Abstract
Nitric oxide synthase (NOS) inhibition with N(G)-monomethyl-L-arginine (L-NMMA) is often used to assess the role of NO in human cardiovascular function. However, the window of effect for L-NMMA on human vascular function is unknown, which is critical for designing and interpreting human-based studies. This study utilized the passive leg movement (PLM) assessment of vascular function, which is predominantly NO-mediated, in 7 young male subjects under control conditions, immediately following intra-arterial L-NMMA infusion (0.24 mg/dl/min), and at 45–60 and 90–105 min post L-NMMA infusion. The leg blood flow (LBF) and leg vascular conductance (LVC) responses to PLM, measured with Doppler ultrasound and expressed as the change from baseline to peak (ΔLBFpeak and ΔLVCpeak) and area under the curve (LBFAUC and LVCACU), were assessed. PLM-induced robust control ΔLBFpeak (1135±324 ml/min) and ΔLVCpeak (10.7±3.6 ml/min/mmHg) responses that were significantly attenuated (704±196 ml/min and 6.7±2 ml/min/mmHg) immediately following L-NMMA infusion. Likewise, control condition PLM ΔLBFAUC (455±202 ml) and ΔLVCAUC (4.0±1.4 ml/mmHg) were significantly attenuated (141±130 ml and 1.3±1.2 ml/mmHg) immediately following L-NMMA infusion. However, by 45–60 min post L-NMMA infusion all PLM variables were not significantly different from control, and this was still the case at 90–105 min post L-NMMA infusion. These findings reveal that the potent reduction in NO bioavailability afforded by NOS inhibition with L-NMMA has a window of effect of less than 45–60 min in the human vasculature. These data are particularly important for the commonly employed approach of pharmacologically inhibiting NOS with L-NMMA in the human vasculature.
Keywords: NOS, L-NMMA, vascular function, vasodilation, NO bioavailability
INTRODUCTION
Nitric oxide (NO), synthesized in the vascular endothelium and catalyzed by NO synthase (NOS), has been determined to be antiatherogenic and an important marker of vascular function 1–8. This positive cardiovascular role has made NO the focus of much research studying the endogenous therapeutic effects of this molecule 9–16. A common investigational approach to better understand the impact of NO has to been to block NO synthesis by inhibiting NOS with the intra-arterial administration of the drug N(G)-monomethyl-L-arginine (L-NMMA) 17, 13, 18–21. This approach has yielded valuable insight into the regulation of vascular function and the physiological effects of NO on blood flow and vascular conductance throughout the vasculature. However, the pharmacodynamics of L-NMMA, particularly with regard to vascular function, are not well understood and L-NMMA is typically administered at the end of an experimental protocol due to presumed long-lasting effects 17, 13, 18–21. The delayed L-NMMA administration in experimental designs involving humans has either limited the scope or has added additional subject burden to work around this limitation. There is, in fact, some evidence supporting long-lasting effects with the intra-venous administration of L-NMMA, perhaps lasting as long as 5 hours, inferred by changes in fundus pulsation amplitude, cardiac output, and exhaled NO 22. In another study, systemic vascular resistance and pulmonary vascular resistance were elevated for at least 60 minutes following intra-venous administration of L-NMMA and the pharmacodynamics of L-NMMA appeared to be organ-specific 23. Thus, in terms of human vascular function and the intra-arterial administration of L-NMMA, the specific window of effect of L-NMMA is, not well understood.
Validated by very good agreement with more technical assessments of vascular function, like intra-arterial drug infusions and flow-mediated dilation, the passive leg movement (PLM) technique to assess vascular function has emerged as a powerful, yet relatively simple, test. Utilizing Doppler ultrasound, the PLM test assesses the hyperemia elicited by the passive movement of the lower leg through a 90° range of motion at the knee 24. Using L-NMMA, our group 18 and others 25 have documented that the PLM-induced increase in leg blood flow (LBF) and leg vascular conductance (LVC) is primarily due to NO release in young healthy subjects and that the decrease in this hyperemic and vasodilatory response with advancing age and disease can largely be attributed to a progressive fall in NO bioavailability 26, 19, 20, 27–31, 24, 32. In fact, utilizing L-NMMA, the PLM-induced hyperemia has, in young healthy subjects, proven to be upwards of 80% dependent on NO bioavailability 18. Thus, in young healthy subjects, measuring the time-course of the impact of L-NMMA on PLM-induced LBF and LVC will elucidate the window of effect of this drug in the human vasculature. Indeed, the change in LBF and LVC over the course of such a protocol can be attributed to L-NMMA and used to characterize the pharmacodynamics of this commonly used drug.
Therefore, the aim of this study was to characterize the direct physiologic pharmacodynamics of L-NMMA in the human vasculature, using PLM to isolate NO as the prime mediator of the hyperemia. Specifically, we tested the hypothesis that the well documented attenuation in PLM-induced hyperemia achieved by the intra-arterial delivery of L-NMMA, which targets NO-mediated vasodilation, would no longer be evident by 90–105 minutes. Such a finding would allow the more flexible and more efficacious use of L-NMMA in human research, reducing experimental design limitations and subject burden in future investigations employing this drug to better understand the role of NO in the human vasculature.
MATERIALS AND METHODS
Subjects:
Seven healthy, young men (age: 26 ± 4 yrs, stature: 180 ± 7 cm, and body mass: 81 ± 9 kg) volunteered to participate in this study. The subjects were all recreationally active and with no history of overt cardiovascular or metabolic disease. The experimental procedures utilized in this study were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veteran’s Affairs Medical Center and was in compliance with clause 35 of the Declaration of Helsinki, except for registration in a database. Informed consent was attained from each subject prior to the start of testing.
Experimental design:
On the morning of the experiment, subjects arrived at the laboratory having fasted and abstained from caffeine for that day and vigorous exercise for 24 hours. Following determination of thigh volume, the subjects underwent sterile catheterization of the common femoral artery using the Seldinger technique. Following catheterization, subjects rested for approximately 30 min in the upright-seated posture, which they maintained throughout the experiments. An occlusion cuff (Hokansen, Bellevue, WA, USA), placed just distal to the knee, was inflated to ≥250 mmHg prior to each test in order to ensure localization of the L-NMMA to the thigh 33. The first PLM assessment was performed in control conditions, and baseline measurements were obtained for the 60 s leading up to PLM, with the leg held at a knee-joint angle of 180 degrees. PLM was performed by a member of the research team who was also able to verify that no voluntary muscle contractions took place during the movements. In order to avoid such active resistance or anticipatory contractions, subjects were made aware that movement would commence in about 1 min, but were not aware of the exact beginning of the PLM assessment. The knee was then guided through the passive flexion-extension cycle with a 90 degree range of motion at a rate of 1 Hz for 60 s. During PLM, the contralateral leg remained supported and motionless at a knee-joint angle of 180 degrees. Following the control test, L-NMMA was administered intra-arterially and the same sequence of baseline measurements followed by PLM was performed. This sequence was again repeated at both 45–60 min and 90–105 min post L-NMMA infusion.
L-NMMA infusion:
L-NMMA was administered intra-arterially to provide localized NOS inhibition with minimal influence on MAP, to avoid systemic effects that could confound the assessment of local vascular function. Dosing of L-NMMA (Bachem, Bubendorf, Switzerland) was calculated for each subject following the anthropometric determination of thigh volume 34. Initially, the L-NMMA was diluted to 5 mg·ml−1 from 250 mg of lyophilized powder. The L-NMMA was then delivered at a loading dose of 0.24 mg·dl−1·min−1 for 5 min, after the inflation of the occlusion cuff below the knee, and before obtaining baseline measurements. Note, as the L-NMMA infusions were turned off prior to all measurements, no vehicle was used during the control and the 45–60 and 90–105 min post L-NMMA infusion conditions.
Measurements:
Peripheral hemodynamics:
A Logic e9 ultrasound system (General Electric Medical Systems, Milkwaukee, WI, USA) was used to measure common femoral artery blood velocity and diameter. Specifically, utilizing a linear array transducer probe operating at an imaging frequency of 9 MHz and a pulse wave frequency of 5 MHz, the Logic e9 was used to assess both blood velocity and common femoral artery diameter of the passively moved leg, distal to the inguinal ligament and proximal to the bifurcation of the deep and superficial femoral arteries. Real-time Duplex visualization of the ultrasound image and the pulse wave spectra allowed for a sustained insonation angle of ≤60 degree, with the sample volume, maximized for vessel size, centered in the common femoral artery.
Central hemodynamics:
Arterial pressure was measured directly, throughout the study, from the common femoral artery catheter, using a pressure transducer (Transpac IV, Hospira, Lake Forest, IL, USA) at the level of the catheter. Heart rate (HR) was measured with a standard 3-lead ECG. A finometer (Finapres Medical Systems, Amsterdam, the Netherlands) was used to determine stroke volume (SV), and cardiac output (CO), was calculated using the Modeflow method (Beatscope; Finapres Medical Systems) 35–39.
Data acquisition and analysis:
Mean arterial pressure (MAP) was calculated as the mean of the arterial pressure recorded by the pressure transducer, while CO, SV, and HR were all recorded at 200 Hz using data acquisition software (AcqKnowledge, Biopac Systems Inc., Goleta, CA, USA). The diameter of the common femoral artery (D) was obtained from the Logic e9 at a perpendicular angle along the central axis of the scanned area and was used, in combination with intensity weighted mean blood velocity (Vmean), to calculate leg blood flow (LBF) as:
Leg vascular conductance (LVC) was calculated as:
Baseline data were attained by averaging each of the variables over the 60 s leading up to the initiation of passive movement. During the PLM tests, variables were collected on a second-by-second basis, then smoothed using a 3 s rolling average. Once LBF and LVC were determined, the peak change in each variable (ΔLBFpeak and ΔLVCpeak, respectively) was determined by subtracting the baseline from the peak. The area under the curve for each variable (LBFAUC and LVCAUC, respectively) was calculated over the 60 s of PLM, following normalization by the baseline.
Statistical analysis:
In order to compare the PLM responses during control conditions, the L-NMMA infusion, and at 45–60 and 90–105 min post infusion, one-way repeated measures ANOVAs were used to compare the ΔLBFpeak, ΔLVCpeak, LBFAUC and LVCAUC. Where appropriate, the Student-Newman-Keuls method was used to identify differences in the one-way repeated measures ANOVAs. Two-way repeated measures ANOVAs (time x condition) were used for the comparison of MAP, HR, SV, and CO between control conditions, the L-NMMA infusion, and at 45–60 and 90–105 min post infusion. Significance for the statistical analyses was accepted at p ≤ 0.05 and the results are presented as mean ± SD or no variance, for clarity in some Figures.
RESULTS
Effect of L-NMMA on the PLM-induced LBF and LVC time-course:
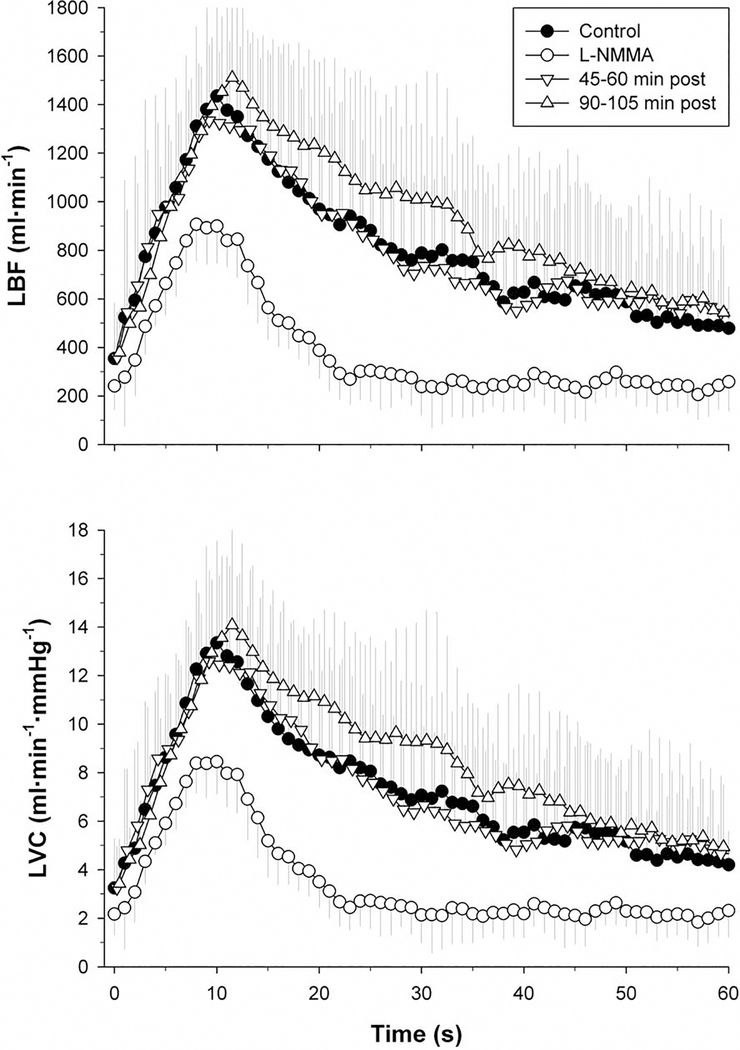
Although it should be recognized that individual differences in kinetics are such that the group average data, as illustrated in Figure 1, may blur the specifics of the individually analyzed data for ΔLBFpeak, ΔLVCpeak, LBFAUC, and LVCAUC, this method of presenting the data offers a clear graphical representation of the general response to PLM over time (Figure 1). Accordingly, the measurements of LBF and LVC, taken over 60 s of PLM, illustrate the attenuated time-course in these variables immediately following the administration of L-NMMA. Indeed, this L-NMMA-induced reduction in both LBF and LVC, with respect to the control condition, can be clearly identified by the blunted initial hyperemic response (≈10 s), and this significant attenuation was then maintained throughout the remainder of the PLM (Figure 1). In contrast, the observed difference in the time-course between the control condition and the L-NMMA infusion were negated 45–60 and 90–105 min after L-NMMA infusion. Collectively, these data reveal a response, over time, which was attenuated compared to the PLM response immediately following the L-NMMA infusion, but were not different from the control condition (Figure 1).
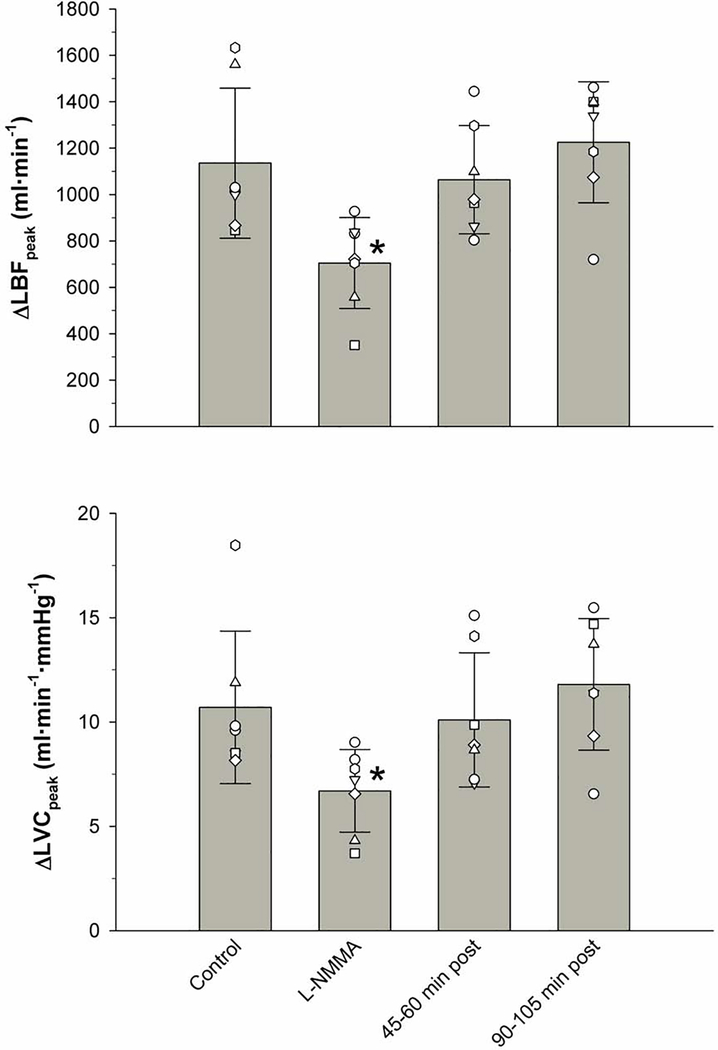
Figure 1. Leg blood flow (LBF) and leg vascular conductance (LVC) responses of the passively moved leg with and without intra-arterial N(G)-monomethyl-L-arginine (L-NMMA) infusion.
Values are mean ± SD. L-NMMA was significantly different from Control, 45–60 min post, and 90–105 min post (p < 0.05).
The Effect of L-NMMA on PLM-induced ΔLBFpeak, ΔLVCpeak, LBFAUC, and LVCAUC:
There were statistically significant decreases in the PLM-induced ΔLBFpeak (1135.7 ± 323.8 ml/min to 704.4 ± 196.2 ml/min) and ΔLVCpeak (10.7 ± 3.6 ml/min/mmHg to 6.7 ± 2 ml/min/mmHg) immediately following the infusion of L-NMMA. These changes equate to a ~38% attenuation in ΔLBFpeak and a similar ~37% attenuation of ΔLVCpeak. However, as documented in Figure 2, following the L-NMMA washout periods of 45–60 and 90–105 min, both ΔLBFpeak and ΔLVCpeak were no longer different from the original responses measured under control conditions.
Figure 2. Peak change in leg blood flow (ΔLBFpeak) and leg vascular conductance (ΔLVCpeak) in response to passive leg movement with and without intra-arterial N(G)-monomethyl-L-arginine (L-NMMA) infusion.
Values are mean ± SD and individual subject. * significantly different from Control, 45–60 min post, and 90–105 min post (p < 0.05).
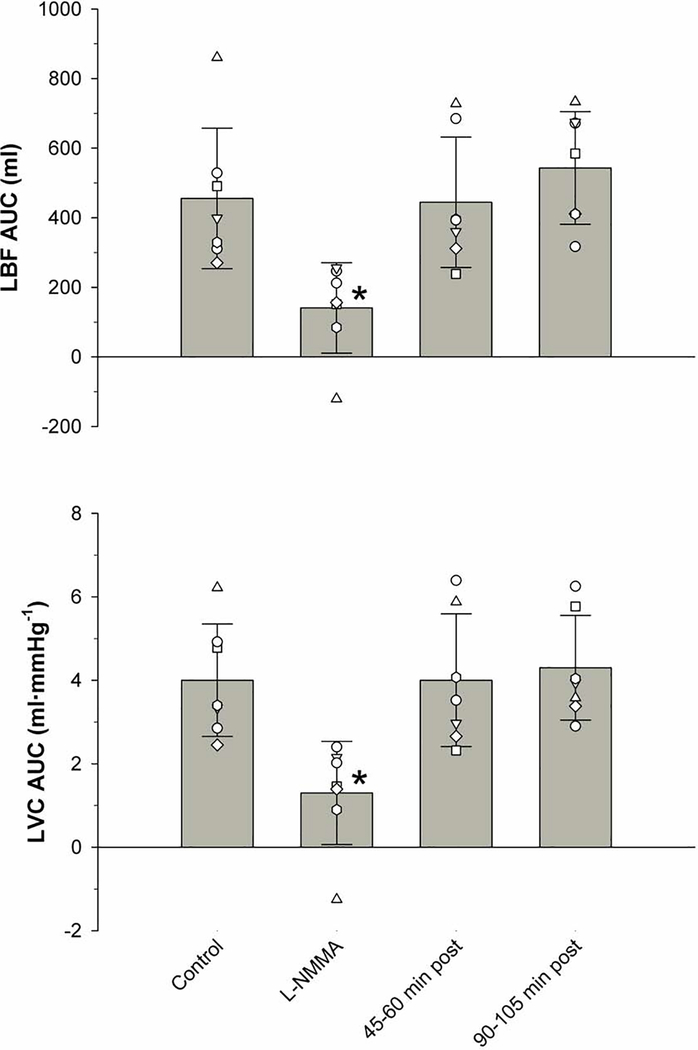
A more robust finding was documented by the integration of the area under the curve for LBF and LVC (Figure 3). Specifically, immediately following the infusion of L-NMMA, the PLM-induced LBFAUC significantly decreased from a control value of 455 ± 202 ml to 141 ± 130 ml, a reduction of ~69%. PLM-induced LVCAUC revealed a similar magnitude of effect, significantly decreasing from a control value of 4.0 ± 1.4 to 1.3 ± 1.2 ml/mmHg, immediately after the L-NMMA administration, a reduction of ~67%. Additionally, there was no significant difference between the PLM-induced LBFAUC and LVCAUC in the control condition compared to those measured at 45–60 and 90–105 min post L-NMMA infusion.
Figure 3. Area under the curve for leg blood flow (LBFAUC) and leg vascular conductance (LVCAUC) in response to passive leg movement with and without intra-arterial N(G)-monomethyl-L-arginine (L-NMMA) infusion.
AUC was calculated as the summed second-by-second response accounting for baseline during the 60 s of PLM. Values are mean ± SD and individual subject. * significantly different from Control, 45–60 min post, and 90–105 min post (p < 0.05).
The effect of L-NMMA on PLM-induced central hemodynamics:
The central hemodynamic variables of MAP, HR, SV, and CO were measured throughout the experimental protocol to assess the impact of the intra-arterial L-NMMA infusion, to the vasculature of a single thigh, on the cardiovascular system as a whole. These data, documented in Table 1 (baseline) and illustrated in Figure 4 (PLM response), provide evidence that this method of L-NMMA infusion did not alter central hemodynamics (HR, SV, CO, and MAP) and the effects remained localized to the vessels of the thigh. While there were some clear affects over time, including changes in MAP, HR, and CO, induced by the PLM, there are no statistically significant differences between the four trials. The exception to this was an apparent effect of Time x Condition exhibited by SV, although there were no significant differences between the experimental conditions for this variable.
Table 1.
Baseline hemodynamics
| Control | Immediately post L-NMMA | 45–60 min post L-NMMA | 90–105 min post L-NMMA | |
|---|---|---|---|---|
| HR (beats·min−1) | 65±13 | 63±12 | 62±13 | 62±15 |
| SV (ml·beat−1) | 105±36 | 105±24 | 95±13 | 101±25 |
| CO (l·min−1) | 6.3±1.5 | 6.2±1.4 | 5.9±1.3 | 5.8±1.4 |
| MAP (mmHg) | 109±7 | 111±7 | 112±7 | 113±9 |
| LBF (ml·min−1) | 354±230 | 241±98* | 358±187 | 379±182 |
| LVC (ml·min−1·mmHg−1) | 3.3±2.0 | 2.2±0.9* | 3.2±1.7 | 3.4±1.8 |
Values are mean ± SD. HR, heart rate, HR; SV, stroke volume; CO, cardiac output; MAP, mean arterial pressure; LBF, leg blood flow; LVC, leg vascular conductance.
significantly different from Control, 45–60 min post, and 90–105 min post L-NMMA infusion (p < 0.05).
Figure 4. Central hemodynamic data for mean arterial pressure (MAP), heart rate (HR), stroke volume (SV) and cardiac output (CO) in response to passive leg movement with and without intra-arterial N(G)-monomethyl-L-arginine (L-NMMA) infusion.
Data are presented as the mean values without an indication of variance, for clarity. No statistical significant difference exists between the experimental conditions within each central hemodynamic parameter. There is an effect of Time x Condition in SV, although there is no statistical significant difference between the experimental conditions.
DISCUSSION
Nitric oxide (NO), synthesized in the vascular endothelium and catalyzed by NO synthase (NOS), is an antiatherogenic and cardioprotective molecule that contributes critically to vascular function. These properties have fueled a burgeoning interest in the endogenous therapeutic effects of NO. Much of the research surrounding NO has utilized the NOS inhibitor N(G)-monomethyl-L-arginine (L-NMMA), to evaluate the consequences of decreased NO bioavailability. Therefore, this study sought to define the time-course of the effect of intra-arterial L-NMMA on the PLM assessment of vascular function, which has been documented to be predominantly NO-mediated, in order to provide a more accurate profile of the pharmacodynamics of this drug. This study revealed that the intra-arterial infusion of L-NMMA resulted in a relatively brief window of effect, as evidenced by the reversal of the significant attenuation of both PLM-induced LBF and LVC by 45–60 min post infusion. This finding is of particular importance in terms of experimental design when using the commonly used pharmacologic approach of blocking NOS with L-NMMA in the human vasculature.
L-NMMA pharmacodynamics in the human vasculature
The pharmacodynamics of L-NMMA, especially in terms of vascular function are not well understood, but are presumed to be long-lasting17, 13, 18–21. The prolonged effects of L-NMMA have been extrapolated based on the results from Mayer et al. 22 using a systemic, intra-venous infusion of L-NMMA to define both pharmacokinetics and pharmacodynamics profiles. The pharmacokinetics data demonstrated that L-NMMA had a relatively short half-life in the blood (≈63 min), yet appeared to have much longer-lasting systemic physiological effects, as measured by the percent reduction from baseline in fundus pulsation amplitude, CO, and exhaled NO. After 300 min there was a return to near-baseline values for fundus pulsation amplitude and CO, while exhaled NO remained reduced until closer to 500 min 22. However, previous results from Blitzer et al. 23 demonstrate that the effect of a systemic, intra-venous, infusion of L-NMMA on systemic vascular resistance, perhaps more pertinent to vascular function than the outcome variables assessed by Mayer et al. 22, had largely dissipated by 40–60 min post infusion. Based on these previous findings, the current study began initial measurements of PLM-induced LBF and LVC with a timeframe of 45–60 min in order to outline the physiologic effects of NOS inhibition. The local vascular measures, assessed in the current study, indicate that the effect of L-NMMA-induced NOS inhibition had returned to baseline values by 45–60 min post infusion (Figures 3 and 4). These results are in-line with the 40–60 min effect of L-NMMA on systemic vascular resistance 23, but are in contrast to the at least 60 min effect on pulmonary vascular resistance 23 and nearly 500 min needed to bring exhaled NO back to control values 22. It should be noted that much of the recent work, performed by our group and others, utilizing L-NMMA to better understand the role of NO bioavailability in the human vasculature, delayed initiating the L-NMMA infusion until the final step in the protocol because of the possibility of long lasting physiological consequences of NOS inhibition 17, 13, 18–21. The results of the current study, however, have changed the understanding of the effects of local NOS inhibition, allowing a more flexible role for L-NMMA in the experimental design of, in vivo, human vascular research.
Influence of administration route and dose of L-NMMA
Two primary differences between the current and previous studies examining the window of effect of L-NMMA are the route of drug administration and the dose. Previous studies administered relatively large, systemic intra-venous doses of L-NMMA. For example, in rats, intravenous L-NMMA bolus injections of 25, 50, and 100 mg/kg body mass elevated plasma L-NMMA concentrations for 100 to over 200 min 40. Whereas, in patients with septic shock, plasma concentrations of L-NMMA remained elevated for over 20 hours after prolonged (up to 8 hours) intra-venous administration of L-NMMA at 1, 2.5, 5, 10, and 20 mg/kg body mass/h 41. Even in the two aforementioned studies 23, 22, with the lowest drug dosing (3–4 mg/kg body mass), L-NMMA was administered intravenously. In contrast, the current study, focused on a relatively small, local intra-arterial dose of L-NMMA (~1 mg/kg body mass), an administration route and dose similar to investigations of NO-mediated vascular function in humans 17, 42, 43, 25, 18, 44, 19. Furthermore, by examining the local vascular consequences, this study was better able to define the pharmacodynamics profile of L-NMMA specific to the human vasculature. The critical influence of the dose and route of administration is exemplified by the effects on resting CO. In the two previous studies, examining the systemic effects of L-NMMA by the intra-venous infusion of the drug 23, 22, baseline CO decreased by 13–28%, which was attributed to an increase in afterload, indicative of the systemic effect of L-NMMA. In the current study baseline CO was not reduced by the intra-arterial administration of L-NMMA (Table 1 and Figure 4). Moreover, none of the measured central hemodynamic variables during PLM were altered by the intra-arterial infusion of L-NMMA (Figure 4). The findings of this study refine the current understanding of the pharmacodynamics of L-NMMA by demonstrating that the window of effect in the local vasculature is relatively short compared to systemic administration.
Organ-specificity and time-course of L-NMMA
An interesting observation that arises from the findings of this and previous studies is that the window of effect for L-NMMA appears to be organ-specific (e.g. brain, lung, muscle). This is evidenced by disparate time-courses for the effect of L-NMMA on systemic vascular resistance and pulmonary vascular resistance within subjects 23. Furthermore, the time-course of the L-NMMA effect differed, within subjects, across fundus pulsation amplitude, CO, and exhaled NO 22. The mechanisms responsible for these differential time-courses of effect of L-NMMA are beyond the scope this study, but may result from differences in the endogenous production/concentration of L-NMMA, NOS isoform expression, or NOS function 45–48. Thus, the relatively short window of effect of L-NMMA reported in the current study is likely specific to the human vasculature, predominantly supplying skeletal muscle. Future studies are needed to determine variations in the L-NMMA window of effect for other specific organ systems. and other non-selective/selective NOS inhibitors.
Experimental Considerations
While it remains difficult to directly test the degree of NOS inhibition with L-NMMA due to the limited specificity of NOS agonists, our group has previously determined the dose-response to L-NMMA in the human vasculature 49. Increasing doses of L-NMMA infused into the brachial artery reduced arm blood flow until a plateau was attained at the dose used in the current study (0.24 mg·dl−1·min−1) and a further doubling of the dose (0.48 mg·dl−1·min−1) did not elicit further reductions in blood flow. Additionally, our group has previously determined that this 0.24 mg·dl−1·min−1 dose significantly decreases the PLM-induced hyperemia and vasodilation in young males 18, 31.
It should be noted that these findings are also likely specific to L-NMMA and should not be assumed to be relevant for other NOS inhibitors (e.g. nitro-L-arginine methyl ester, L-NAME). Furthermore, the non-selective NOS inhibition with L-NMMA, used in this study, precludes differentiating the roles of the neuronal and endothelial NOS isoforms (nNOS and eNOS, respectively) and such differentiation may be important for identifying the specific mechanisms of vascular dysfunction in health and disease 50–58. However, although isoform differentiation was not a focus of the current study, interestingly, there is evidence that in the human vasculature the role of nNOS is likely most evident at rest, while eNOS becomes more critical during a perturbation such as an increase in shear stress 59, as evoked here by PLM. Based upon these findings, it is likely that the effect of L-NMMA on PLM was predominantly through the inhibition of eNOS.
Finally, although the primary focus was on the physiological effects of L-NMMA, the assessment of plasma nitrate, nitrite, and L-NMMA would have been potentially useful additions to this investigation, providing additional information regarding NO bioavailability and pharmacodynamics. However, unfortunately, such measurements were not performed in the current investigation.
NO-mediated vascular function and L-NMMA
The importance of NO-mediated vascular function and NO bioavailability continues to increase with the growing recognition of the positive cardiovascular effects of this molecule, in addition to important roles in other organs 9–16. The PLM assessment of vascular function is predominantly NO-mediated, as demonstrated by the ~70% reduction in both LBFAUC and LVCAUC (Figures 2 and 3). Moreover, the PLM assessment is able to quantify differences in NO-bioavailability with aging and disease 26, 19, 20, 27–31, 24, 32. The relatively high contribution of NO to the PLM-induced LBF and LVC responses in the current study is in agreement with previous studies in young healthy subjects 25, 18, 19. The power of the implications regarding NO-mediated vascular function and NO-bioavailability afforded by the combination of PLM and L-NMMA are exemplified by comparing the PLM response in young healthy subjects to that of subjects experiencing healthy aging. Indeed, L-NMMA administration attenuates the PLM-induced response in young healthy subjects to that which is strikingly similar to old healthy subjects 19, 20. In turn, L-NMMA has minimal effect on the PLM-induced response in old healthy subjects 19, 20. These findings support the concept that healthy aging is associated with an attenuation in NO-mediated vascular function and NO-bioavailability. Thus, the combination of PLM and L-NMMA highlights the growing consensus that PLM is a powerful, yet relatively simple, assessment of NO-mediated vascular function and NO-bioavailability, which is able to provide important insight into cardiovascular health with aging and disease.
Conclusion
To provide a more accurate profile of the pharmacodynamics of L-NMMA, this study sought to define the time-course of the effect of intra-arterial L-NMMA on the, predominantly NO-mediated, PLM assessment of vascular function. By focusing on the local vascular effects of NOS inhibition this study revealed that the potent attenuation of NO bioavailability afforded by NOS inhibition with intra-arterial L-NMMA has a window of effect of less than 45–60 min in the human vasculature. These data are of particular importance in terms of experimental design when using the commonly used pharmacologic approach of blocking NOS with L-NMMA in the human vasculature.
Highlights.
The nitric oxide synthase (NOS) inhibitor N(G)-monomethyl-L-arginine (L-NMMA) is presumed to have long-lasting effects and is, therefore, typically administered at the end of experimental protocols. The current data demonstrate that the window of effect for L-NMMA inhibition in the human vasculature is markedly shorter (<45–60 min) than previously thought.
The current findings will help strengthen experimental design employing the intra-arterial infusion of L-NMMA in humans by facilitating more flexible and, therefore, more insightful procedures.
By focusing on the local vascular effects of NOS inhibition this study revealed that the potent attenuation of NO bioavailability afforded by NOS inhibition with intra-arterial L-NMMA has a window of effect of less than 45–60 min in the human vasculature.
Acknowledgments:
We are indebted to all of the participants for their time and effort expended to complete this study.
Funding: This study was supported by National Heart, Lung, and Blood Institute grants (P01 HL-091830 and R01 HL142603) and Ruth L. Kirschstein National Research Service Awards (T32 HL139451 and T32 HL007576), Veterans Affairs Merit (I01 CX001999, E6910-R, E1697-R, E1572-P, and E3207-R) and Senior Research Career Scientist (E9275-L) Awards.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA and Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–72. PMC329981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naruse K, Shimizu K, Muramatsu M, Toki Y, Miyazaki Y, Okumura K, Hashimoto H and Ito T. Long-term inhibition of NO synthesis promotes atherosclerosis in the hypercholesterolemic rabbit thoracic aorta. PGH2 does not contribute to impaired endothelium-dependent relaxation. Arterioscler Thromb. 1994;14:746–52. [DOI] [PubMed] [Google Scholar]
- 3.Tsao PS, McEvoy LM, Drexler H, Butcher EC and Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation. 1994;89:2176–82. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC and Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. [DOI] [PubMed] [Google Scholar]
- 5.Tsao PS, Wang B, Buitrago R, Shyy JY and Cooke JP. Nitric oxide regulates monocyte chemotactic protein-1. Circulation. 1997;96:934–40. [DOI] [PubMed] [Google Scholar]
- 6.Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, Schenke W and Quyyumi AA. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension. 1998;32:9–15. [DOI] [PubMed] [Google Scholar]
- 7.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH and Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–54. [DOI] [PubMed] [Google Scholar]
- 8.Broxterman RM, Witman MA, Trinity JD, Groot HJ, Rossman MJ, Park SY, Malenfant S, Gifford JR, Kwon OS, Park SH, Jarrett CL, Shields KL, Hydren JR, Bisconti AV, Owan T, Abraham A, Tandar A, Lui CY, Smith BR and Richardson RS. Strong Relationship Between Vascular Function in the Coronary and Brachial Arteries. Hypertension. 2019;74:208–215. PMC6716528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy RM, Leszczynska-Piziak J and Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–21. PMC329973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsao PS, Theilmeier G, Singer AH, Leung LL and Cooke JP. L-arginine attenuates platelet reactivity in hypercholesterolemic rabbits. Arterioscler Thromb. 1994;14:1529–33. [DOI] [PubMed] [Google Scholar]
- 11.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr., Shin WS and Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–8. PMC185173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von der Leyen HE, Gibbons GH, Morishita R, Lewis NP, Zhang L, Nakajima M, Kaneda Y, Cooke JP and Dzau VJ. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci U S A. 1995;92:1137–41. PMC42653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesauro M, Schinzari F, Rovella V, Di Daniele N, Lauro D, Mores N, Veneziani A and Cardillo C. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension. 2009;54:995–1000. [DOI] [PubMed] [Google Scholar]
- 14.Ward MR, Thompson KA, Isaac K, Vecchiarelli J, Zhang Q, Stewart DJ and Kutryk MJ. Nitric oxide synthase gene transfer restores activity of circulating angiogenic cells from patients with coronary artery disease. Mol Ther. 2011;19:1323–30. PMC3129566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung C, Quitter F, Lichtenauer M, Fritzenwanger M, Pfeil A, Shemyakin A, Franz M, Figulla HR, Pfeifer R and Pernow J. Increased arginase levels contribute to impaired perfusion after cardiopulmonary resuscitation. Eur J Clin Invest. 2014;44:965–71. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Varga M, Wang X, Haddad DJ, An S, Medzikovic L, Derakhshandeh R, Kostyushev DS, Zhang Y, Clifford BT, Luu E, Danforth OM, Rafikov R, Gong W, Black SM, Suchkov SV, Fineman JR, Heiss C, Aschbacher K, Yeghiazarians Y and Springer ML. Overexpression of Nitric Oxide Synthase Restores Circulating Angiogenic Cell Function in Patients With Coronary Artery Disease: Implications for Autologous Cell Therapy for Myocardial Infarction. J Am Heart Assoc. 2016;5:e002257 PMC4859354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM and Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol (1985). 2002;92:2019–25. [DOI] [PubMed] [Google Scholar]
- 18.Trinity JD, Groot HJ, Layec G, Rossman M, J., Ives SJ, Runnels S, Gmelch BS, Bledsoe A and Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol. 2012;590:1413–1425. PMC3382331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groot HJ, Trinity JD, Layec G, Rossman M, J., Ives SJ, Morgan DE, Bledsoe A and Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol. 2015;593:3917–3928. PMC4575577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trinity JD, Groot HJ, Layec G, Rossman M, J., Ives SJ, Morgan DE, Gmelch BS, Bledsoe A and Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol. 2015;308:H672–H679. PMC4360052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey RE, Ranadive SM, Limberg JK, Baker SE, Nicholson WT, Curry TB, Barnes JN and Joyner MJ. Forearm vasodilatation to a beta2 -adrenergic receptor agonist in premenopausal and postmenopausal women. Exp Physiol. 2020;105:886–892. PMC7210782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer BX, Mensik C, Krishnaswami S, Derendorf H, Eichler HG, Schmetterer L and Wolzt M. Pharmacokinetic-pharmacodynamic profile of systemic nitric oxide-synthase inhibition with L-NMMA in humans. Br J Clin Pharmacol. 1999;47:539–44. PMC2014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blitzer ML, Loh E, Roddy MA, Stamler JS and Creager MA. Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans. J Am Coll Cardiol. 1996;28:591–6. [DOI] [PubMed] [Google Scholar]
- 24.Gifford JR and Richardson RS. CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol. 2017;123:1708–1720. PMC5814681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortensen SP, Askew CD, Walker M, Nyberg M and Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol. 2012;590:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venturelli M, Amann M, Layec G, McDaniel J, Trinity JD, Fjeldstad AS, Ives SJ, Yonnet G and Richardson RS. Passive leg movement-induced hyperaemia with a spinal cord lesion: evidence of preserved vascular function. Acta Physiol (Oxf). 2014;210:429–39. PMC3972380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J and Richardson RS. Heart failure and movement-induced hemodynamic: partitioning the impact of central and peripheral dysfunction. Int J Cardiol. 2015;178:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groot HJ, Rossman M, J., Garten RS, Wang E, Hoff J, Helgerud J and Richardson RS. The effect of physical activity on passive leg movement-induced vasodilation with age. Med Sci Sports Exerc. 2016;48:1548–57. PMC4949157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J and Richardson RS. The Mechanoreflex and Hemodynamic Response to Passive Leg Movement in Heart Failure. Med Sci Sports Exerc. 2016;48:368–76. PMC4760919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson AD, Rossman M, J., Witman MA, Barrett-O’Keefe Z, Groot HJ, Garten RS and Richardson RS. Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross sectional study. J Appl Physiol. 2016;120:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD and Richardson RS. Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol. 2017;123:1468–1476. PMC5814686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hydren JR, Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC and Richardson RS. Delineating the age-related attenuation of vascular function: Evidence supporting the efficacy of the single passive leg movement as a screening tool. J Appl Physiol. 2019;126:1525–1532. PMC6620662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shields KL, Broxterman RM, Jarrett CL, Bisconti AV, Park SH and Richardson RS. The passive leg movement technique for assessing vascular function: defining the distribution of blood flow and the impact of occluding the lower leg. Exp Physiol. 2019;104:1575–1584. PMC6773497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrenson L, Poole JG, Kim J, Brown C, Patel P and Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–31. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R and Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol. 2003;179:361–366. [DOI] [PubMed] [Google Scholar]
- 36.van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L and Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol. 2003;90:131–137. [DOI] [PubMed] [Google Scholar]
- 37.Azabji KM, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D and Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin Sci. 2004;106:365–369. [DOI] [PubMed] [Google Scholar]
- 38.de Vaal JB, de Wilde RBP, van den Berg PCM, Schreuder JJ and Jansen JRC. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth. 2005;95:326–331. [DOI] [PubMed] [Google Scholar]
- 39.de Wilde RBP, Geerts BF, Cui J, van den Berg PCM and Jansen JRC. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia. 2009;64:762–769. [DOI] [PubMed] [Google Scholar]
- 40.Maurer TS, Mishra Y and Fung HL. Nonlinear pharmacokinetics of L-N(G)-methyl-arginine in rats: characterization by an improved HPLC assay. Biopharm Drug Dispos. 1999;20:397–400. [DOI] [PubMed] [Google Scholar]
- 41.Hussein Z, Beerahee M, Grover R, Jordan B, Jeffs R, Donaldson J, Zaccardelli D, Colice G, Guntupalli K, Watson D and Vincent JL. Pharmacokinetics of the nitric oxide synthase inhibitor L-NG-methylarginine hydrochloride in patients with septic shock. Glaxo Wellcome International Septic Shock Study Group. Clin Pharmacol Ther. 1999;65:1–9. [DOI] [PubMed] [Google Scholar]
- 42.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O’Driscoll G and Tschakovsky ME. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol. 2010;298:H119–H126. [DOI] [PubMed] [Google Scholar]
- 43.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD and Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol. 2011;301:H1118–H1126. PMC3191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray DW, Witman MAH, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA and Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension. 2013;62:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moncada S, Palmer RM and Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 46.Nathan C and Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–8. [DOI] [PubMed] [Google Scholar]
- 47.Lloyd-Jones DM and Bloch KD. The vascular biology of nitric oxide and its role in atherogenesis. Ann Rev Med. 1996;47:365–375. [DOI] [PubMed] [Google Scholar]
- 48.Kittel A and Maas R. Pharmacology and clinical pharmacology of methylarginines used as inhibitors of nitric oxide synthases. Curr Pharm Des. 2014;20:3530–47. [DOI] [PubMed] [Google Scholar]
- 49.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA and Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol. 2011;300:H1101–7. 3064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furfine ES, Harmon MF, Paith JE, Knowles RG, Salter M, Kiff RJ, Duffy C, Hazelwood R, Oplinger JA and Garvey EP. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J Biol Chem. 1994;269:26677–83. [PubMed] [Google Scholar]
- 51.Wakefield ID, March JE, Kemp PA, Valentin JP, Bennett T and Gardiner SM. Comparative regional haemodynamic effects of the nitric oxide synthase inhibitors, S-methyl-L-thiocitrulline and L-NAME, in conscious rats. Br J Pharmacol. 2003;139:1235–43. PMC1573945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P and Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–62. [DOI] [PubMed] [Google Scholar]
- 53.Copp SW, Hirai DM, Schwagerl PJ, Musch TI and Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol. 2010;588:1321–31. PMC2872736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirai DM, Copp SW, Holdsworth CT, Ferguson SK, Musch TI and Poole DC. Effects of neuronal nitric oxide synthase inhibition on microvascular and contractile function in skeletal muscle of aged rats. Am J Physiol Heart Circ Physiol. 2012;303:H1076–84. PMC3469646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC and Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol. 2013;591:2885–96. PMC3690692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shabeeh H, Melikian N, Dworakowski R, Casadei B, Chowienczyk P and Shah AM. Differential role of endothelial versus neuronal nitric oxide synthase in the regulation of coronary blood flow during pacing-induced increases in cardiac workload. Am J Physiol Heart Circ Physiol. 2013;304:H1277–82. PMC3652090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shabeeh H, Seddon M, Brett S, Melikian N, Casadei B, Shah AM and Chowienczyk P. Sympathetic activation increases NO release from eNOS but neither eNOS nor nNOS play an essential role in exercise hyperemia in the human forearm. Am J Physiol Heart Circ Physiol. 2013;304:H1225–30. PMC3652092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, Hageman KS, Poole DC and Musch TI. Neuronal nitric oxide synthase regulation of skeletal muscle functional hyperemia: exercise training and moderate compensated heart failure. Nitric Oxide. 2018;74:1–9. PMC5825278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seddon MD, Chowienczyk PJ, Brett SE, Casadei B and Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–6. [DOI] [PubMed] [Google Scholar]