Abstract
Human malignant pleural mesothelioma (HMPM) is highly resistant to conventional therapy, and therefore novel therapies are required. We previously reported that overexpression of the FUSE-binding protein-interacting repressor (FIR), a c-myc transcriptional repressor, induces apoptosis via c-Myc suppression, and is thus a suitable cancer therapy. In the current preclinical trial, a fusion gene deleted non-transmissible Sendai virus vector encoding FIR (SeV/ΔF/FIR) was prepared and its cytotoxic activity against an orthotopic xenograft model of HMPM, in combination with cisplatin, was assessed. SeV/ΔF/FIR and a fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein (SeV/ΔF/GFP) were prepared. The transduction efficiency of these agents in terms of dose-dependent cytotoxicity and/or apoptosis induction was then assessed in a few HMPM cells. Combination therapy with SeV/ΔF/FIR plus cisplatin was evaluated in vitro and in a mouse model. SeV/ΔF/FIR significantly reduced cell viability in three HMPM cell lines but was less effective in non-tumor immortalized mesothelial cells. SeV/ΔF/FIR cytotoxicity was partly due to apoptosis induction via c-Myc suppression. In addition, SeV/ΔF/FIR showed synergistic antitumor effects in combination with cisplatin, as was revealed by isobologram analysis in MSTO-211H. Moreover, combination therapy with SeV/ΔF/FIR plus cisplatin demonstrated significant tumor reduction and improvement in survival rate in an animal model. Combination therapy with SeV/ΔF/FIR plus cisplatin has therapeutic potential against HMPM. SeV/ΔF/FIR plus cisplatin will be an attractive modality against HMPM in the future.
Human malignant pleural mesothelioma (HMPM), which commonly originates from mesothelial cells lining the pleural cavity, is an aggressive tumor that is difficult to treat.(1) The number of HMPM patients is predicted to increase because of the long latency of the disease and historical exposure to asbestos(2) To date, standard therapies against HMPM are surgery, chemotherapy and/or radiation, but all are unsatisfactory (median survival, 6–12 months).(3) Gene therapy has been considered for HMPM treatment because of easy accessibility to the intrapleural cavity.(4,5) For example, intrapleural delivery of replication-deficient adenoviral vectors expressing the suicide gene herpes simplex thymidine kinase followed by the administration of ganciclovir was reported as an antiviral therapy.(6)
Safe and highly efficient transduction of viral vectors is required for the success of gene therapies. The Sendai virus (SeV) is a candidate vector for this purpose; it is a member of the Paramyxoviridae family with a non-segmented negative-strand RNA genome, and it infects targets via sialic acid residues through glycoproteins or asialoglycoproteins, which are present on the cell surface of most mammalian cells.(7,8) Fusion protein is necessary for SeV transmission and SeV is relatively safe because it replicates only in the cytoplasm and is not incorporated into the host cell’s genomic DNA.(9)
Elevated c-Myc expression has been detected in a broad range of human cancers, indicating its key role in tumor development.(10) Human malignant pleural mesothelioma also shows elevated c-Myc expression (11) Previous reports have indicated that the FUSE-binding protein-interacting repressor (FIR) strongly represses c-myc transcription by inhibiting TFIIH (P89) DNA helicase activity’(12) and induces apoptosis;(13) thus, the FIR expression vector is a potential candidate for cancer gene therapy.(14) In the current preclinical study, a fusion gene deleted non-transmissible Sendai virus vector encoding FIR (SeV/ΔF/FIR) was prepared and its efficacy against HMPM was determined. Specifically, the cytotoxic activity of SeV/ΔF/FIR against HMPM in vitro and in vivo was explored; we also determined its therapeutic feasibility with and without cisplatin.
Materials and Methods
Cell lines and culture conditions.
Three HMPM cell lines, MSTO-211H (211H), H2452 and H226, and a non-tumorigenic mesothelial cell line, Met5A, were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were grown in RPMI 1640, supplemented with 10% fetal bovine serum (FBS), 1% (v/v) penicillin and streptomycin (100 U/mL; Invitrogen, Carlsbad, CA, USA), and grown in a humidified atmosphere containing 5% CO2 at 37°C.
Preparation of non-transmissible recombinant Sendai virus vectors.
A fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein (SeV/ΔF/GFP) was prepared as previously described.(15) SeV/ΔF/FIR was prepared according to the same procedure. In brief, human FIR cDNA was amplified with a pair of NotI site-tagged primers containing SeV-specific transcriptional regulatory signal sequences (end and start italicized), 5′-ATTGCGGCCGCCAAGGTTCAATGGCGACGGCGACCATAGC-3′ and 5′-ATTGCGGCCGCGATGAACTTTCACCCTAAGTTTTTCTTACTACGGTCACGCAGAGAGGTCACTGTTATCAAAACGC-3′. The amplified fragment was introduced into the NotI site of the parent SeV vector cDNA, pSeV18+b(+)/ΔF to generate pSeV18+hFIR-SeV/ΔF. pSeV18+hFIR-SeV/ΔF was then transfected into LLC-MK2 cells that were preliminarily infected with psoralen- and long-wave UV-treated vaccinia virus vTF7-3 expressing T7 polymerase. The cells were then washed twice with minimum essential medium (MEM) and cultured for 24 h in MEM containing cytosine β-D-arabinofuranoside (AraC; 40 μg/mL) and trypsin (7.5 μg/mL). LLC-MK2/F7/A cells expressing the F protein were suspended in MEM containing AraC and trypsin, layered onto the transfected cells, and cultured at 37°C for an additional 48 h. The virus titer was determined by infectivity and expressed using cell-infectious units (CIU). Titers of the recovered viral vectors were also expressed as CIU. These vectors were kept frozen at −80°C until use.
Fluorescent microscopy.
Expression of the GFP was assessed and photographed using the LSM 510-V3.5 fluorescent microscope (Carl Zeiss, Goettingen, Germany).
SeV/ΔF/GFP transduction efficiency.
One million 211H, H2452, H226 and Met5A cells were seeded in six-well plates and transduced with SeV/ΔF/GFP. Monolayer cells were washed twice with phosphate-buffered saline (PBS) and overlaid with serum-free medium containing SeV/ΔF/GFP at a multiplicity of infection (MOI) of 0, 10, 100 and 1000. The cells were incubated for over 72 h at 37°C. Transduction studies were performed in triplicate for each MOI. Transduction efficiency was calculated by counting the GFP-positive cells per 1000 cells at ×40 magnification under the LSM 510-V3.5 fluorescent microscope.
Western blotting and antibodies.
Protein extracts were separated by electrophoresis on a 7.5–15% Perfect NT Gel (DRC, Tokyo, Japan). Proteins were then transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) in a tank transfer apparatus (Bio-Rad, Hercules, CA, USA), and the membranes were blocked with 0.5% skim milk in PBS. The primary mouse monoclonal antibody against FIR C-terminus (6B4) was prepared by Dr Nozaki (Mitsubishi Kasei Institute of Life Sciences, Machida, Japan).(16) In brief, the synthetic peptide C+KVVAEVYDQERFDNSDLSA (C+541–559; numbers indicate the amino acids of PUF60)(17) was used as the immunization antigen. Primary antibodies, anti-FIR (6B4), mouse polyclonal anti-c-Myc, rabbit polyclonal anti-P89, goat polyclonal anti-[β-actin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), mouse monoclonal anti-caspase-9, and mouse monoclonal anti-caspase-3 (Upstate Biotechnology, Lake Placid, NY, USA) were diluted at 1:100, 1:1000, 1:1000, 1:500, 1:1000 and 1:1000, respectively. Rabbit polyclonal anti-mouse IgG horseradish peroxidase conjugate (HRP; Dako, Glostrup, Denmark) diluted 1:1000, rabbit polyclonal anti-goat IgG HRP (Cappel, West Chester, PA, USA) diluted 1:500 and donkey polyclonal anti-rabbit IgG HRP (Amersham Pharmacia Biotech, Piscata-way, NJ, USA) diluted 1:2000 were used as secondary antibodies. Antigens on the membrane were detected with enhanced chemiluminescence detection reagents (GE Healthcare, UK Ltd, Buckinghamshire, UK).
In vitro cytotoxicity assay.
Cytopathic effects were assessed by the CellTiter 96® AQueous One Solution Cell Proliferation Assay reagent 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymeth-oxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega, Madison, WI, USA) according to the manufacturer’s instructions. Relative viability was calculated based on absorbance without any treatment.
In brief, 3 × 103 cells/well from 211H, H2452, H226 and Met5A cell lines were seeded in 96-well plates and infected with SeV/ΔF/GFP and SeV/ΔF/FIR at 10, 100 and 1000 MOI. The cells were incubated for over 72 h at 37°C. To detect cell viability, 10 μL of MTS (CellTiter 96® AQueous; Promega Corporation, Madison, WI, USA) solution (5 mg/mL) was added to each well and incubated for 4 h at 37°C. Thereafter, 100 μL of 10% sodium dodecylsulfate was added and the absorbance was measured at 490 nm using an enzyme-linked immunosorbent assay plate reader.
Apoptosis detection assay.
Cells were treated with SeV/ΔF/GFP or SeV/ΔF/FIR for 72 h. DNA was stained with 4V, 6-diamidino-2-phenylindole (DAPI) II (Vysis, Abbott Park, IL, USA) and observed by immunofluorescence microscopy (Leica QFISH; Leica Microsystems, Tokyo, Japan). Apoptosis was assessed using the APOPercentage apoptosis assay kit (Biocolor, County Antrim, Northern Ireland) (18) and observed with an inverted microscope.
Isobologram analysis of synergistic effects.
The 50% effective dose (ED50) for SeV/ΔF/FIR (MOI) was defined as the initial viral dose that induced 50% cell viability at 72 h after inoculation compared with the control. Cisplatin (Nihon Kayaku Corporation, Tokyo, Japan) sensitivity of mesothelioma cells was expressed as the drug concentration that inhibited cell growth by 50% compared with untreated controls (IC50). The combined effects of SeV/ΔF/FIR plus cisplatin were assessed by isobologram analysis (19) and the combination index (CI) method.(20,21) In brief, 211H cells in 96-well microplates were exposed in triplicate to serial dilutions of each agent or both agents using a constant ratio combination design for 72 h; the MTS assay was then performed to determine cell viability. Calculated CI was used to ascertain the presence of synergism (CI < 1), an additive effect (CI = 1) or antagonism (CI > 1) between SeV/ΔF/FIR and cisplatin.(22)
Orthotopic xenograft HMPM implantation model.
Eight-week-old male BALB/c/nunu mice were purchased from Clea Japan (Tokyo, Japan). All animal experiments were approved by the Committee of Ethics on Animal Experiments in the Faculty of Medicine, Chiba University, and carried out according to the Guidelines for Animal Experiments of Chiba University, Chiba, Japan and the Law and Notification of the Government. 211H cells were washed twice and resuspended in PBS. Thereafter, 1 × 106 211H cells in 100 μL PBS were injected with a 27-gauge needle into the thoracic cavity of nude mice.(23) The same technique was used for SeV/ΔF/FIR and SeV/ΔF/GFP injections. The mice were killed 4 weeks after tumor cell inoculation, and the thoracic tumors were carefully removed and weighed on day 29. For future histological analysis, the tumors were excised and embedded with Optimal Cutting Temperature compound (Sakura Finetechnical, Tokyo Japan) for frozen sections. Histological sections of the tumors were stained with hematoxylin and eosin (HE). To detect apoptotic cells, the sections were subjected to a terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay (Roche Applied Science, Mannheim, Germany).
Blood analysis in the animal model.
To evaluate the adverse effects of SeV/ΔF/FIR, hematological values and blood biochemistry, including total bilirubin, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, blood urea nitrogen and creatinine, were examined (SRL, Tokyo, Japan). The hemogram was assessed by May-Giemsa staining.
Statistical analysis.
Summary statistics were constructed using frequencies and proportions for categorical data, and means and standard deviations (SD) for continuous variables. We compared control and test groups using Fisher’s exact test for categorical outcomes and t-tests for continuous variables as appropriate. For time-to-event outcomes, the distribution of time to a first event was compared using the log-rank test, while the Kaplan–Meier method was used to estimate the absolute risk of each event for each group, and hazard ratios and 95% confidence intervals were estimated by the Cox proportional hazards model. All comparisons were planned and the tests were two-sided. A P-value of <0.05 was considered to be statistically significant. All statistical analyses were performed using the SPSS software version 16 (SPSS Institute, Chicago, IL, USA) and SAS software program, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Transduction efficiency and cytotoxicity of the SeV/ΔF/GFP vector against HMPM and mesothelial cells in vitro.
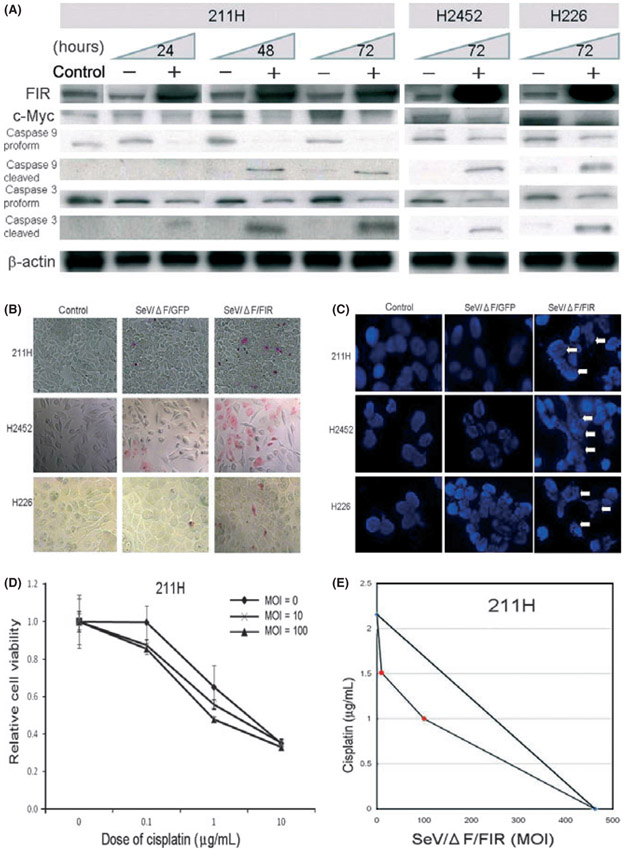
GFP-expressing positive cells were counted under the microscope (Fig. 1A). Optimal expression of a dose-dependent increase in GFP-positive cells was obtained at 10, 100 and 1000 MOI. More than 90% of GFP-positive cells were detected at MOI over 1000 (Fig. 1B). SeV/ΔF/FIR at 100 MOI significantly reduced the number of viable cancer cells (Movie S1) compared with SeV/ΔF/GFP and the control (PBS; Fig. 1A). As FIR is a c-myc transcriptional repressor, the efficiency of SeV/ΔF/FIR might differ depending on the c-Myc expression level. Thus, expression levels of c-Myc and other proteins (FIR and P89) were determined in three different HMPM cells and immortalized non-tumor mesothelial cells. c-Myc expression was relatively upregulated in 211H compared with other cell lines (H2452, H226, Met5A; Fig. 1C). With this knowledge, SeV/ΔF/FIR cytotoxicity was examined using a MTS assay. Although the SeV/ΔF/GFP infection rate and c-Myc expression level differed among cells (Fig. 1B,C), all the mesothelioma cells (211H, H2452 and H226) were more efficiently killed by SeV/ΔF/FIR than SeV/ΔF/GFP in a dose-dependent manner; however, this was not the case against the immortalized non-tumor mesothelial cells (Met5A; Fig. 1D, Table 1).
Fig. 1.
(A) Microscopic observation (×400) of human malignant pleural mesothelioma (HMPM) cell lines (211H, H2452 and H226), a mesothelial cell line (Met5A) 72 h after infection with fusion gene deleted non-transmissible Sendai virus vector encoding FUSE-binding protein-interacting repressor (SeV/ΔF/FIR) or fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein (SeV/ΔF/GFP) at 100 multiplicity of infection (MOI), and the control (PBS). GFP-transduced cells were observed under fluorescence microscopy. SeV/ΔF/FIR induced significant reduction in cells. (B) Transduction efficiency of 211H, H2452, H226 and Met5A with SeV/ΔF/GFP at 10, 100 and 1000 MOI. The bar depicts the percentage of GFP-positive cells at 72 h after transduction with SeV/ΔF/GFP (error bars, SD). (C) Endogenous expression of FIR, c-Myc and P89 among three HMPM cells and immortalized non-tumor mesothelial cells. β-actin was used as the loading control. (D) Cytotoxic activities of SeV/ΔF/FIR in 211H, H2452, H226 and Met5A. Cells were infected with different doses of SeV/ΔF/FIR (■) or SeV/ΔF/GFP (♦) at 10, 100 and 1000 MOI; a MTS cell viability assay was performed 72 h after infection. Relative cell viability was calculated based on the value of uninfected cells as 100% by the MTS assay (error bars, SD). One hundred MOI of SeV/ΔF/FIR for 72 h was sufficient to induce apoptosis.
Table 1.
ED50 values for SeV/ΔF/FIR in three human malignant pleural mesothelioma cell lines and a mesothelial cell line
| Cell line | ED50 (MOI) | P-value |
|---|---|---|
| 211H | 472.1 ± 171.4 | |
| H2452 | 124.3 ± 19.9 | 0.026 |
| H226 | 164.7 ± 13.5 | 0.037 |
| Met5A | 1.96 × 105 ± 2.27 × 105 | 0.274 |
Each value represents the median ± SD. P-value, compared with 211H. ED50, 50% effective dose; MOI, multiplicity of infection; SeV/ΔF/FIR, fusion gene deleted non-transmissible Sendai virus vector encoding FUSE-binding protein-interacting repressor.
SeV/ΔF/FIR induced apoptosis in HMPM with endogenous c-Myc suppression.
We previously reported that FIR induces apoptosis in HeLa cells and human cervical squamous carcinoma cells via c-Myc suppression.(13) FIR-adenoviral vector also demonstrates strong cytotoxicity against human esophageal cancer cells.(14) To determine whether SeV/ΔF/FIR also induces apoptosis in HMPM cells, we examined the expression levels of FIR, c-Myc, caspase-3 and caspase-9 after SeV/ΔF/FIR transduction (Fig. 2A). As expected, SeV/ΔF/FIR transduction increased FIR expression and decreased expressions of c-Myc and proforms of caspase-3 and caspase-9, but increased cleaved caspase-3 and caspase-9 expressions compared with SeV/ΔF/GFP in all HMPM cells examined (Fig. 2A). Furthermore, APOPercentage assay (Fig. 2B) and DNA fragmentation (Fig. 2C) indicated that SeV/ΔF/FIR transduction induced apoptotic cells. These results indicate that SeV/ΔF/FIR cytotoxicity is induced at least in part through apoptosis, even in HMPM cells.
Fig. 2.
(A) Expression of FUSE-binding protein-interacting repressor (FIR), c-Myc, caspase 9 and caspase 3 after SeV/ΔF transduction. Lysate samples (20 μg/lane) from HMPM cells (211H, H2452 and H226) after fusion gene deleted non-transmissible Sendai virus vector encoding FIR (SeV/ΔF/FIR) transduction (+) at 100 MOI for 24, 48 and 72 h, medium only (control) and fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein (SeV/ΔF/GFP) (−) were subjected to western blot analysis. β-actin was used as the loading control. (B) APOPercentage Assay was used to detect early apoptosis in 211H, H2452 and H226 cells. More apoptotic cells were observed within 72 h after infection with SeV/ΔF/FIR at 100 multiplicity of infection (MOI) compared with SeV/ΔF/GFP and controls (PBS; original magnification, ×400). (C) DNA fragmentation as shown by DAPI stain. 211H, H2452 and H226 cells were infected with SeV/ΔF/FIR at 100 MOI, SeV/ΔF/GFP and controls (PBS). DAPI staining was performed 72 h after treatment with SeV/ΔF/FIR or SeV/ΔF/GFP. DNA fragmentation (arrows) was observed in SeV/ΔF/FIR-infected cells. (D) Synergistic activity of SeV/ΔF/FIR plus cisplatin in 211H cells, as shown by the leftward shift of the dose response curve of the MTS assay (error bars, SD). (E) Isobologram analysis (for ED50). The combined effects of SeV/ΔF/FIR and cisplatin were examined in 211H cells using at least two different dosing ratios.
SeV/ΔF/FIR has a synergistic effect in combination with cisplatin against 211H.
Cisplatin is used clinically for HMPM treatment. If SeV/ΔF/FIR can increase cisplatin cytotoxicity, combination therapy with SeV/ΔF/FIR might reduce the dosage of cisplatin required for HMPM treatment. Hence, we investigated the effects of SeV/ΔF/FIR plus cisplatin on 211H cells.(20,21) SeV/ΔF/FIR plus cisplatin caused a leftward shift of the dose response curve determined by the MTS assay (Fig. 2D), and moderate to slight synergism was revealed by isobologram analysis (Fig. 2E, Table 2).(21,22) Although further experiments are required, these results indicate that the combination of SeV/ΔF/FIR and cisplatin is synergistic in 211H and is promising for future clinical applications.
Table 2.
IC50 values for SeV/ΔF/FIR chemotherapeutic (cisplatin) combination regimens in 211H
| Cell line | Cisplatin IC50 (μg/mL) | Combination index |
|---|---|---|
| 211H | 2.22 ± 0.09 | 0.72 |
Value represents the median ± SD. SeV/ΔF/FIR, fusion gene deleted non-transmissible Sendai virus vector encoding FUSE-binding protein-interacting repressor.
Effects of SeV/ΔF/FIR injection in mice orthotopic xenograft HMPM.
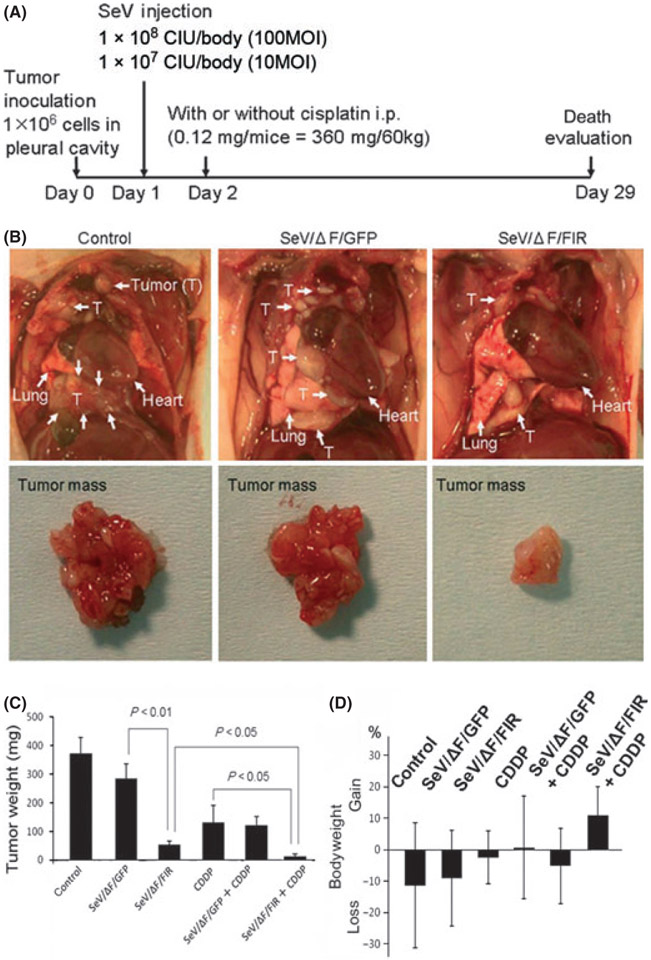
To examine the therapeutic effect of SeV/ΔF/FIR in an animal model, orthotopic xenograft HMPM mouse models were established (Fig. 3A).(23) As expected, SeV/ΔF/FIR at 1 × 108 cell-infectious units (CIU)/body (100 MOI) drastically reduced tumor growth (Fig. 3B,C). Interestingly, the mice treated with the combination of SeV/ΔF/FIR plus cisplatin only gained bodyweight on day 29 after tumor implantation (Fig. 3D).
Fig. 3.
(A) Design of the animal experiment used to examine the effect of fusion gene deleted non-transmissible Sendai virus vector encoding FIR (SeV/ΔF/FIR) in an orthotopic xenografted mouse model. 211H cells were injected into the pleural cavity with or without cisplatin. Sendai virus (SeV) was injected into the intrathoracic cavity on day 1 after tumor cell inoculation. Intraperitoneal administration (i.p.) of cisplatin (0.12 mg/body) was performed on day 2. (B) Representative photographs of tumors from control (PBS) and SeV (1 × 108 cell-infectious units [CIU]/body) treatment groups on day 29. (C) Weights of tumors treated with SeV (1 × 108 CIU/body) with or without cisplatin (CDDP) in vivo (n = 10). Significant reductions in tumor weight were observed on day 29 (error bars: SD). (D) Bodyweight changes among mice during SeV treatment (1 × 108 CIU/body) on day 29. Control (PBS), SeV/ΔF/FIR and fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein (SeV/ΔF/GFP) groups (n = 7 per group). Cisplatin (CDDP), SeV/ΔF/FIR plus CDDP, and SeV/ΔF/GFP plus CDDP groups (n = 9 per group) (error bars, SD).
Enhanced antitumor effect of SeV/ΔF/FIR plus cisplatin in an orthotopic HMPM animal model.
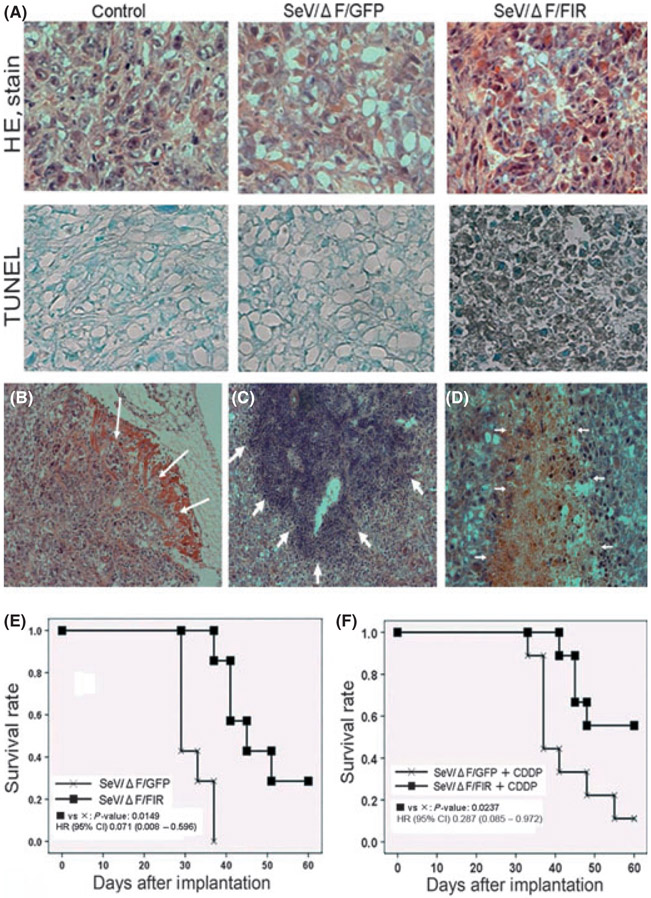
Next, thoracic tumors grown in the pleural cavity were histologically examined. SeV/ΔF/FIR transduction induced acidophilic cells on HE staining that were revealed to be apoptotic by TUNEL assay (Fig. 4A). We confirmed that the tumor was a biphasic type of malignant mesothelioma that had locally invaded the muscle (Fig. 4B). Lymphocyte infiltration (Fig. 4C) and necrotic changes (Fig. 4D) were more prominent in SeV/ΔF/FIR than in SeV/ΔF/GFP and controls (PBS). Further analysis is necessary to determine the mechanism behind these histological changes and the apoptosis induction by SeV/ΔF/FIR. To evaluate the future therapeutic potential of SeV/ΔF/FIR, we also examined the combined effect of SeV/ΔF/FIR and cisplatin. SeV/ΔF/FIR alone (Fig. 4E) was less effective than when it was administered in combination with cisplatin (Fig. 4F).
Fig. 4.
(A) Histological analysis of thoracic tumors in 211H cells with Sendai virus (SeV) on day 29 (1 × 10s cell-infectious units [CIU]/body). The thoracic tumors were analyzed by HE staining (top panels) and terminal deoxynucleotidyl transferase-mediated nick end labeling assay (TUNEL) (bottom panels). (B) The tumor cells invaded the muscle tissue in the group treated with control (PBS). Lymphocytic infiltration (C) and necrotic changes (D) were prominent in the group treated with fusion gene deleted non-transmissible Sendai virus vector encoding FIR (SeV/ΔF/FIR) (1 × 10s CIU/body). (E) Survival rate analysis. Mice with mesothelioma were treated with intrathoracic injection of SeV/ΔF/FIR (■) or fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein (SeV/ΔF/GFP) (×) at 1 × 10s CIU/body on day 1. Survival data analyzed by log-rank test (n = 7 per group). HR, hazard ratio; CI, confidence interval. (F) Survival rate analysis. Mice with mesothelioma were treated with intrathoracic injection of SeV/ΔF/FIR or SeV/ΔF/GFP at 1 × 10s CIU/body on day 1 and intra-peritoneal administration with cisplatin (0.12 mg/body) on day 2. SeV/ΔF/FIR plus cisplatin (CDDP; ■) or SeV/ΔF/GFP plus CDDP (×). Survival data analyzed by log-rank test (n = 9 per group).
SeV/ΔF/FIR showed no significant side-effects in the mouse model.
The side-effects of SeV/ΔF/FIR treatments were also investigated. SeV/ΔF/FIR-treated mice were in a fair condition (Fig. S1A). Hematological values between these three groups were not significantly different on day 29 (Table 3). No significant difference in liver and kidney toxicity was revealed by serum samples from these three groups (Table 4). No atypical cells were observed by May-Giemsa staining after SeV/ΔF/FIR transduction (Fig. S1B). However, when the viral amount was reduced to 1 × 107 CIU/body (10 MOI), SeV/ΔF/FIR did not show a significant tumor reduction effect compared with SeV/ΔF/GFP (Fig. S1C). During these experiments, no mouse died immediately after SeV/ΔF/FIR or SeV/ΔF/GFP injection.
Table 3.
Hematological values for each treatment group in vivo on day 29 (n = 5)
| Control (PBS) | SeV/ΔF/GFP | SeV/ΔF/FIR | P-value* | P-value** | |
|---|---|---|---|---|---|
| White blood cells (×104/μL) | 9780 ± 3502.4 | 6020 ± 2730.8 | 6340 ± 2846.6 | 0.095 | 0.861 |
| Hemoglobin (g/dL) | 14.30 ± 0.36 | 14.04 ± 0.23 | 14.08 ± 0.48 | 0.217 | 0.874 |
Each value represents the median ± SD.
SeV/ΔF/GFP (1 × 108 CIU/body) compared with the control.
SeV/ΔF/FIR (1 × 108 CIU/body) compared with SeV/ΔF/GFP. CIU, cell-infectious units; SeV/ΔF/FIR, fusion gene deleted non-transmissible Sendai virus vector encoding FUSE-binding protein-interacting repressor; SeV/ΔF/GFP, fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein.
Table 4.
Blood biochemistry of each treatment group in vivo on day 29 (n = 7)
| Control (PBS) | SeV/ΔF/GFP | SeV/ΔF/FIR | P-value* | P-value** | |
|---|---|---|---|---|---|
| T-Bil (mg/dL) | 0.067 ± 0.023 | 0.041 ± 0.020 | 0.076 ± 0.040 | 0.043 | 0.064 |
| AST (IU/L) | 141.7 ± 53.9 | 112.1 ± 46.2 | 181.0 ± 238.3 | 0.292 | 0.467 |
| ALT (IU/L) | 70.0 ± 43.9 | 71.3 ± 31.7 | 90.6 ± 131.1 | 0.951 | 0.712 |
| LDH (IU/L) | 2676.9 ± 1265.4 | 1712.1 ± 463.8 | 2286.4 ± 1319.1 | 0.083 | 0.299 |
| BUN (mg/dL) | 22.40 ± 3.49 | 21.94 ± 2.30 | 21.70 ± 3.32 | 0.778 | 0.876 |
| Cr (mg/dL) | 0.170 ± 0.019 | 0.123 ± 0.026 | 0.167 ± 0.062 | 0.002 | 0.11 |
Each value represents the median ± SD.
SeV/ΔF/GFP (1 × 108 CIU/body) compared with the control.
SeV/ΔF/FIR (1 × 108 CIU/body) compared with SeV/ΔF/GFP. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; LDH, lactate dehydrogenase; CIU, cell-infectious units; SeV/ΔF/FIR, fusion gene deleted non-transmissible Sendai virus vector encoding FUSE-binding protein-interacting repressor; SeV/ΔF/GFP, fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein; T-Bil, total bilirubin.
Discussion
In this study, we showed that SeV/ΔF/FIR induced a significant antitumor effect on HMPM cells in a dose-dependent manner in vitro and in vivo. In addition, we identified the anti-tumor effect of SeV/ΔF/FIR plus cisplatin against tumor growth without significant side-effects in an orthotopic xenograft mesothelioma mouse model.
Sendai virus has a high gene transduction capacity,(7,8,24) and SeV/ΔF/GFP showed high transduction efficiency in three different HMPM cell lines (Fig. 1B). In the present study, SeV/ΔF/FIR activated caspase-3 and caspase-9 and decreased c-Myc expression in three different HMPM cell lines (Fig. 2A), and induced apoptosis (Fig. 2B) and DNA fragmentation (Fig. 2C). In addition, histological observations indicated that SeV/ΔF/FIR suppressed tumor growth by apoptosis (Fig. 4A) as well as necrosis (Fig. 4D) in vivo. These observations indicate that SeV/ΔF/FIR cytotoxicity is derived from apoptosis through c-Myc suppression, even in HMPM cells, as revealed by a previous study.(13,14) SeV/ΔF/GFP itself showed a slight growth inhibitory effect against at 211H and H2452 (Fig. 1D). Thus, SeV/ΔF/GFP itself might have non-specific viral vector effects against certain HMPM cells.
It is clinically important to examine the combined effects of SeV/ΔF/FIR and cisplatin, which is a key drug used for HMPM patients.(25) Alterations of DNA repair processes have been known to be important to platinum resistance.(26) In this context, P89 might be important because it has a dual role in DNA helicase and DNA repair.(27) Interestingly, FIR was found to engage in P89 helicase suppression, and thus repress c-Myc transcription by delaying promoter escape.(12) c-Myc suppression enhanced the effectiveness of cisplatin in osteosarcoma and melanoma.(28,29) Thus, FIR might alter cisplatin sensitivity by P89 and/or c-Myc suppression because the synergistic effect of SeV/ΔF/FIR plus cisplatin is quite evident (Figs 2E,3C, Table 2). Further detailed studies on this mechanism are necessary.
c-Myc expression is low in quiescent normal cells whereas it is elevated in a broad range of human cancers, including HMPM.(10) As SeV/ΔF/FIR targets c-Myc, SeV/ΔF/FIR is expected to have a greater influence on cancer cells than normal cells. As expected, SeV/ΔF/FIR had less toxicity in non-tumor immortalized mesothelial cells (Met5A), which indicated low c- Myc expression (Fig. 1C,D, Table 1). Moreover, hematological values and blood biochemistry indicated no significant side-effects (Tables 3,4). Therefore, SeV/ΔF/FIR might be relatively safe in normal cells.
In conclusion, our data clearly indicate that SeV/ΔF/FIR has significant therapeutic potential against HMPM. Furthermore, combination therapy with SeV/ΔF/FIR plus cisplatin significantly enhanced the antitumor effect in vitro and in vivo. Thus, SeV/ΔF/FIR plus cisplatin will be an attractive modality against HMPM in the future.
Supplementary Material
Fig. S1. (A) Outlooks of representative mice from each experiment. (B) Hemogram from May-Giemsa-stained samples. (C) Tumor weights of SeV/ΔF/FIR (1 × 107 CIU/body) in vivo (n = 7) on day 29.
Movie S1. Time-lapse microscopy.
Acknowledgments
The authors are grateful to Ms Mai Tamura, Ms Nobuko Tanaka and Dr Toshiko Kajiwara for their technical assistance (Department of Molecular Diagnosis, Graduate School of Medicine, Chiba University, Chiba, Japan).
Footnotes
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005; 353: 1591–603. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson JT, McElvenny DM, Damton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005; 92: 587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramalingam SS, Belani CP. Recent advances in the treatment of malignant pleural mesothelioma. J Thorac Oncol 2008; 3: 1056–64. [DOI] [PubMed] [Google Scholar]
- 4.Tada Y, Takiguchi Y, Hiroshima K et al. Gene therapy for malignant pleural mesothelioma: present and future. Oncol Res 2008; 17: 239–46. [DOI] [PubMed] [Google Scholar]
- 5.Vachani A, Moon E, Wakeam E, Albelda SM. Gene therapy for mesothelioma and lung cancer. Am J Respir Cell Mol Biol 2010; 42: 385–93. [DOI] [PubMed] [Google Scholar]
- 6.Sterman DH, Recio A, Vachani A et al. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res 2005; 11: 7444–53. [DOI] [PubMed] [Google Scholar]
- 7.Markwell MA, Svennerholm L, Paulson JC. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci USA 1981; 78: 5406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai Y Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev Med Virol 1999; 9: 83–99. [DOI] [PubMed] [Google Scholar]
- 9.Bitzer M, Armeanu S, Lauer UM, Neubert WJ. Sendai virus vectors as an emerging negative-strand RNA viral vector system. J Gene Med 2003; 5: 543–53. [DOI] [PubMed] [Google Scholar]
- 10.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol 2005; 6: 635–45. [DOI] [PubMed] [Google Scholar]
- 11.Ramael M, Van den Bossche J, Buysse C, Deblier I, Segers K, Van Marck E. Immunoreactivity for c-fos and c-myc protein with the monoclonal antibodies 14E10 and 6E10 in malignant mesothelioma and non-neoplastic mesothelium of the pleura. Histol Histopathol 1995; 10: 639–43. [PubMed] [Google Scholar]
- 12.Liu J, He L, Collins I et al. The FBP interacting repressor targets TFIIH to inhibit activated transcription. Mol Cell 2000; 5: 331–41. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K, Tomonaga T, Shimada H et al. An essential role of alternative splicing of c-myc suppressor FUSE-binding protein-interacting repressor in carcinogenesis. Cancer Res 2006; 66: 1409–17. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita K, Tomonaga T, Kajiwara T et al. c-myc suppressor FBP-interacting repressor for cancer diagnosis and therapy. Front Biosci 2009; 14: 3401–8. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Iga M, Nabeta H et al. Non-transmissible Sendai virus encoding granulocyte macrophage colony-stimulating factor is a novel and potent vector system for producing autologous tumor vaccines. Cancer Sci 2008; 99: 2315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura K, Nozaki N, Enomoto T, Tanaka M, Kikuchi A. Analysis of M phase-specific phosphorylation of DNA topoisomerase II. J Biol Chem 1996; 271: 21439–45. [DOI] [PubMed] [Google Scholar]
- 17.Page-McCaw PS, Amonlirdviman K, Sharp PA. PUF60: a novel U2AF65-related splicing activity. RNA 1999; 5: 1548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisseleva MV, Cao L, Majerus PW. Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/protein kinase B phosphorylation and leads to apoptotic cell death. J Biol Chem 2002; 277: 6266–72. [DOI] [PubMed] [Google Scholar]
- 19.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther 2001; 298: 865–72. [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 21.Matsunaga N, Kanno J, Yoshimura I. A statistical method for judging synergism: application to an endocrine disruptor animal experiment. Environmetrics 2003; 14: 213–22. [Google Scholar]
- 22.Pandha HS, Heinemann L, Simpson GR et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res 2009; 15: 6158–66. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Yano S, Ogino H et al. The therapeutic efficacy of anti vascular endothelial growth factor antibody, bevacizumab, and pemetrexed against orthotopically implanted human pleural mesothelioma cells in severe combined immunodeficient mice. Clin Cancer Res 2007; 13: 5918–25. [DOI] [PubMed] [Google Scholar]
- 24.Iwadate Y, Inoue M, Saegusa T et al. Recombinant Sendai virus vector induces complete remission of established brain tumors through efficient interleukin-2 gene transfer in vaccinated rats. Clin Cancer Res 2005; 11: 3821–7. [DOI] [PubMed] [Google Scholar]
- 25.Steele JP, Klabatsa A. Chemotherapy options and new advances in malignant pleural mesothelioma. Ann Oncol 2005; 16: 345–51. [DOI] [PubMed] [Google Scholar]
- 26.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res 2008; 14: 1291–5. [DOI] [PubMed] [Google Scholar]
- 27.Drapkin R, Reardon JT, Ansari A et al. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature 1994; 368: 769–72. [DOI] [PubMed] [Google Scholar]
- 28.Zupi G, Scarsella M, Semple SC, Mottolese M, Natali PG, Leonetti C. Antitumor efficacy of bcl-2 and c-myc antisense oligonucleotides in combination with cisplatin in human melanoma xenografts: relevance of the administration sequence. Clin Cancer Res 2005; 11: 1990–8. [DOI] [PubMed] [Google Scholar]
- 29.Xie XK, Yang DS, Ye ZM, Tao HM. Recombinant antisense C-myc adenovirus increase in vitro sensitivity of osteosarcoma MG-63 cells to cisplatin. Cancer Invest 2006; 24: 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (A) Outlooks of representative mice from each experiment. (B) Hemogram from May-Giemsa-stained samples. (C) Tumor weights of SeV/ΔF/FIR (1 × 107 CIU/body) in vivo (n = 7) on day 29.
Movie S1. Time-lapse microscopy.