Abstract
Background
Atopic dermatitis (AD) is a common chronic, inflammatory skin condition. The pathogenesis of AD involves many cytokines that utilize the Janus kinase/signal transducer and activator of transcription (JAK-STAT) signaling cascade; therefore, JAK inhibitors may be used in the treatment of AD. This review aims to evaluate the pathophysiology, efficacy, and safety of JAK inhibitors and their emerging role as a therapeutic option for patients with AD.
Methods
A PubMed search of Phase I, II, and III clinical trials was conducted for relevant literature published between January 2015 and June 2020 utilizing the key terms: JAK inhibitors, atopic dermatitis, efficacy, safety, and treatment. The search was subsequently expanded to include additional terms.
Results
In multiple Phase II and III clinical trials, JAK inhibitors were more efficacious than placebo or vehicle controls and slightly more efficacious in direct comparisons to corticosteroids. Overall, JAK inhibitors have a moderate safety profile for use in AD. Some of the more severe theoretical adverse events included thrombosis and reactivation of viral infections. While data remain limited for the long-term efficacy and safety of JAK inhibitor use in patients with AD, many ongoing clinical trials have promising preliminary results.
Discussion
Short-term data suggest that both topical and oral JAK inhibitors are efficacious and safe for use in patients with AD, although cases of thrombosis and viral disease have been reported. While the current standard treatments for AD are likely preferred, failed therapy with these agents or corticosteroid phobia may be indications for the use of JAK inhibitors in patients with AD.
Keywords: atopic dermatitis, eczema area and severity index, Janus kinase inhibitor, pruritus
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin condition with a relapsing and remitting course; it is often associated with a family or personal history of additional atopic conditions such as asthma and allergies.1 An estimated 7.3% of adults in the United States have AD2 and 85% of these individuals develop the disease before the age of five years old.3 AD can have a substantial negative physical and psychological impact on patients, resulting in impaired social interactions, sleep disturbances, pruritus, and dietary restrictions.4
The pathogenesis of AD is multifactorial, resulting from a combination of skin barrier defects, genetic susceptibility, environmental triggers, and hypersensitivity.3,5 Many cytokines involved in the pathophysiology of AD utilize the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway and the spleen tyrosine kinase pathway (SYK), a non-receptor tyrosine kinase pathway.6 The JAK-STAT pathway consists of a signaling cascade following the binding of a ligand or cytokine to various receptors including the JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2) enzymes.
Management of AD includes the avoidance of environmental triggers, use of topical and systemic medications, and non-medication-based treatments (Table 1).3,7–13 Common topical treatments include corticosteroids and calcineurin inhibitors (CIs).14 Although corticosteroids are highly efficacious and are the most common topical treatment for AD, their long-term side effect profile discourages extended use.15,16 Additionally, some patients and caregivers of pediatric patients may have negative feelings and beliefs regarding the use of corticosteroids, a concept known as “corticosteroid phobia” which can affect medication adherence and efficacy. Systemic therapies, such as monoclonal antibodies and Janus kinase (JAK) inhibitors, are more recently developed therapies for AD. JAK inhibitors include both topical and systemic oral therapies that block the downstream effects of cytokines involved in the pathogenesis of AD.6 Several JAK inhibitors are currently undergoing investigation in trials assessing their efficacy and long-term safety for use in AD.6
Table 1.
Current Therapies Available for AD
| Medication Examples | Mechanism of Action | Common Adverse Events | |
|---|---|---|---|
| Topical | |||
| Calcineurin inhibitors | Cyclosporine Tacrolimus Pimecrolimus |
Inhibits production of IL-2 | Cutaneous infections |
| Bleach bath | Sodium hypochlorite (NaOCl) | Eradicates bacteria superimposed on AD | Dry skin Rash |
| Phototherapy | Narrowband UVB light decreases expression of IL-5, IL-13, IL-31, induces T-cell apoptosis, and decreases dendritic cells | Sunburn Photodamage Actinic keratosis |
|
| Oral/Injection | |||
| Monoclonal antibodies | Dupilumab Lebrikizumab |
Inhibits IL-4 and IL-13 | Nasopharyngitis URI Conjunctivitis |
| Azathioprine* | Interferes with purine synthesis as a precursor purine analog and selectively inhibits lymphocytes | GI disturbances Increased LFTs Myelosuppression |
|
| Mycophenolate mofetil* | Blocks IMP dehydrogenase, which is required for de novo purine synthesis, and exerts immunomodulatory effects on the body | GI disturbances Increased predisposition for infection Myelosuppression |
|
| Methotrexate* | Inhibits DHFR, disrupting THFA synthesis and causing immunosuppression; interferes with lymphocyte activation | GI disturbances Hepatotoxicity Headache/fatigue Pancytopenia |
|
| Both | |||
| Corticosteroids | Hydrocortisone | Suppresses immune and inflammatory axis | Skin atrophy Immune axis suppression Rebound disease flare-ups Cutaneous infections |
| JAK inhibitors | Baricitinib Tofacitinib Delgocitinib Upadacitinib Abrocitinib Gusacitinib (ASN002) |
Inhibits JAKs and downstream cytokine pathways | Nasopharyngitis Nausea Headaches |
Note: *Indicates off-label use of agent as AD treatment.
Abbreviations: AD, atopic dermatitis; DHFR, dihydrofolate reductase; IL, interleukin; IMP, inosine monophosphate; JAK, Janus kinase; LFTs, liver function tests; THFA, tetrahydrofolic acid; URI, upper respiratory infection; UVB, ultraviolet B.
There are many ongoing challenges to treating AD. The biggest limitations of current therapies include poor adherence, adverse effects, and rebound disease flare-ups upon medication discontinuation.3 There is also a large economic burden of disease, as the difference in expenditure between AD patients and matched controls may be as high as $3302 annually.17 Medications, visits to the doctor’s office and emergency room, and time away from work all contribute to the financial burden of disease.18 This review aims to evaluate the pathophysiology of the JAK-STAT pathway, review the efficacy and safety of JAK inhibitors (abrocitinib, baricitinib, delgocitinib, gusacitinib, ruxolitinib, tofacitinib, upadacitinib), and discuss the emerging role of JAK inhibitors as a therapeutic option for AD.
Methods
A PubMed search of phase I, II, and III, randomized, double-blind, clinical trials published between January 2015 and June 2020 was conducted for relevant literature assessing the efficacy and safety of JAK inhibitors for use in AD treatment. The initial search utilized the key terms: “JAK inhibitors and atopic dermatitis.” Subsequently, each of the following terms were individually added to the primary search terms “JAK inhibitors” and “atopic dermatitis” to yield additional results: “efficacy,” “safety,” “treatment,” “eczema area and severity index” (EASI), “investigators global assessment” (IGA), “scoring AD” (SCORAD), and “body surface area” (BSA). The generic name of each JAK inhibitor used as an AD therapy was further included with the term “atopic dermatitis” to locate any additional trials. The search terms “EASI,” “IGA,” and “SCORAD” were included because they encompass the most common assessment instruments utilized in AD trials.19–21 Results were limited to a five-year period to aid in the identification of recent clinical trials evaluating JAK inhibitors for AD treatment, as JAK inhibitors are an emerging therapy in this context. Only trials investigating JAK inhibitors for AD treatment and articles in which JAK inhibitors were compared with placebo and corticosteroids were included. Trials evaluating JAK inhibitor use in conditions other than AD and use of alternate medications for AD treatment were excluded. Relevant article selection and data extraction were performed by the primary author. The combined PubMed search criteria yielded a total of 101 peer-reviewed journal articles. Of these articles, a total of 35 sources were included in the study. Finally, a Google search was conducted utilizing the name of each drug and the term “atopic dermatitis” to include new data from press releases by pharmaceutical companies, yielding an additional four sources. The assessment instruments (EASI, mEASI, IGA) presented in this review were based on each respective trials’ primary efficacy endpoints.
Pathophysiology of the JAK-STAT Pathway
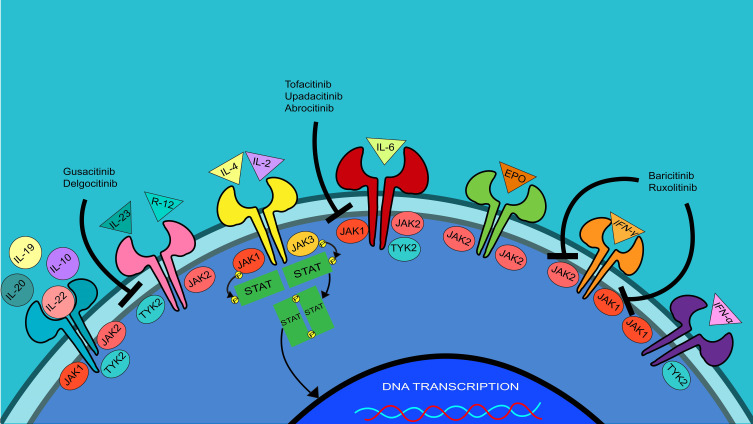
JAK receptors are activated via transphosphorylation which results in subsequent phosphorylation of the STAT transcription factors; the STATs then dimerize and travel from the cytosol into the nucleus of the cell to modify gene transcription (Figure 1).22,23 Inhibition of one or more JAK receptors in this pathway can potentially treat AD and other inflammatory diseases.22,23 Each of the four JAK receptors has a different downstream effect on cytokine signaling. The interleukin (IL)-4Rα and JAK1 signaling pathways control chronic itch or pruritus.24 IL-31, a cytokine heavily involved in pruritus, is dependent on JAK1 in its signaling pathway.6 JAK2 affects erythropoiesis, platelet activation, and myelopoiesis; JAK2 inhibition is associated with adverse hematological effects such as anemia and thrombocytopenia.6 JAK3 is mainly expressed by B- and T-lymphocytes; the inhibition of JAK3 is associated with cluster of differentiation (CD)8+ T-cell dysfunction and a decrease in the production of natural killer cells.25 TYK2 regulates many ILs and interferons, and inhibition results in suppression of multiple cytokines.6,26,27 The STAT family consists of seven transcription factors; each is phosphorylated and activated by different JAK receptors.6,26 SYK aids in releasing cytokines IL-1B, IL-10, and IL-17 and impacts differentiation and proliferation of dendritic cells, keratinocytes, and B-lymphocytes.28
Figure 1.
JAK-STAT signaling pathways. JAK inhibitors stop the downstream functions of inflammatory cytokines in AD. Inhibition of JAKs blocks their transphosphorylation, further inhibiting the phosphorylation and dimerization of STATs. This ultimately inhibits the dimerized STATs from acting as transcription factors for the inflammatory cytokines and blunts the overall inflammatory response.
Abbreviations: DNA, deoxyribonucleic acid; EPO, erythropoietin; IFN, interferon; IL, interleukin; JAK, Janus kinase; P, phosphate; STAT, signal transducer and activator of transcription; SYK, spleen tyrosine kinase; TYK2, non-receptor tyrosine-protein kinase.
JAK1 and JAK3 receptors modulate the pathway for cytokines IL-2 and IL-4 via phosphorylation of STAT3, STAT5, and STAT6.6 IL-4 is a key player in antibody class switching and potentiates T helper type 2 (Th2) cell differentiation, stimulating the release of additional cytokines.26 IL-4 is one of the main cytokines involved in the pathogenesis of AD, thus many of the JAK inhibitors investigated for the treatment of AD affect this pathway.29 Similarly, JAK2 and TYK2 modulate the pathway for cytokines IL-12 and IL-23 via phosphorylation and activation of STAT4. IL-12 is a proinflammatory cytokine that upregulates the activity of Th1 cells to further secrete inflammatory cytokines; inhibition of this cytokine’s signaling pathway reduces inflammation.30
Efficacy of Topical JAK Inhibitors for Treatment of AD
Delgocitinib
Delgocitinib is a JAK1, JAK2, JAK3, and TYK2 inhibitor approved for treatment of AD in Japan, making it the only JAK inhibitor currently approved as a topical agent for the treatment of moderate-to-severe AD (Table 2).31 Delgocitinib also inhibits mast cells, monocytes, T-cells, and B-cells and increases filaggrin production, a protein implicated in the maintenance of the skin barrier.32,33 In a phase II multicenter, randomized, double-blind, vehicle-controlled study of children aged 16 years or younger assessing the safety and efficacy of delgocitinib, baseline modified EASI (mEASI) scores improved by 61.8% in the 0.5% delgocitinib group and 4.8% in the vehicle group after four weeks of treatment (p<0.001).34 The mEASI score considers both acute and chronic AD lesions, and incorporates patient evaluation of pruritus.35 In an alternate Phase III, randomized, double-blind, vehicle-controlled study of delgocitinib use in patients 16 years and older, baseline mEASI scores improved by 44.3% from baseline (p<0.001) in the delgocitinib group and 1.7% in the vehicle group after four weeks of treatment.33
Table 2.
JAK Inhibitor Therapies Used in AD
| Primary Target of Inhibition | Other Uses | Study | Clinical Trial Phase | Length of Study (Weeks) | Sample Size | Endpoint | Dosages | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Topical | |||||||||
| Delgocitinib | JAK1, JAK2, JAK3, and Tyk2 | Alopecia areata, chronic hand eczema, discoid lupus erythematous* | Nakagawa, et al.34 | II | 4 | 103 (pediatric) |
mEASI | 0.25% BID | 54.2%+ |
| 0.5% BID | 61.8%+ | ||||||||
| Vehicle | 4.8%+ | ||||||||
| Nakagawa, et al.33 | III | 4-week (controlled) 24-week (extension) | 158 | mEASI | 0.5% BID | 44.3%+ | |||
| Vehicle | 1.7%+ | ||||||||
| Ruxolitinib | JAK1 and JAK2 | Myelofibrosis, polycythemia vera** | Kim, et al.36 | II | 8 | 307 | EASI | 0.15% | 50.7%+ |
| 0.5% | 58.7%+ | ||||||||
| 1.5% | 67.0%+ | ||||||||
| 1.5 BID | 78.5%+ | ||||||||
| 0.1% Triamcinolone BID | N/A+ | ||||||||
| TRuE-AD138 | III | 8 | 622 | IGA (0,1) | Vehicle | 26.9%+ | |||
| 0.75% BID | 50%++ | ||||||||
| 1.5% BID | 53.8%++ | ||||||||
| TRuE-AD238 | III | 8 | 618 | IGA (0,1) | Placebo | 15.1%++ | |||
| 0.75% BID | 39%++ | ||||||||
| 1.5% BID | 51.3%++ | ||||||||
| Tofacitinib | JAK3 and/or JAK1 | RA, ulcerative colitis, psoriatic arthritis** | Bissonnette, et al.41 | II | 4 | 69 | EASI | 2% | 81.7%+ |
| Placebo | 29.9%+ | ||||||||
| Oral | |||||||||
| Abrocitinib | JAK1 | Psoriasis* | Gooderham, et al.42 | II | 12 | 267 | IGA (0,1) | 10 mg | 10.9%++ |
| 30 mg | 8.9%++ | ||||||||
| 100 mg | 29.6%++ | ||||||||
| 200 mg | 43.8%++ | ||||||||
| Placebo | 5.8%++ | ||||||||
| JADE MONO-145 | III | 12 | 387 | EASI-75 | 100 mg | 39.7%++ | |||
| 200 mg | 62.7%++ | ||||||||
| Placebo | 11.8%++ | ||||||||
| IGA (0,1) | 100 mg | 23.7%++ | |||||||
| 200 mg | 43.8%++ | ||||||||
| Placebo | 7.9%++ | ||||||||
| Silverberg et al.46 JADE MONO-2 |
III | 12 | 391 | EASI-75 | 100 mg | 44.5%++ | |||
| 200 mg | 61.0%++ | ||||||||
| Placebo | 10.4%++ | ||||||||
| IGA (0,1) | 100 mg | 28.4%++ | |||||||
| 200 mg | 38.1%++ | ||||||||
| Placebo | 9.1%++ | ||||||||
| Baricitinib | JAK1 and JAK2 | RA** | Guttman-Yassky, et al.48 | II | 16 | 124 | EASI-50 | 2 mg | 57%++ |
| 4 mg | 61%++ | ||||||||
| Placebo | 37%++ | ||||||||
| Simpson et al.50 BREEZE-AD1 |
III | 16 | 624 | IGA (0,1) | 1 mg | 11.8%++ | |||
| 2 mg | 11.4%++ | ||||||||
| 4 mg | 16.8%++ | ||||||||
| Placebo | 4.8%++ | ||||||||
| Simpson et al.50 BREEZE-AD2 |
III | 16 | 615 | IGA (0,1) | 1 mg | 8.8%++ | |||
| 2 mg | 10.6%++ | ||||||||
| 4 mg | 13.8%++ | ||||||||
| Placebo | 4.5%++ | ||||||||
| BREEZE-AD451 | III | 16 | 463 | EASI-75 | 1 mg | 22.6%++ | |||
| 2 mg | 27.6%++ | ||||||||
| 4 mg | 31.5%++ | ||||||||
| Placebo | 17.2%++ | ||||||||
| Gusacitinib (ASN002) | JAK1, JAK2, JAK3, Tyk2, and SYK | None | Bissonnette, et al.52 | Ib | 4 | 36 | EASIǂ*** | 20 mg | 9.6%+ |
| 40 mg | 17.5%+ | ||||||||
| 80 mg | 16.5%+ | ||||||||
| Placebo | 7.6%+ | ||||||||
| Upadacitinib | JAK1 | RA** | Guttman-Yassky, et al.53 | IIb | 16 | 167 | EASI | 7.5 mg | 39%+ |
| 15 mg | 62%+ | ||||||||
| 30 mg | 74%+ | ||||||||
| Placebo | 23%+ | ||||||||
Notes: *Under investigation. **FDA-approved. ***EASI score was a secondary endpoint in this study, adverse events were the primary endpoint. +Endpoint improvement from baseline. ++Percent of patients achieving the outcome. ǂSecondary endpoint.
Abbreviations: BID, twice daily; EASI, Eczema Area and Severity Index; IGA, Investigator Global Assessment; JAK, Janus kinase; mEASI, modified EASI; mg, milligram; RA, rheumatoid arthritis.
Ruxolitinib
Ruxolitinib is a selective JAK1 and high potency JAK2 inhibitor especially efficacious for the treatment of pruritus in AD.36,37 In a phase II randomized, double-blind, vehicle- and active-controlled trial assessing the safety and efficacy of ruxolitinib, baseline EASI scores improved by 78.5% (p<0.0001) in the 1.5% twice daily ruxolitinib group and 26.9% in the vehicle group after eight weeks of treatment.37 Baseline EASI scores in the triamcinolone twice-daily group improved by 40.0% and 59.8% at weeks two and four of treatment, respectively; comparatively, baseline EASI scores in the 1.5% once-daily ruxolitinib cream improved by 49.9% and 67.0% and baseline EASI scores in the 1.5% twice daily ruxolitinib group improved by 52.7% and 71.6% at the same respective timepoints.37 Statistical significance of these improvements against the triamcinolone group was not achieved.37 Two Phase III randomized, double-blind, vehicle-controlled studies, TRuE-AD1 and TRuE-AD2, assessed achievement of IGA scores of 0 or 1 at the end of eight weeks of treatment.38 In the TRuE-AD1 trial, IGA scores of 0 or 1 were achieved by 50% of patients in the 0.75% twice daily ruxolitinib group (p<0.0001), 53.8% of patients in the 1.5% twice daily ruxolitinib group (p<0.0001) and 15.1% of the patients in the vehicle group after eight weeks of treatment.38 In the TRuE-AD2 trial, an IGA of 0 or 1 was achieved by 39% of the patients in the 0.75% twice daily ruxolitinib group (p<0.0001), 51.3% of patients in the 1.5% twice daily ruxolitinib group (p<0.0001) and 7.6% of the patients in the vehicle group after eight weeks of treatment.38
Tofacitinib
Tofacitinib is a JAK3 and/or JAK1 inhibitor, with possible minimal JAK2 inhibition.39,40 In a phase IIa randomized, double-blind, vehicle-controlled study assessing the safety and efficacy of tofacitinib, baseline EASI scores improved by 81.7% (p<0.001) in the 2% twice daily tofacitinib group and 29.9% in the vehicle-controlled group after four weeks of treatment.41 Furthermore, improvements in pruritus were achieved within as little as two days of treatment,41 suggesting that tofacitinib may be appealing therapeutic options for patients with debilitating or severe pruritus.
Efficacy of Oral JAK Inhibitors for Treatment of AD
Abrocitinib
Abrocitinib is a JAK1 inhibitor.42,43 A phase II randomized, double-blind, placebo-controlled trial assessed the safety and the efficacy of abrocitinib in AD patients via improvement in IGA scores, with a focus on the proportion of patients achieving IGA scores of 0 or 1 (clear or almost clear skin).44 IGA scores of 0 or 1 were achieved by 43.8% of patients in the 200 mg abrocitinib group and by 5.8% in the placebo group at the end of 12 weeks of treatment (p<0.001).42 Two phase III randomized, double-blind, placebo-controlled trials, JADE MONO-1 and JADE MONO-2, assessed co-primary efficacy endpoints of the percentage of patients achieving an improvement of 75% in the EASI scores (EASI-75) and IGA scores of 0 or 1 at the end of 12 weeks of treatment. Per preliminary results from the JADE MONO-1 study, EASI-75 was achieved by 62.7% of patients in the 200 mg abrocitinib group, 39.7% of patients in the 100 mg abrocitinib group, and 11.8% of patients in the placebo group after 12 weeks of treatment.45 IGA scores of 0 or 1 were achieved by 43.8% of patients in the 200 mg abrocitinib group, 23.7% of patients in the 100 mg abrocitinib group, and 7.9% of patients in the placebo group after 12 weeks of treatment. Analysis of statistical significance has not yet been released for this data.45 Preliminary data from the JADE MONO-2 trial reported achievement of EASI-75 in 61% of patients in the 200 mg abrocitinib group (p<0.001), 44.5% in the 100 mg abrocitinib group (p<0.001), and 10.4% in the placebo group at 12 weeks.46 IGA scores of 0 or 1 were achieved by 38.1% of patients in the 200 mg abrocitinib group (p<0.001), 28.4% in the 100 mg abrocitinib group (p<0.001), and 9.1% in the placebo group at 12 weeks.46 The results of these trials suggest the most efficacious dosing for abrocitinib is either 100 mg or 200 mg oral tablets once daily.42,45,47
Baricitinib
Baricitinib is a potent JAK1 and JAK2 inhibitor with additional minimal inhibition of JAK3 and TYK2.48,49 A phase II randomized, double-blind, placebo-controlled trial assessing the safety and efficacy of baricitinib reported achievement of EASI-50 after 16 weeks of treatment in 61% (p=0.027) of patients receiving 4 mg baricitinib plus topical corticosteroid compared to 37% of the patients receiving placebo plus topical corticosteroid.48 Two phase III randomized, double-blind, placebo-controlled trials, BREEZE-AD1 and BREEZE-AD2, assessed the efficacy and safety of baricitinib per proportion of patients achieving IGA scores of 0 or 1 at the end of 16 weeks of treatment.50 In BREEZE-AD1, an IGA score of 0 or 1 was achieved by 16.8% of the patients in the 4 mg baricitinib group (p<0.001) and 4.8% of the patients in the placebo group after 16 weeks of treatment.50 In BREEZE-AD2, an IGA score of 0 or 1 was achieved by 13.8% of the patients in the 4 mg baricitinib group (p<0.001) and 4.5% of the patients in the placebo group after 16 weeks of treatment.50 Preliminary data were recently released for the BREEZE-AD4 trial, a multicenter, randomized, double-blind, placebo-controlled phase III trial that assessed the efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe AD and contraindications to or failure of therapy with CIs.51 The primary efficacy endpoint of proportion of patients achieving EASI-75 after 16 weeks of treatment was 27.6% of patients (p>0.05) in the 2 mg baricitinib plus corticosteroid group, 31.5% (p<0.05) in the 4 mg baricitinib plus corticosteroid group, and 17.2% in the placebo plus corticosteroid group.51
Gusacitinib
Gusacitinib (ASN002) is a JAK1, JAK2, JAK3, TYK2, and SYK inhibitor.28,52 In a phase Ib randomized, double-blind, placebo-controlled trial assessing the safety and efficacy of gusacitinib, baseline EASI scores improved by 17.5% in the 40 mg once daily ASN002 group (p=0.02), 16.5% in the 80 mg once daily ASN002 group (p=0.17), and 7.6% in the once-daily placebo group after four weeks of treatment.52
Upadacitinib
Upadacitinib maximizes inhibition of JAK1 and minimizes the inhibition of JAK2 and JAK3; this helps to prevent unwanted side effects associated with JAK2 inhibition such as anemia and thrombocytopenia.53 Upadacitinib is a promising therapy not only for AD but also for additional inflammatory diseases, with 37 clinical trials currently underway evaluating its use in various disorders. A phase IIb multicenter, randomized, double-blind, placebo-controlled, parallel-group phase trial assessing the safety and efficacy of upadacitinib reported an improvement from baseline EASI scores of 62% in the upadacitinib 15 mg group, 74% in the upadacitinib 30 mg group, and 23% in the placebo group at the end of 16 weeks of treatment (p<0.001).53
Other
Additional JAK inhibitors are in the early phases of clinical trials for use in AD patients. Cerdulatinib (RVT-502) is both an SYK and JAK1, JAK3, and TYK2 inhibitor currently being investigated in a phase IIa clinical trial for use in AD; however, no data is currently available for review.23,54 The medication is also currently being investigated for its use in T-cell leukemia, B-cell malignancies, leukemia, and lymphoma.54,55 Momelotinib is an adenosine triphosphate (ATP)-competitive JAK1 and JAK2 inhibitor currently being tested in murine models as a topical medication for multiple skin disorders, including AD.56 SNA-125 is a topical JAK3 and tropomyosin receptor kinase A inhibitor which mediates neurogenic inflammation and pruritus; it is being tested in a phase I/II proof of concept clinical trial for AD and no data is currently available for review.6 As JAK inhibitors are relatively new therapeutic options for AD, there is a general lack of substantial long-term data for both efficacy and safety of the drugs in adult populations and even less available data regarding their use in pediatric populations.
Safety/Adverse Events
While JAK inhibitors typically exhibit a tolerable safety profile, their use is associated with various adverse events. In the phase III JADE MONO trials, approximately 10% to 20% of patients experienced adverse events (AEs), primarily nausea, nasopharyngitis, and headaches (Table 3).45,46 There were zero, two (herpangina and pneumonia), and one (eczema herpeticum and staphylococcal infection) serious AEs in the 200 mg, 100 mg, and placebo groups, respectively, in the JADE MONO-2 trial.46 No malignant neoplasms or venous thromboembolisms were reported in any of the groups.46 In the phase III BREEZE trials for baricitinib, approximately 55% to 60% of patients in the baricitinib groups reported treatment-emergent (TE) AEs; the most commonly reported AEs included nasopharyngitis, upper respiratory infections (URIs), increases in creatinine phosphokinase (CPK), and headaches, which reported in less than 10% of patients in the baricitinib groups across both trials.50 There were no deaths, deep vein thromboses, pulmonary embolisms, or opportunistic infections in any of the groups in either trials.50
Table 3.
Adverse Events Associated with JAK Inhibitors
| Trial | Percent of Patients Reporting TEAEs | Frequency of Serious Adverse Events | Adverse Events | Frequency of Adverse Events | Rate of Discontinuation | |
|---|---|---|---|---|---|---|
| Topical | ||||||
| Delgocitinib | Nakagawa et al.34 Pediatric | 41.7% | 0% | Nasopharyngitis | 0.25% (17.6%) 0.5% (20.6%) Vehicle (8.6%) |
4.9% |
| Impetigo | 0.25% (5.9%) 0.5% (2.9%) Vehicle (8.6%) |
|||||
| Urticaria | 0.25% (5.9%) 0.5% (0%) Vehicle (0%) |
|||||
| Nakagawa et al.33 Adult at the end of 4 weeks |
18.4% | 0% | Nasopharyngitis | 0.5% (5.7%) Placebo (3.8%) |
2.5% | |
| Kaposi varicelliform eruption | 0.5% (1.9%) Placebo (0%) |
|||||
| Dental carries | 0.5% (1.9%) Placebo (0%) |
|||||
| Ruxolitinib | Kim et al.36 | 30.4% | 0% | Application site pain | 0.1% Triamcinolone BID (0%) 0.15% (2.0%) 0.5% (0%) 1.5% (3.9%) 1.5 BID (2.0%) Vehicle (3.8%) |
N/A |
| Nasopharyngitis | 0.1% Triamcinolone BID (0%) 0.15% (5.9%) 0.5% (2.0%) 1.5% (7.8%) 1.5% BID (4.0%) Vehicle (7.7%) |
|||||
| TRuE-AD138 | N/A | N/A | N/A | N/A | N/A | |
| TRuE-AD238 | N/A | N/A | N/A | N/A | N/A | |
| Tofacitinib | Bissonnette et al.41 | 43.5% | 0% | Infections (mostly nasopharyngitis) | 2% (8.7%) Vehicle (4.3%) |
5.8% |
| CPK increase | 2% (0%) Vehicle (4.3%) |
|||||
| Contact dermatitis | 2% (0%) Vehicle (4.3%) |
|||||
| Headache | 2% (1.4%) Vehicle (2.9%) |
|||||
| Oral | ||||||
| Abrocitinib | Gooderham, et al.42 | 68.9% | 3.4% | Gastrointestinal disorders | 10 mg (8.2%) 30 mg (9.8%) 100 mg (10.7%) 200 mg (21.8%) Placebo (7.1%) |
41.2% |
| Diarrhea | 10 mg (6.1%) 30 mg (2.0%) 100 mg (1.8%) 200 mg (9.1%) Placebo (1.8%) |
|||||
| Nausea | 10 mg (6.1%) 30 mg (5.9%) 100 mg (1.8%) 200 mg (14.5%) Placebo (1.8%) |
|||||
| Infections and infestations | 10 mg (46.9%) 30 mg (37.3%) 100 mg (42.9%) 200 mg (41.8%) Placebo (23.2%) |
|||||
| JADE MONO-145 | N/A | 3.0% | Short-lasting nausea | 100 mg (9.0%) 200 mg (20.9%) Placebo (N/A) |
6.5% | |
| Headaches | 100 mg (7.7%) 200 mg (9.7%) Placebo (N/A) |
|||||
| Nasopharyngitis | 100 mg (14.7%) 200 mg (11.7%) Placebo (N/A) |
|||||
| Silverberg et al.46 JADE MONO-2 |
62.1% | 2.1% | Nausea | 100 mg (7.6%) 200 mg (14.2%) Placebo (2.6%) |
15.6% | |
| Nasopharyngitis | 100 mg (12.7%) 200 mg (7.7%) Placebo (6.4%) |
|||||
| Headache | 100 mg (5.7%) 200 mg (7.7%) Placebo (2.6%) |
|||||
| Thrombocytopenia | 100 mg (0%) 200 mg (3.2%) Placebo (0%) |
|||||
| Baricitinib | Guttman-Yassky et al.48 | 54.8% | 0.8% | Headaches | 2 mg + TCS (5%) 4 mg + TCS (13%) Placebo (0%) |
31.45% |
| CPK increase | 2 mg + TCS (3%) 4 mg + TCS (13%) Placebo (0%) |
|||||
| Nasopharyngitis | 2 mg + TCS (3%) 4 mg + TCS (8%) Placebo (2%) |
|||||
| Simpson et al.50 BREEZE-AD1 |
55.8% | 2.7% | Nasopharyngitis | 1 mg (17.3%) 2 mg (9.8%) 4 mg (9.6%) Placebo (10.4%) |
7.9% | |
| URIs | 1 mg (0.8%) 2 mg (2.4%) 4 mg (3.2%) Placebo (2.4%) |
|||||
| CPK increase | 1 mg (0.8%) 2 mg (0.8%) 4 mg (3.2%) Placebo (0.8%) |
|||||
| Headaches | 1 mg (5.5%) 2 mg (11.4%) 4 mg (8.0%) Placebo (6.4%) |
|||||
| Simpson et al.50 BREEZE-AD2 |
55.4% | 3.8% | Nasopharyngitis | 1 mg (10.5%) 2 mg (13.0%) 4 mg (8.1%) Placebo (12.3%) |
7.3% | |
| URIs | 1 mg (4.8%) 2 mg (4.1%) 4 mg (3.3%) Placebo (2.0%) |
|||||
| CPK increase | 1 mg (3.2%) 2 mg (0.8%) 4 mg (5.7%) Placebo (0.4%) |
|||||
| Headaches | 1 mg (4.8%) 2 mg (7.3%) 4 mg (8.9%) Placebo (2.0%) |
|||||
| BREEZE-AD451 | N/A | N/A | Nasopharyngitis | N/A | N/A | |
| Headache | N/A | |||||
| Influenza | N/A | |||||
| Gusacitinib (ASN002) | Bissonnette et al52 | N/A | 2.8% | Headache | 20 mg (11%) 40 mg (44%) 80 mg (22%) Placebo (33%) |
N/A |
| Nausea | 20 mg (0%) 40 mg (11%) 80 mg (44%) Placebo (22%) |
|||||
| Nasopharyngitis | 20 mg (22%) 40 mg (11%) 80 mg (0%) Placebo (11%) |
|||||
| Diarrhea | 20 mg (0%) 40 mg (11%) 80 mg (22%) Placebo (11%) |
|||||
| Back pain | 20 mg (0%) 40 mg (22%) 80 mg (0%) Placebo (0%) |
|||||
| Upadacitinib | Guttman-Yassky, et al.53 | 72.9% | 2.4% | URI | 7.5 mg (17%) 15 mg (12%) 30 mg (12%) Placebo (10%) |
24% |
| Worsening AD | 7.5 mg (14%) 15 mg (4.8%) 30 mg (9.5%) Placebo (5%) |
|||||
| Acne | 7.5 mg (9.5%) 15 mg (4.8%) 30 mg (14%) Placebo (2.5%) |
|||||
| Headache | 7.5 mg (7.1%) 15 mg (7.1%) 30 mg (9.5%) Placebo (2.5%) |
|||||
| Neutropenia | 7.5 mg (2.4%) 15 mg (4.8%) 30 mg (4.8%) Placebo (0%) |
|||||
Abbreviations: AD, atopic dermatitis; BID, twice daily; CPK, creatinine phosphokinase; mg, milligram; N/A, not available; TCS, topical corticosteroid; TEAE, treatment emergent adverse event; URI, upper respiratory infection.
Pooled safety data from the phase III delgocitinib trials over 52 weeks reported either mild or moderate TEAEs in 69% of patients, while one individual had a severe, non-treatment-related TEAE.31 Approximately 25%, 5%, and 4% of patients experiences nasopharyngitis, contact dermatitis, and acne, respectively.31 The most common reasons for treatment discontinuations due to AEs were contact dermatitis and application-site irritation.31 Although this medication is effective in both children and adult Japanese patients, the impact and efficacy of delgocitinib in individuals of other ethnicities and skin types is not well established. There are on-going phase II trials in Denmark, Germany, and the United States investigating the efficacy and safety of delgocitinib in moderate-to-severe hand AD.31,33,34 In a phase Ib trial of gusacitinib, the most commonly experienced TEAEs related to gusacitinib included headaches, nausea, diarrhea, nasopharyngitis, and back pain.52 Treatment discontinuation due to AEs in patients receiving gusacitinib were primarily due to mild hypertension and low lymphocyte levels.52
The most common AEs reported from the phase II ruxolitinib trial included nasopharyngitis and pain at the site of application.37 Approximately 30% of patients from the phase III TRuE-AD1 and TRuE-AD2 trials reported TEAEs; incidences were comparable among the patients receiving different doses of ruxolitinib and the vehicle control in both studies over eight weeks.57 Serious AEs were reported by less than one percent of patients in each group.57 A 44-week extension period for the TRuE-AD trials is currently ongoing to examine longer-term efficacy and safety.57 In a phase II trial of tofacitinib, 31% of patients in the 2% tofacitinib group and 56% of patients in the vehicle group experienced TEAEs.41 The most common TEAEs in patients receiving tofacitinib were infections, specifically nasopharyngitis.41 Other common TEAEs included increases in CPK, contact dermatitis, and headaches.41 There were no deaths, reactivation of herpes infections, malignancies, or opportunistic infections reported.41 In a phase IIb trial for upadacitinib, approximately 40–50% of patients receiving upadacitinib reported mild-to-moderate infections.53 The most commonly reported AEs included URIs, worsening AD, and mild-to-moderate acne. Neutropenia was among the more serious AEs reported in patients receiving upadacitinib; there were no deaths, malignancies, opportunistic or tuberculosis infections, gastrointestinal perforations, cardiovascular, or thromboembolic events reported in the trial.53
Discussion
JAK inhibitors demonstrate considerable efficacy for the treatment of AD, both alone and in combination with topical corticosteroids. After just four weeks of treatment, the topical JAK inhibitor tofacitinib improved baseline EASI scores by approximately 80%.41 Although not statistically significant, approximately 10% more patients achieved IGA scores of 0 or 1 after four weeks of treatment with 1.5% daily ruxolitinib cream compared to triamcinolone cream.37 While topical JAK inhibitors are efficacious therapeutic agents for the treatment of AD, the current first-line therapies (topical corticosteroids and CIs) are highly efficacious, making it unlikely that topical JAK inhibitors will become first-line therapeutic alternatives. If patients fail treatment with multiple first-line topical agents, it is possible that insurance will cover the use of topical JAK inhibitors; however, poor adherence is often a primary cause of medication failure, and it is unlikely that patients who failed all other topical therapies will achieve disease stability with use of a topical JAK inhibitor. Topical JAK inhibitors can be a beneficial alternative for patients with corticosteroid phobia, although insurance coverage may pose challenges.
Oral JAK inhibitors are also efficacious for treating AD and may be an appropriate therapy for patients with moderate-to-severe disease. After four months of treatment, baseline EASI scores improved by approximately 75% with the use of upadacitinib.53 Addition of the JAK inhibitor, baricitinib, to a topical corticosteroid may increase efficacy via the proportion of patients achieving EASI-50 by nearly 25% and proportion of patients achieving EASI-75 by nearly 15% versus topical corticosteroids alone.48 Trials directly comparing corticosteroids and CIs with JAK inhibitors are scarce for many of the therapeutic options currently being studied. However, the preliminary data from the ruxolitinib and baricitinib studies comparing the efficacy of JAK inhibitors to one of the current standards of therapy, topical corticosteroids, is promising.
The most current data from clinical trials on JAK inhibitor use in AD suggest that headaches, nausea, and mild-to-moderate infections, most commonly nasopharyngitis, are the most commonly reported AEs; these effects may be experienced by a sizeable proportion of patients. Although the overall incidence of serious AEs with the use of oral JAK inhibitors for AD treatment is low, data from studies evaluating use of JAK inhibitors for other dermatologic conditions suggest a potentially slightly increased risk of more serious AEs including reactivation of herpes zoster, thromboembolic events, cardiovascular events, and hematological events.6 Additionally, studies assessing JAK inhibitors as treatments for patients with rheumatoid arthritis also disclose an increased risk of thromboembolic events with the use of ruxolitinib, tofacitinib, and baricitinib.58 Thus, JAK inhibitor use may potentially be contraindicated in patients at an increased risk for thromboembolic disease, although these results have not been observed in trials assessing use of JAK inhibitors for AD. In addition to thromboembolisms, thrombocytopenia, anemia, and neutropenia are theoretical risks with JAK2 inhibition, as JAK2 is implicated in the pathways for erythropoiesis, myelopoiesis, and platelet activation.6 AEs of this nature occurred in a small proportion of patients receiving upadacitinib and tofacitinib in two of the above-listed studies.52,53 Although upadacitinib is a JAK1 selective inhibitor, research suggests JAK2 is dependent on JAK1 for transphosphorylation and activation; the intertwined pathways of JAKs could explain why neutropenia is reported as an AE for upadacitinib.59 The often younger and relatively healthier patient populations in AD are likely less susceptible to the more severe AEs observed with use of JAK inhibitors compared to elderly individuals or individuals predisposed to autoimmune conditions such as psoriasis or rheumatoid arthritis, potentially explaining why severe AEs are uncommon or unobserved in AD trials.6
Corticosteroids and CIs are often the first-line therapeutic agents for AD with moderate safety profiles. In a 2018 systemic review of oral corticosteroids in AD, the most commonly reported AEs were rebound AD flare-ups upon drug discontinuation in 81% of prednisone-treated patients.60 Adrenal insufficiency or suppression, opportunistic infections, and immunosuppression are other reported severe AEs more commonly associated with long-term use of systemic corticosteroids (> one year);60 these AEs have not been observed with JAK inhibitor use in AD patients. Low rates of skin infections may occur in individuals using topical CIs (≤5–10%) and topical corticosteroids (≤2%); skin atrophy with topical corticosteroid use is a common cutaneous symptom reported as frequently as 8% to 12% in some studies of low to medium potency corticosteroids.61 Various bacterial, viral, respiratory tract, and gastrointestinal infections are the most commonly reported systemic AEs in children treated with topical corticosteroids (17%) and CIs (20%); there is no increased risk of adrenal or immune suppression in children using these agents for treatment of AD when compared to the general population.61 The Food and Drug Administration (FDA) is unable to rule out the possibility of an increased risk of lymphoma with use of topical CIs; however, recent data suggest the use of topical tacrolimus in AD does not increase the risk of cancer.61,62 Mild-to-moderate infections, namely nasopharyngitis and URIs, are AEs common to use of JAK inhibitors, topical corticosteroids, and topical CIs. However, skin atrophy and cutaneous infections, common AE manifestations of topical corticosteroids or CIs, are not commonly seen with JAK inhibitor use. As there is limited long-term safety data currently available for JAK inhibitors, it is unknown if cutaneous infections could be a long-term AE of JAK inhibitors.6,32–34,41,42,49,50,52,53,60,63
While efficacy and safety profiles are important considerations when selecting a therapy, cost and convenience may also impact patient preference. JAK inhibitors have a lower production cost compared to monoclonal antibodies, and a higher production cost compared to corticosteroids.29,64,65 JAK inhibitors may be administered orally or topically, allowing for convenient routes of administration.6 As the testing of JAK inhibitors as therapies for AD continues, it will be important for longer-term trials to achieve a better understanding of any time-related AE and efficacy concerns, as this information is currently largely unavailable given the recent development of many of these agents.
Limitations of this study include the lack of establishment of an a priori research protocol prior to performing a literature search, as well as the use of only one MEDLINE indexed electronic database for systematic searches and one author for article selection and data extraction. Such limitations may affect the internal validity of this review article. Additionally, this review is limited in its ability to determine the long-term safety and efficacy of JAK inhibitors compared to many of the first-line medications for AD. As additional data emerge, the role of JAK inhibitors in AD compared to the current standard of care may be more clearly defined. In the present, JAK inhibitors have a moderate short-term safety profile and a comparable efficacy to current first-line AD therapies.
Conclusion
The increasing use of JAK inhibitors in adults and children with moderate-to-severe AD can provide an efficacious therapeutic option when used alone or in conjunction with topical corticosteroids, although these agents are unlikely to become first-line therapies given the currently available therapeutic options for patients with AD. Current evidence suggests JAK inhibitors may also lack association with some of the more serious AEs associated with corticosteroids and CIs which are the current first-line treatments for moderate-to-severe AD, although there is potential for increased risk of thromboembolism, neutropenia, and lymphocytopenia. For patients with AD who have failed or had an inadequate response to therapy with corticosteroids or CIs, or with corticosteroid phobia, JAK inhibitors may be an appropriate consideration for symptomatic and long-term relief. The use of JAK inhibitors in the treatment of AD is still largely experimental, as the drugs mentioned in this article are currently not FDA-approved for use in AD. With the continued advancement of clinical trials, we anticipate that JAK inhibitors will be beneficial additions to the armamentarium of therapeutic options available for the treatment of moderate-to-severe AD.
Funding Statement
This article has no funding source.
Disclosure
Dr Lindsay Strowd has received consulting fees or research funding from Galderma, Lilly, Pfizer, Sanofi, and Actelion, and reports grants from Pfizer, CME writing from Galderma, personal fees from Actelion and Sanofi, and Phase 3 clinical trial PI from Lilly, outside the submitted work. Dr. Feldman received research, speaking and/or consulting support from Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Helsinn, Arena, Forte, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He consults for others through Guidepoint Global, Gerson Lehrman and other consulting organizations. He is founder and majority owner of (www.DrScore.com). He is also a founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. The authors report no other potential conflicts of interest for this work.
References
- 1.Waldman AR, Ahluwalia J, Udkoff J, Borok JF, Eichenfield LF. Atopic dermatitis. Pediatr Rev. 2018;39(4):180–193. doi: 10.1542/pir.2016-0169 [DOI] [PubMed] [Google Scholar]
- 2.Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in america study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi: 10.1016/j.jid.2018.08.028 [DOI] [PubMed] [Google Scholar]
- 3.Mayba JN, Gooderham MJ. Review of atopic dermatitis and topical therapies. J Cutan Med Surg. 2017;21(3):227–236. [DOI] [PubMed] [Google Scholar]
- 4.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the national eczema association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 5.David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21–37. [DOI] [PubMed] [Google Scholar]
- 6.He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20(2):181–192. doi: 10.1007/s40257-018-0413-2 [DOI] [PubMed] [Google Scholar]
- 7.Silverberg NB. Atopic dermatitis prevention and treatment. Cutis. 2017;100(3):192. [PubMed] [Google Scholar]
- 8.Maarouf M, Shi VY. Bleach for atopic dermatitis. Dermatitis. 2018;29(3):120–126. doi: 10.1097/DER.0000000000000358 [DOI] [PubMed] [Google Scholar]
- 9.Rodenbeck DL, Silverberg JI, Silverberg NB. Phototherapy for atopic dermatitis. Clin Dermatol. 2016;34(5):607–613. doi: 10.1016/j.clindermatol.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Salvador JM, Pérez-Ferriols A. Phototherapy in atopic dermatitis. Adv Exp Med Biol. 2017;996:279–286. [DOI] [PubMed] [Google Scholar]
- 11.Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863–871.e811. doi: 10.1016/j.jaad.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 12.Seegraber M, Srour J, Walter A, Knop M, Wollenberg A. Dupilumab for treatment of atopic dermatitis. Expert Rev Clin Pharmacol. 2018;11(5):467–474. doi: 10.1080/17512433.2018.1449642 [DOI] [PubMed] [Google Scholar]
- 13.Slater NA, Morrell DS. Systemic therapy of childhood atopic dermatitis. Clin Dermatol. 2015;33(3):289–299. doi: 10.1016/j.clindermatol.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Guenther L, Lynde C, Poulin Y. Off-label use of topical calcineurin inhibitors in dermatologic disorders. J Cutan Med Surg. 2019;23(4_suppl):27s–34s. doi: 10.1177/1203475419857668 [DOI] [PubMed] [Google Scholar]
- 15.Drucker AM, Eyerich K, de Bruin-weller MS, et al. Use of systemic corticosteroids for atopic dermatitis: international Eczema council consensus statement. Br J Dermatol. 2018;178(3):768–775. doi: 10.1111/bjd.15928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta AB, Nadkarni NJ, Patil SP, Godse KV, Gautam M, Agarwal S. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82(4):371–378. doi: 10.4103/0378-6323.178903 [DOI] [PubMed] [Google Scholar]
- 17.Drucker AM, Qureshi AA, Amand C, et al. Health care resource utilization and costs among adults with atopic dermatitis in the United States: a claims-based analysis. J Allergy Clin Immunol Pract. 2018;6(4):1342–1348. doi: 10.1016/j.jaip.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 18.Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39(6):406–410. doi: 10.2500/aap.2018.39.4175 [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Zheng Y, Zhang X, He Y, Li C. Efficacy and safety of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults. Oncotarget. 2017;8(65):108480–108491. doi: 10.18632/oncotarget.22499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema area and severity index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172(5):1353–1357. doi: 10.1111/bjd.13662 [DOI] [PubMed] [Google Scholar]
- 21.Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74(2):288–294. doi: 10.1016/j.jaad.2015.09.062 [DOI] [PubMed] [Google Scholar]
- 22.Gunduz O. JAK/STAT pathway modulation: does it work in dermatology? Dermatol Ther. 2019;32(3):e12903. doi: 10.1111/dth.12903 [DOI] [PubMed] [Google Scholar]
- 23.Montilla AM, Gomez-Garcia F, Gomez-Arias PJ, et al. Scoping review on the use of drugs targeting JAK/STAT pathway in atopic dermatitis, vitiligo, and alopecia areata. Dermatol Ther (Heidelb). 2019;9(4):655–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228.e213. doi: 10.1016/j.cell.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;11(10):e0164080. doi: 10.1371/journal.pone.0164080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jakstat. 2013;2(3):e24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wöss K, Simonović N, Strobl B, Macho-Maschler S, Müller M. TYK2: an upstream kinase of STATs in cancer. Cancers (Basel). 2019;11(11):1728. doi: 10.3390/cancers11111728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavel AB, Song T, Kim HJ, et al. Oral Janus kinase/SYK inhibition (ASN002) suppresses inflammation and improves epidermal barrier markers in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144(4):1011–1024. doi: 10.1016/j.jaci.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 29.Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishizaki M, Akimoto T, Muromoto R, et al. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J Immunol. 2011;187(1):181–189. doi: 10.4049/jimmunol.1003244 [DOI] [PubMed] [Google Scholar]
- 31.Dhillon S. Delgocitinib: first Approval. Drugs. 2020;80(6):609–615. doi: 10.1007/s40265-020-01291-2 [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa H, Nemoto O, Igarashi A, Nagata T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. Br J Dermatol. 2018;178(2):424–432. doi: 10.1111/bjd.16014 [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82(4):823–831. doi: 10.1016/j.jaad.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144(6):1575–1583. doi: 10.1016/j.jaci.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 35.Chopra R, Vakharia PP, Sacotte R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177(5):1316–1321. doi: 10.1111/bjd.15641 [DOI] [PubMed] [Google Scholar]
- 36.Kim BS, Sun K, Papp K, Venturanza M, Nasir A, Kuligowski ME. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82(6):1305–1313. doi: 10.1016/j.jaad.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 37.Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. 2020;145(2):572–582. doi: 10.1016/j.jaci.2019.08.042 [DOI] [PubMed] [Google Scholar]
- 38.Incyte Corporation. Incyte Announces Positive Topline Results From Phase 3 TRuE-AD Program Evaluating Ruxolitinib Cream in Patients With Atopic Dermatitis [press release]. Wilmington, DE: Incyte Corporation; 2020 [February 19].Available from: https://investor.incyte.com/press-releases/press-releases/2020/Incyte-Announces-Positive-Topline-Results-From-Phase-3-TRuE-AD-Program-Evaluating-Ruxolitinib-Cream-in-Patients-With-Atopic-Dermatitis/default.aspx. Accessed May 15, 2020.
- 39.Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73(3):395–399. doi: 10.1016/j.jaad.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 40.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–550. doi: 10.1016/j.immuni.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175(5):902–911. doi: 10.1111/bjd.14871 [DOI] [PubMed] [Google Scholar]
- 42.Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371. doi: 10.1001/jamadermatol.2019.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmieder GJ, Draelos ZD, Pariser DM, et al. Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: phase II, randomized, double-blind, placebo-controlled study. Br J Dermatol. 2018;179(1):54–62. doi: 10.1111/bjd.16004 [DOI] [PubMed] [Google Scholar]
- 44.Peeva E, Hodge MR, Kieras E, et al. Evaluation of a Janus kinase 1 inhibitor, PF-04965842, in healthy subjects: a phase 1, randomized, placebo-controlled, dose-escalation study. Br J Clin Pharmacol. 2018;84(8):1776–1788. doi: 10.1111/bcp.13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfizer. Pfizer Presents Positive Phase 3 Data At The 28th Congress Of The European Academy Of Dermatology And Venereology For Abrocitinib In Moderate To Severe Atopic Dermatitis [press release]. New York, NY: Pfizer;2019 [October 12]. Available from: https://investors.pfizer.com/investor-news/press-release-details/2019/Pfizer-Presents-Positive-Phase-3-Data-at-the-28th-Congress-of-the-European-Academy-of-Dermatology-and-Venereology-for-Abrocitinib-in-Moderate-to-Severe-Atopic-Dermatitis/default.aspx. Accessed May 15, 2020.
- 46.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863. doi: 10.1001/jamadermatol.2020.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfizer. Pfizer Announces Positive Top-Line Results From Second Pivotal Phase 3 Study Of Investigational Oral Jak1 Candidate, Abrocitinib, In Patients Aged 12 And Older With Moderate To Severe Atopic Dermatitis [press release]. New York, NY: Pfizer; 2019 [September 27]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_positive_top_line_results_from_second_pivotal_phase_3_study_of_investigational_oral_jak1_candidate_abrocitinib_in_patients_aged_12_and_older_with_moderate_to_severe_atopic_dermatitis. Accessed May 15, 2020.
- 48.Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913–921.e919. doi: 10.1016/j.jaad.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 49.Markham A. Baricitinib: first Global Approval. Drugs. 2017;77(6):697–704. doi: 10.1007/s40265-017-0723-3 [DOI] [PubMed] [Google Scholar]
- 50.Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255. doi: 10.1111/bjd.18898 [DOI] [PubMed] [Google Scholar]
- 51.Timmins P. Lilly and Incyte Announce Top-Line Results from Phase 3 Study (BREEZE-AD4) of Oral Selective JAK Inhibitor Baricitinib in Combination with Topical Corticosteroids in Patients with Moderate to Severe Atopic Dermatitis Not Controlled with Cyclosporine [Press Release]. Indianapolis: Eli Lilly and Company; 2020. [Google Scholar]
- 52.Bissonnette R, Maari C, Forman S, et al. The oral Janus kinase/spleen tyrosine kinase inhibitor ASN002 demonstrates efficacy and improves associated systemic inflammation in patients with moderate-to-severe atopic dermatitis: results from a randomized double-blind placebo-controlled study. Br J Dermatol. 2019;181(4):733–742. doi: 10.1111/bjd.17932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guttman-Yassky E, Thaci D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi: 10.1016/j.jaci.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 54.Coffey GP, Feng J, Betz A, et al. Cerdulatinib pharmacodynamics and relationships to tumor response following oral dosing in patients with relapsed/refractory B-cell malignancies. Clin Cancer Res. 2019;25(4):1174–1184. doi: 10.1158/1078-0432.CCR-18-1047 [DOI] [PubMed] [Google Scholar]
- 55.Ito Y, Makita S, Tobinai K. Development of new agents for peripheral T-cell lymphoma. Expert Opin Biol Ther. 2019;19(3):197–209. doi: 10.1080/14712598.2019.1572746 [DOI] [PubMed] [Google Scholar]
- 56.Jin W, Huang W, Chen L, et al. Topical application of JAK1/JAK2 inhibitor momelotinib exhibits significant anti-inflammatory responses in DNCB-induced atopic dermatitis model mice. Int J Mol Sci. 2018;19(12):3973. doi: 10.3390/ijms19123973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papp K, Szepietowski JC, Kircik L, et al.Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from two phase 3, randomized, double-blind studies. 2020. [DOI] [PubMed]
- 58.Verden A, Dimbil M, Kyle R, Overstreet B, Hoffman KB. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf. 2018;41(4):357–361. doi: 10.1007/s40264-017-0622-2 [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Liang R, Chen CW, et al. JAK1-dependent transphosphorylation of JAK2 limits the antifibrotic effects of selective JAK2 inhibitors on long-term treatment. Ann Rheum Dis. 2017;76(8):1467–1475. [DOI] [PubMed] [Google Scholar]
- 60.Yu SH, Drucker AM, Lebwohl M, Silverberg JI. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J Am Acad Dermatol. 2018;78(4):733–740.e711. doi: 10.1016/j.jaad.2017.09.074 [DOI] [PubMed] [Google Scholar]
- 61.Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15(4):303–310. doi: 10.1007/s40272-013-0013-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83(2):375–381. doi: 10.1016/j.jaad.2020.03.075 [DOI] [PubMed] [Google Scholar]
- 63.Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016;16:75. doi: 10.1186/s12887-016-0607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muller R. JAK inhibitors in 2019, synthetic review in 10 points. Eur J Intern Med. 2019;66:9–17. doi: 10.1016/j.ejim.2019.05.022 [DOI] [PubMed] [Google Scholar]
- 65.Skojec A, Foulke G, Kirby JS. Variation in the cost of generic topical corticosteroids. JAMA Dermatol. 2015;151(11):1255–1256. doi: 10.1001/jamadermatol.2015.2394 [DOI] [PubMed] [Google Scholar]