2A1, which had the highest levels in Panc 3.014. Compared with KLM-1, Panc 3.014, and BxPc-3 also had lower proapoptotic BAK and a trend toward higher MCL1. Proapoptotic BAX was similar in KLM-1 and BxPc-3, but lower in Panc 3.014. In conclusion, combining SS1P with ABT-737 overcomes SS1P-resistance in PDAC, although to a variable extent. The efficacy of the combination is mainly associated with the RIT-associated inhibition of protein synthesis and the ability to downregulate MCL1 and BCL2A1, while levels of other key apoptotic proteins may also be important. Our data support the combination of an RIT and a BH3-mimetic, and identify factors that potentially limit the efficacy of such therapeutic approach.
Keywords: BH3-mimetic, mesothelin, immunotoxin, pancreatic Cancer
Pancreatic cancer is the fourth leading cause of cancer death in the United States and results in an estimated 227,000 deaths per year worldwide.1 The most common subtype, pancreatic ductal adenocarcinoma (PDAC), is notoriously resistant to most chemotherapeutic agents, and alternative treatment options are clearly needed.
Our laboratory focuses on the development of recombinant immunotoxins (RITs) for cancer treatment. These RITs are composed of an antigen-binding Fv fused to a 38 kDa portion of Pseudomonas exotoxin A (PE38).2 RITs are internalized by receptor-mediated endocytosis, and traffic through the endocytic compartments to the endoplasmic reticulum (ER), during which the toxin gets separated from the Fv by the action of furin. PE38 translocates to the cytosol, where it ADP-ribosylates and inactivates elongation factor 2. This halts protein synthesis and eventually leads to cell death.3
SS1P is an RIT that targets mesothelin, a 40 kDa cell surface glycoprotein.4 In normal tissue, the expression of mesothelin is limited to mesothelial cells that line the pleura, peritoneum, and pericardium. In malignant mesothelioma, PDAC and several other malignancies, mesothelin is highly expressed, making these malignancies interesting targets for SS1P.5,6 SS1P has been evaluated in 2 phase I clinical trials, predominantly focusing on mesothelioma. The drug was well tolerated, but had limited antitumor activity as a single agent.7,8
SS1P kills mesothelioma cells from patients and many mesothelioma cell lines.9 In contrast, PDAC cell lines HTB-80 and Panc 3.014 were found to be resistant to SS1P and to a variant of SS1P (SS1P-KDEL) that profoundly inhibits protein synthesis.10 Lower levels of BAK, a proapoptotic member of the BCL2 protein family, were found to protect these cell lines from apoptosis, despite the induction of complete protein synthesis inhibition.10 We found that one approach to overcome resistance was combining SS1P with TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), an activator of the extrinsic apoptotic pathway.10 This finding fuelled our efforts to identify drugs that can be combined with SS1P to tip the balance toward PDAC cell death.
The BCL2 family proteins are an attractive target for this purpose because they regulate cellular homeostasis, tumorigenesis and cellular responses to anticancer therapy, including apoptosis.11 BCL2 family members are divided into 3 groups. The prosurvival members (BCL2, BCLXL, BCLW, MCL1, and BCL2A1) inhibit apoptosis, whereas a second group (BAX, BAK, and BOK) are considered the promoters and effectors of apoptosis. Both groups contain 4 BCL2 homology domains (BH1–4). A third group are the BH3-only proteins, which can interact with both antiapoptotic and proapoptotic proteins to promote apoptosis.11 Two pathways of apoptosis can be distinguished: the intrinsic pathway, which is activated by several different cytotoxic insults and is strictly controlled by the BCL2 family proteins, and the extrinsic pathway, which is mainly triggered by ligation of members of the TNF-receptor family.12
ABT-737 is a BH3-mimetic that binds with high affinity to BCL2, BCLXL, and BCLW, but has low affinity for MCL1 and BCL2A1.13 High levels of MCL1 or BCL2A1 can therefore play a role in resistance to ABT-737.14,15 Both are short-lived proteins16: hence compounds that inhibit protein synthesis, like RITs, can lead to the loss of these prosurvival proteins. We previously reported that the downregulation of MCL1 is an important event in RIT-associated cell death.17 RITs and ABT-737 thus seem highly complementary, and combining ABT-737 with some RITs has indeed been shown to overcome resistance in other cancer cell lines, including melanoma, colon, ovarian, small cell lung, and epidermoid cancer.18–20
The aim of this study was to evaluate the combination of SS1P with ABT-737 in a panel of PDAC cell lines, and to identify factors that play a role in the efficacy of this combination. We found that combining SS1P with ABT-737 overcomes SS1P-resistance in PDAC. The efficacy of the combination is mainly associated with the RIT-associated inhibition of protein synthesis.
MATERIALS AND METHODS
Reagents
SS1P [SS1(dsFv)-PE38] and LMB-2 [anti-Tac(Fv)-PE38] were expressed in Escherichia coli BL21 (λDE3) from vectors encoding VH-PE38 and VL.21 Clinical-grade SS1P and LMB-2 was manufactured by Advanced BioScience Laboratories Inc. (Kensington, MD) and the Monoclonal Antibody and Recombinant Protein Production Facility [National Cancer Institute (NCI), Frederick, MD], respectively. ABT-737 was purchased from Selleck Chemicals LLC (Houston, TX), dissolved in DMSO at 10 mmol/L stock concentration, and stored at −80°C.
Cell Lines
PDAC cell lines KLM-122 and BxPc-3 were provided by Dr Udo Rudloff (NCI, Bethesda, MD) and maintained in RPMI-1640 with 10% FBS. These cell lines were originally obtained from the RIKEN Cell Bank and the American Type Culture Collection, respectively. Panc 3.014 was obtained from Dr Elizabeth Jaffee (Department of Oncology, Johns Hopkins University, Baltimore, MD) and maintained in RPMI-1640 with 20% FBS and 0.2 U/mL human insulin. Medium was also supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). All cell lines were grown at 37°C with 5% CO2. Cell line identity was verified using short tandem repeat analysis (NCI, Frederick, MD).
Cell Proliferation, Cell Death, and Protein Synthesis Inhibition Assays
For each of these assays, cells were incubated with SS1P, ABT-737, or both, 16 hours after plating. Cell proliferation was measured with the Cell Counting Kit-8 WST-8 assay (Dojindo Molecular Technologies Inc., Gaithersburg, MD). After incubation, CCK-8 reagent was added and the 96-well plates were incubated at 37°C until the untreated wells reached values of ~1 Abs450. Values were normalized between controls of 1 μM staurosporine (Sigma-Aldrich, St Louis, MO) and buffer [Dulbecco’s phosphate buffered saline without Ca and Mg (D-PBS), Quality Biological Inc. (Gaithersburg, MD) containing 0.2% human serum albumin (HSA)]. Cell death was quantified using the Annexin V-PE Apoptosis Detection Kit I (BD Pharmingen, San Jose, CA). After incubation, both floating and adherent cells were collected, stained with Annexin V-PE and 7-aminoactinomycin D (7-AAD) according to manufacturer’s instructions, and analyzed on a FACSCalibur. Viable cells were considered Annexin V and 7-AAD negative, as determined by gating the untreated cells. Protein synthesis inhibition was quantified by incubating cells with 2 μCi/mL 3H leucine (Perkin Elmer, Boston, MA) for 2.5 hours. The 96-well plates were subsequently put on dry ice for at least 30 minutes, and allowed to thaw for 1 hour. Cells were collected on filter mats, and samples were counted using a Wallac Beta plate reader (Boston, MA). Values are presented relative to controls of D-PBS 0.2% HSA.
Quantification of Mesothelin Expression and SS1P Internalization
To quantify the cell surface mesothelin expression, cells were grown for 2 days, harvested, washed with FACS buffer (PBS with 5% FBS and 0.1% NaN3), and incubated with 5 μg/mL of mouse antimesothelin MN antibody23 (Rockland Immunochemicals Inc., Gilbertsville, PA) on ice for 30 minutes. After washing, cells were incubated with goat antimouse IgG-R-PE (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) on ice for 30 minutes in the dark, and washed again twice. Mesothelin expression was analyzed on a FACSCalibur. QuantiBRITE PE beads (BD Pharmingen) were used to quantitate the number of mesothelin sites per cell. To evaluate RIT internalization and the effect of ABT-737 on uptake, cells were incubated for 3 hours at 37°C with 2 μg/mL of SS1P-Alexa647 alone or in combination with 10 μM ABT-737. SS1P was labeled with the Alexa Fluor 647 Labeling Kit (Invitrogen) according to manufacturer’s instructions. After incubation, cells were washed with FACS buffer, and surface-bound SS1P was removed by stripping cells with glycine buffer (0.2 mol/L glycine, pH 2.5 and 1 mg/mL of BSA) for 10 minutes, neutralized with Tris-HCl 1M pH 8, and washed with FACS buffer. Fluorescence intensity was analyzed on a FACSCalibur.
Western Blot
Cells were collected after plating, washed with D-PBS, and solubilized in RIPA buffer with protease inhibitors (Roche Applied Science, Indianapolis, IN). Protein concentrations were determined using a Coomassie Plus kit (Pierce, Rockford, IL). Equal amounts of protein were loaded onto NuPAGE 4%–12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes (Invitrogen). The following primary antibodies were used: rabbit anti-furin #36–1800 (Invitrogen), rabbit anti-MCL1 #4572 and rabbit anti-BCLXL (54H6) #2764 (Cell Signaling Technology, Danvers, MA), rabbit anti-BAK NT #06–536 and rabbit anti-BAX NT #06–499 (Millipore/Upstate, Lake Placid, NY), mouse anti-BCL2 #610539 (BD Transduction Laboratories, Lexington, KY), rabbit anti-BCL2A1 #PA5–20269 (Thermo Scientific, Rockford, IL), and mouse anti-β-actin #8226 (Abcam, Cambridge, MA). HRP-conjugated goat antirabbit or mouse secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were visualized with ECL or ECL Plus substrates (GE Healthcare, Piscataway, NJ). Protein levels were quantified and adjusted for β-actin levels with Image J.24
Statistics
Data are presented as mean ± SE of measurement of replicate experiments. Each experiment was performed independently at least twice, and representative or average data are displayed. Applied statistics include Student t tests and 1-way analysis of variance with Bonferroni multiple comparison tests. Statistical analysis and figure drafting was performed using GraphPad PRISM 5 software (GraphPad Software Inc., La Jolla, CA). A P-value of < 0.05 was considered statistically significant.
RESULTS
Combining SS1P and ABT-737 Decreases Cell Proliferation
The effects of SS1P and ABT-737 on cell proliferation were evaluated with the WST-8 assay. PDAC cell lines KLM-1, BxPc-3, and Panc 3.014 were treated for 24 hours with either SS1P (10, 50, or 100 ng/mL), ABT-737 (1, 5, or 10 μM), or both (Fig. 1). With KLM-1, increasing concentrations of SS1P caused up to a 40% decrease in proliferation (Fig. 1A). In BxPc-3 (Fig. 1B) and Panc 3.014 (Fig. 1C), SS1P caused a very small decrease in cell proliferation (< 10%), and a dose-response was absent. At the highest concentrations, ABT-737 induced a minor decrease in KLM-1 proliferation, whereas it slightly increased proliferation in BxPc-3 and Panc 3.014. When SS1P and ABT-737 were combined there was a substantial dose-dependent decrease in KLM-1 proliferation. At the highest concentrations of SS1P and ABT-737, KLM-1 proliferation was virtually completely arrested. In BxPc-3, a similar dose-response effect was observed when combining both compounds, although to a lesser extent. In this cell line, the benefit was especially obvious when combining 10 μM ABT-737 with either 50 or 100 ng/mL SS1P. In Panc 3.014, no decrease in cell proliferation was observed when combining SS1P with ABT-737. These data indicate that the combination of SS1P and ABT-737 induces a dose-response decrease in proliferation, although efficacy varies among the cell lines.
FIGURE 1.
Combining SS1P and ABT-737 induces dose-response decrease in cell proliferation. KLM-1 (A), BxPc-3 (B), and Panc 3.014 cell (C) proliferation data are presented after a 24-hour incubation with SS1P (10, 50, or 100 ng/mL), and/or ABT-737 (1, 5, or 10 μM). WST-8 data are normalized for staurosporine controls (1 μM) and untreated controls. Combining SS1P and ABT-737 had a significant dose-response effect on KLM-1 (A) and a moderate effect on BxPc-3 (B). In Panc 3.014 (C), no effect was observed.
Combining SS1P and ABT-737 Induces Cell Death
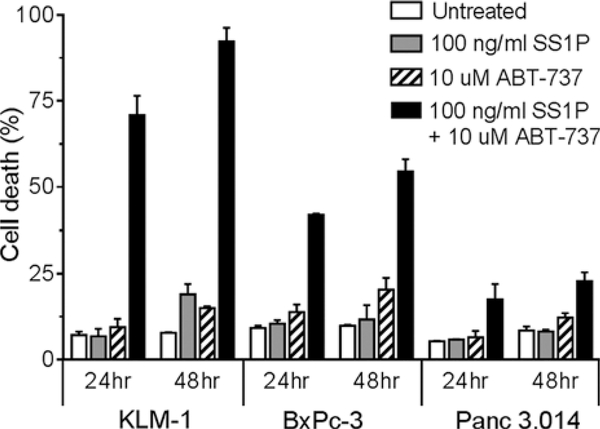
Short-term growth assays cannot differentiate between proliferation arrest and real cell death.25 Therefore, we characterized the cytotoxic effect of SS1P and ABT-737 by staining cells with Annexin V-PE and 7-AAD. Cell lines were incubated with 100 ng/mL SS1P, 10 μM ABT-737 or both for 24 and 48 hours. Results showed that at both time points, SS1P and ABT-737 alone induced little or no cell death, compared with the untreated controls (Fig. 2). Compared with SS1P, the combination with ABT-737 increased cell death in all 3 cell lines. After 24 hours, cell death was 71% in KLM-1 (P < 0.01), 42% in BxPc-3 (P < 0.01), and 18% in Panc 3.014 cells (P = 0.12). At 48 hours, the combination further increased cell death, up to 92% in KLM-1, resulting in a significant increase in cell death in all 3 cell lines compared with SS1P alone (KLM-1, P < 0.01; BxPc-3 and Panc 3.014, P < 0.05). These data indicate that the combination with ABT-737 induces cell death in SS1P-resistant PDAC cell lines.
FIGURE 2.
Combining SS1P and ABT-737 increases cell death. KLM-1, BxPc-3, and Panc 3.014 cell death data are presented after a 24- and 48-hour incubation with 100 ng/mL SS1P and/or 10 μM ABT-737, as evaluated with Annexin V-PE and 7-AAD. Summary data of 2 independent experiments is presented. SS1P and ABT-737 separately did not induce meaningful cell death compared with the untreated cells. The combination increased cell death in all 3 cell lines, most markedly in KLM-1.
Enhancement of Killing by ABT-737 is SS1P-specific in KLM-1
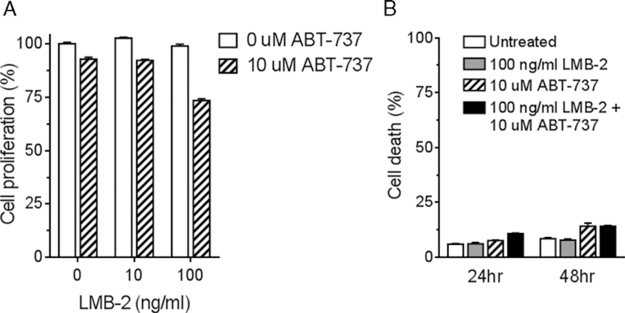
To examine the specificity of the ABT-737-enhanced increase in cell death, we incubated KLM-1 with ABT-737 and LMB-2 [anti-Tac(Fv)-PE38], an anti-CD25 RIT designed to target lymphocytic malignancies26 that does not bind KLM-1. We found that after 24 hours, 10 or 100 ng/mL LMB-2 had no effect on cell proliferation. Similar to earlier findings, 10 μM ABT-737 caused a minor decrease in KLM-1 proliferation. The combination with a 100 ng/mL LMB-2 induced an additional decrease in cell proliferation (−20%; Fig. 3A). Using Annexin V-PE and 7-AAD staining, however, no meaningful increase in apoptotic cells was observed when cells were treated for 24 hours with 100 ng/mL LMB-2 and 10 μM ABT-737. Similarly, at 48 hours there was no difference in cell death between ABT-737 alone and the combination with LMB-2 (Fig. 3B). This is in clear contrast with the effect that the combination of SS1P and ABT-737 had on KLM-1 (Figs. 1A, 2). These data demonstrate that ABT-737 only enhances cell death when combined with an RIT that binds to the cell.
FIGURE 3.
Enhancement of cell death by ABT-737 is SS1P-specific. KLM-1 cells were incubated with ABT-737 (10 μM) and/or LMB-2 (10 or 100 ng/mL). LMB-2 is an immunotoxin targeted at CD25, a B-cell-specific antigen. A, Cell proliferation was evaluated after 24 hours with the WST-8 assay. B, Cell death was evaluated after 24 and 48 hours by Annexin V-PE and 7-AAD staining. The combination of LMB-2 and SS1P did not increase cell death.
Mesothelin Expression, SS1P Internalization, and Furin Levels
Mesothelin is required for the binding of SS1P, and the receptor-mediated internalization has been shown to affect the cytotoxicity of RITs.27 Panc 3.014 had the highest mesothelin expression (92,000 ± 4500 mesothelin-binding sites/cell), followed by KLM-1 (58,000 ± 3700 mesothelin-binding sites/cell), and BxPc-3 (3000 ± 600 mesothelin-binding sites/cell). All 3 cell lines internalized SS1P, and the addition of ABT-737 did not improve the uptake of the RIT after a 3-hour incubation (Fig. 4). Once SS1P is internalized, furin is required to process and activate the toxin. In all 3 PDAC cell lines, furin was present in similar levels (Supplementary material, Supplemental Digital Content 1, http://links.lww.com/JIT/A308). These data indicate that the low cytotoxicity of the SS1P and ABT-737 combination in Panc 3.014 is not due to a lack of mesothelin or furin, or an impaired SS1P internalization.
FIGURE 4.
SS1P internalization is not enhanced by ABT-737. KLM-1, BxPc-3, and Panc 3.014 cells were incubated for 3 hours at 37°C with 2 μg/mL SS1P-Alexa647, alone and in combination with 10 μM ABT-737, after which cells were stripped from surface-bound SS1P and the internalized SS1P was evaluated by flow cytometry. All 3 cell lines internalize SS1P in accordance with their mesothelin expression, and the addition of ABT-737 did not enhance SS1P uptake. Gray dotted line: unstained control; gray solid line: SS1P-Alexa647 + ABT-737; black line: SS1P-Alexa647.
Combining SS1P and ABT-737 Enhances Protein Synthesis Inhibition
After trafficking to the cytosol, SS1P inhibits protein synthesis, a crucial step in the initiation of apoptosis.3 Previous research in non-PDAC cell lines found that ABT-737 can enhance RIT-induced protein synthesis inhibition.18 To examine whether this also applies to PDAC, KLM-1, BxPc-3, and Panc 3.014 were incubated for 8 hours with 100 ng/mL SS1P, 10 μM ABT-737, or both, after which 3H leucine incorporation was measured. SS1P induced a small and variable decrease in protein synthesis, up to 18% in KLM-1 (Fig. 5). ABT-737 had no significant effect on protein synthesis in the cell lines (P > 0.05). When cells were treated with both SS1P and ABT-737, protein synthesis inhibition was increased by 25% in KLM-1 (P < 0.01), 18% in BxPc-3 (P = 0.10), and 4% in Panc 3.014 (P = 0.62), compared with the effect of SS1P alone. These data demonstrate that ABT-737 enhances SS1P-induced protein synthesis in PDAC to a variable extent, in accordance with the cytotoxicity of this combination.
FIGURE 5.
Combining SS1P and ABT-737 enhances protein synthesis inhibition. Protein synthesis inhibition data of KLM-1, BxPc-3, and Panc 3.014 cells after an 8-hour incubation with 100 ng/mL SS1P and/or 10 μM ABT-737. 3H leucine incorporation is presented relative to untreated controls. Combining SS1P and ABT-737 enhanced the SS1P-induced protein synthesis inhibition. Summary data of 3 independent experiments is presented.
BCL2 Family Protein Levels in PDAC Cell Lines
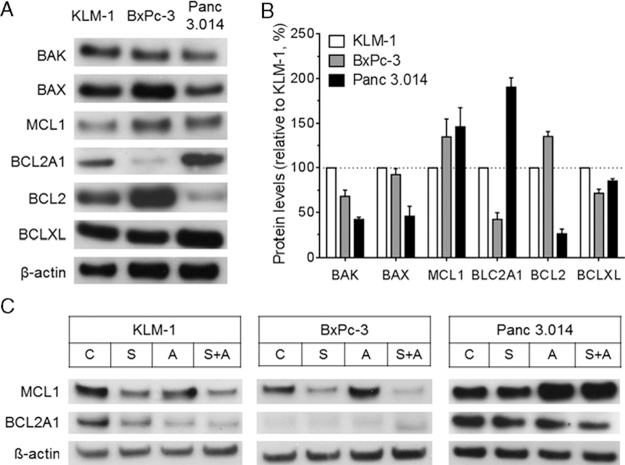
In addition to protein synthesis inhibition, the balance between proapoptotic and antiapoptotic proteins is crucial for the induction of cell death. We previously found that BAK plays an important role in resistance to SS1P.10,17 To determine whether differences in the intrinsic apoptotic pathway are present among the studied PDAC cell lines, we analyzed proapoptotic proteins BAK and BAX, and antiapoptotic proteins MCL1, BCL2A1, BCL2, and BCLXL (Figs. 6A, B). BAK was significantly lower in both BxPc-3 (−32%, P < 0.01) and Panc 3.014 (−58%, P < 0.001), compared with KLM-1. BAX was similar in KLM-1 and BxPc-3 (P = 0.45), but markedly lower in Panc 3.014 (−54%, P < 0.01). MCL1 was higher in BxPc-3 (+ 35%, P = 0.27) and Panc 3.014 (+46%, P = 0.19) than in KLM-1, although these differences did not reach statistical significance. BCL2A1 was significantly lower in BxPc-3 (−58%, P < 0.001) and higher in Panc 3.014 (+ 90%, P < 0.001), relative to KLM-1. BCL2 was higher in BxPc-3 (+ 35%, P < 0.01), and lower in Panc 3.014 (−74%, P < 0.001) compared with KLM-1. BCLXL was lower in BxPc-3 (−28%, P < 0.05) and Panc 3.014 (−15%, P < 0.05). These data suggest that the levels of BAK, BAX, BCL2A1, and possibly MCL1 are associated with the efficacy of the SS1P and ABT-737 combination.
FIGURE 6.
BCL2 family proteins in PDAC cell lines. A, Western blot data of untreated KLM-1, BxPc-3, and Panc 3.014 cell lysates probed for BAK, BAX, MCL1, BCL2A1, BCL2, BCLXL, and β-actin. B, Summary data of 3 independent experiments is presented. Protein levels are adjusted for β-actin and presented relative to KLM-1. C, Western blot data for lysates of KLM-1, BxcPc-3, and Panc 3.014 cells that were treated for 24 hours with 20 ng/mL SS1P (S), 10 μM ABT-737 (A) or the combination (S + A), probed for MCL1, BCL2A1, and β-actin. PDAC indicates pancreatic ductal adenocarcinoma.
We evaluated how SS1P and ABT-737 treatment affected the levels of short-lived antiapoptotic MCL1 and BCL2A1, 2 proteins with a low affinity to ABT-737 (Fig. 6C). Cells were treated for 24 hours with 20 ng/mL SS1P, 10 μM ABT-737 or the combination. Results show that SS1P markedly downregulated MCL1 in KLM-1 and BxPc-3, but not in Panc 3.014. ABT-737 alone induced an upregulation of MCL1, most obvious in Panc 3.014. The combination of SS1P and ABT-737 further improved the downregulation of MCL1 in KLM-1 and BxPc-3, but had no effect in Panc 3.014. For BCL2A1, similar observations were made. These data are in close accordance with the protein synthesis inhibition as shown previously (Fig. 5), and help to explain why Panc 3.014 is most resistant to the combination of SS1P and ABT-737.
DISCUSSION
We report that combining the antimesothelin RIT SS1P and BH3-mimetic ABT-737 induces cell death in SS1P-resistant PDAC cell lines.
Cell death appeared quickly, with significant differences noted after 24 hours. However, the efficacy of the combination varied greatly among the 3 cell lines. With KLM-1, virtually all cells died after 48 hours, whereas roughly 50% of BxPc-3 and 75% of Panc 3.014 cells remained alive. ABT-737 only enhanced cell death in combination with an RIT that can bind to the cell. Previous reports show that ABT-737 combined with TRAIL28 or with actinomycin D29 increased cell death in 2 PDAC cell lines, Panc-1 and BxPc-3. TRAIL activates the extrinsic apoptotic pathway, whereas actinomycin D decreases MCL1 levels, similar to SS1P. In agreement with our observations, these studies showed that high concentrations of ABT-737 (up to 20 μM) had no effect, and none of the 2 combinations completely killed the PDAC cell lines.28,29 Of interest, not all combinations with ABT-737 have proven successful in PDAC. The combination with 2-deoxyglucose, which partially blocks glycolysis, enhanced cell death in several cancer cell lines, but not in Panc-1.30
SS1P and ABT-737 have to overcome many hurdles to successfully kill a cancer cell. We investigated several of these potential blocks, in a search for clues that could explain the observed differences in efficacy among PDAC cell lines. A first step that can affect cytotoxicity is RIT internalization.27 However, the cell line least sensitive to the combination, Panc 3.014, internalized most SS1P at a 3-hour time point and had the highest mesothelin expression (1.6-fold and 30-fold higher than KLM-1 and BxPc-3, respectively). In addition, the combination of SS1P with ABT-737 did not enhance RIT internalization in any of the cell lines. These data indicate that neither mesothelin expression nor SS1P internalization can account for the differences in the cytotoxicity of SS1P and ABT-737 among the PDAC cell lines.
After internalization, the protease furin cleaves SS1P, separating the Fv from the toxin. RITs are consequently less active in a furin-deficient or furin-inhibited cell line.31 We found that furin was present at similar levels in all 3 PDAC cell lines, and therefore does not present a limiting factor. After furin cleavage, the toxin moves from the endocytic compartment to the ER and translocates to the cytosol, where it inactivates protein synthesis, a key step in apoptosis. In agreement with previous work,10 SS1P alone induced little protein synthesis inhibition in PDAC cell lines. The addition of ABT-737 had an enhancing effect that was largest in KLM-1, smaller in BxPc-3, and negligible in Panc 3.014, which clearly correlated with the cytotoxicity of the combination. These data are in agreement with the previous findings in non-PDAC cell lines, where ABT-737 is thought to promote the transfer of toxin to the cytosol.18 Our findings indicate this could be a common mechanism in many cell types. We evaluated the effect of protein synthesis inhibition on 2 critical antiapoptotic proteins, MCL1 and BCL2A1. Both are short-lived proteins and have a low affinity to ABT-737, making them particularly relevant to our combination. MCL1 was significantly downregulated by SS1P in KLM-1 and BxPc-3, while the combination with ABT-737 further enhanced this decrease. This was not the case in Panc 3.014, which correlates with the cytotoxicity and protein synthesis inhibition data. Similar observations were made for BCL2A1.
However, even profound inhibition of protein synthesis and the associated decrease of short-lived antiapoptotic proteins do not guarantee cell death in PDAC cell lines or other cancer types.10,18 The cytotoxicity of any compound or combination also depends on the balance between proapoptotic and antiapoptotic proteins.32,33 We previously reported that low or absent proapoptotic BAK is associated with resistance to PE-mediated cell death.3,17 In this study, the 2 least sensitive cell lines, BxPc-3 and Panc 3.014, had lower levels of BAK than KLM-1. The proapoptotic BAX had similar levels in KLM-1 and BxPc-3, but was lower in Panc 3.014. Thus, both members of the apoptosis effector arm were significantly lower in Panc 3.014. In addition, Panc 3.014 had higher BCL2A1 and a trend toward higher MCL1 levels compared with KLM-1. These observations, combined with the inability of SS1P or the combination with ABT-737 to significantly downregulate both MCL1 and BCL2A1 can explain the high resistance in Panc 3.014. The levels of the antiapoptotic BCL2 and BCLXL did not correlate with SS1P and ABT-737 cytotoxicity. These two proteins levels might play less of a role in resistance to SS1P and ABT-737, as ABT-737 binds both with great affinity.13
Overall, these findings provide clues to the variable efficacy of SS1P and ABT-737 that we observed among PDAC cell lines, and outline options for further research. First, the variability in protein synthesis inhibition suggests cell-line differences upstream of this event, other than mesothelin and furin expression, and SS1P internalization. Topics for further study include differences in intracellular trafficking and aberrations in the protein synthesis inhibition process.34,35 Second, the correlation with key BCL2 family proteins suggests that mitochondrial apoptosis could be a limiting factor for the efficacy of SS1P and ABT-737. Other apoptotic proteins might also play a role, and can provide additional targets to tip the balance further toward death. Third, to assess the therapeutic use of this combination, in vivo evaluation is required in appropriate animal models.
In conclusion, our findings support the combination of SS1P and a BH3-mimetic to overcome resistance in PDAC cells, and identify factors that potentially limit the efficacy of such therapeutic approach.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr Xiufen Liu for technical assistance, Dawn A. Walker for reading and editing the manuscript, and Anna Mazzuca for help in the submission process.
Footnotes
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by the Intramural Research Program of the National Institutes of Health, NCI, CCR.
All authors have declared there are no financial conflicts of interest with regard to this work.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.immunotherapy-journal.com.
REFERENCES
- 1.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;13:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastan I, Hassan R, Fitzgerald DJ, et al. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. [DOI] [PubMed] [Google Scholar]
- 3.Weldon JE, Pastan I. A guide to taming a toxin—recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. [DOI] [PubMed] [Google Scholar]
- 6.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 7.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P a recombinant anti-mesothelin immunotoxin given as a bolus IV infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. [DOI] [PubMed] [Google Scholar]
- 8.Kreitman RJ, Hassan R, FitzGerald DJ, et al. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Verschraegen CF, Mendoza J, et al. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv) PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004; 24:1327–1335. [PubMed] [Google Scholar]
- 10.Du X, Xiang L, Mackall C, et al. Killing of resistant cancer cells with low Bak by a combination of an antimesothelin immunotoxin and a TRAIL receptor 2 agonist antibody. Clin Cancer Res. 2011;17:5926–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008; 9:47–59. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. [DOI] [PubMed] [Google Scholar]
- 14.Vogler M, Dinsdale D, Dyer MJ, et al. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–367. [DOI] [PubMed] [Google Scholar]
- 15.Letai A Restoring cancer’s death sentence. Cancer Cell. 2006;10:343–345. [DOI] [PubMed] [Google Scholar]
- 16.Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007; 282:6192–6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du X, Youle RJ, FitzGerald DJ, et al. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol Cell Biol. 2010;30:3444–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traini R, Ben-Josef G, Pastrana DV, et al. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of pseudomonas exotoxin-based proteins to the cell cytosol. Mol Cancer Ther. 2010;9:2007–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risberg K, Fodstad O, Andersson Y. Synergistic anticancer effects of the 9.2.27PE immunotoxin and ABT-737 in melanoma. PLoS One. 2011;6:e24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattoo AR, Fitzgerald DJ. Combination treatments with ABT-263 and an immunotoxin produce synergistic killing of ABT-263-resistant small cell lung cancer cell lines. Int J Cancer. 2013;132:978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–518. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y, Kobari M, Yusa T, et al. Establishment of an experimental liver metastasis model by intraportal injection of a newly derived human pancreatic cancer cell line (KLM-1). Int J Pancreatol. 1996;20:43–50. [DOI] [PubMed] [Google Scholar]
- 23.Onda M, Willingham M, Nagata S, et al. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting,Western blotting, and ELISA. Clin Cancer Res. 2005;11:5840–5846. [DOI] [PubMed] [Google Scholar]
- 24.Rasband WS. ImageJ. Bethesda, MD: US National Institutes of Health; 1997–2012. Available at: http://rsbweb.nih.gov/ij/. [Google Scholar]
- 25.Kepp O, Galluzzi L, Lipinski M, et al. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011;10:221–237. [DOI] [PubMed] [Google Scholar]
- 26.Kreitman RJ, Wilson WH, Robbins D, et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood. 1999;94:3340–3348. [PubMed] [Google Scholar]
- 27.Du X, Beers R, Fitzgerald DJ, et al. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008; 68:2944–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olberding KE, Wang X, Zhu Y, et al. Actinomycin D synergistically enhances the efficacy of the BH3 mimetic ABT-737 by downregulating Mcl-1 expression. Cancer Biol Ther. 2010;10:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi R, Janssen E, Perkins G, et al. Efficient elimination of cancer cells by deoxyglucose-ABT-263/737 combination therapy. PLoS One. 2011;6:e24102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiron MF, Fryling CM, FitzGerald D. Furin-mediated cleavage of Pseudomonas exotoxin-derived chimeric toxins. J Biol Chem. 1997;272:31707–31711. [DOI] [PubMed] [Google Scholar]
- 32.Chipuk JE, Moldoveanu T, Llambi F, et al. The BCL-2 family reunion. Molecular Cell. 2010;37:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. [DOI] [PubMed] [Google Scholar]
- 34.Wei H, Xiang L, Wayne AS, et al. Immunotoxin resistance via reversible methylation of the DPH4 promoter is a unique survival strategy. Proc Natl Acad Sci U S A. 2012;109:6898–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tortorella LL, Pipalia NH, Mukherjee S, et al. Efficiency of immunotoxin cytotoxicity is modulated by the intracellular itinerary. PLoS One. 2012;7:e47320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.