Abstract
Background
Anti-IL-5 antibodies represent an established therapy for severe eosinophilic asthma (SEA), but some patients show inadequate response. The objective of this study was to assess the effects of a switch to anti-IL-5Rα therapy in patients with inadequate response to anti-IL-5 therapy.
Methods
In this retrospective multi-centre, real-life study, we analysed all SEA patients switched from anti-IL-5 to anti-IL-5Rα therapy due to inadequate response or intolerability. Pulmonary function tests, blood gas analyses, asthma control tests (ACT) and oral corticosteroid (OCS) usage were analysed and compared at three timepoints: baseline (BL, before anti-IL-5 therapy), timepoint 1 (T1, under anti-IL-5 therapy) and timepoint 2 (T2, under anti-IL-5Rα therapy).
Results
Of 665 patients treated with anti-IL-5 antibodies, 70 were switched to anti-IL-5Rα and 60 were included in the analysis. Median treatment duration was 8 months [IQR 5; 15] for anti-IL-5 and 5 months [IQR 4; 6] for anti-IL-5Rα therapy. FEV1 was 61% of predicted at BL [IQR 41; 74], 61% [IQR 43; 79] at T1 and 68% [IQR 49; 87] at T2 (pT1-T2=0.011). ACT score was 10 [IQR 8; 13], 16 [IQR 10; 19] and 19 [IQR 14; 22], respectively (both p<0.001). The number of patients requiring OCS was reduced from 41 (BL) to 32 (T1) and 19 (T2) (both p<0.001). Ten patients discontinued anti-IL-5Rα therapy due to insufficient efficacy (n=7) and adverse events (n=3).
Conclusion
Switching from anti-IL-5 to anti-IL-5Rα therapy in patients with inadequate response was associated with significantly improved FEV1, asthma control and OCS reduction.
Keywords: benralizumab, eosinophils, mepolizumab, reslizumab, severe asthma
Introduction
Asthma is a common chronic airway disease, known as a heterogeneous condition with diverse characteristics and pathological mechanisms affecting up to 30 million people in Western Europe.1 Different asthma phenotypes have been defined with severe eosinophilic asthma (SEA) with elevated numbers of blood or sputum eosinophils2 being in the centre of interest over the last years. Both, blood and sputum eosinophilia are associated with higher airflow limitation and worse asthma control.3 Eosinophil granulocytes are key-regulated by interleukin 5 (IL-5), which plays a central role in proliferation, activation and maturation of eosinophils.4 Since 2016, three monoclonal antibodies targeting the interaction between IL-5 and eosinophils have been approved for clinical use by the European Medicines Agency (EMA). Mepolizumab and reslizumab bind directly to IL-5 leading to a reduced production and survival of eosinophils,5 whereas benralizumab targets the IL-5 receptor alpha (IL-5Rα) directly inducing cell cytotoxicity and depleting eosinophils and other IL-5Rα bearing cells.6 Various clinical trials have proven the clinical benefit of anti-IL-5 or anti-IL-5Rα therapy in SEA leading to a decrease of exacerbation rate, an increase in FEV1 and a reduction of oral corticosteroids (OCS), all with favourable safety profiles and tolerability.7–9 Their benefit in daily clinical practice outside trial settings has also been demonstrated.10,11 Nevertheless, some patients fulfilling requirements for anti-eosinophilic treatment, do not respond to therapy. Recent studies found that first of all daily prednisone requirement, but also sinus disease, and late-onset asthma diagnoses were the strongest predictors of sub-optimal response.12,13
Given a lack of head-to-head comparison, the choice of initial antibody therapy is primarily based on patients’ and physicians’ individual preferences. Recently, several meta-analysis with conflicting results were published.14–19 In our retrospective multi-centre study, we investigated whether benralizumab, due to its different mode of action, is a reasonable treatment option for patients with SEA who showed inadequate response or adverse effects to either mepolizumab or reslizumab.
Methods
Aim, Design and Setting
In this multi-centre, retrospective analysis, clinical efficacy of IL-5Rα antibody therapy with benralizumab in patients with severe eosinophilic asthma previously treated with anti-IL-5 therapy, either mepolizumab or reslizumab, was examined anonymised. All patients were treated in severe asthma outpatient clinics at 6 different university hospitals in Germany (Berlin, Essen, Hannover, Heidelberg, Mainz, Munich). The study was conducted in accordance with the principles of the Declaration of Helsinki. This retrospective analysis was performed with approval of the local ethic committee of the Hannover Medical School (8656_BO_K_2019). All patients provided written informed consent before inclusion in the study.
Treatment
All patients had physician-diagnosed severe asthma, according to ATS/ERS guidelines,20 with an eosinophilic phenotype and were treated with medium to high-dose inhaled glucocorticoids and a long-acting β2-agonist and could receive a second or third controller and/or additional oral corticosteroid (OCS) therapy. All patients underwent patient education programme and had inhaler techniques and adherence checked regularly at clinical visits. Thereby, all patients fulfilled requirements for anti-IL-5 therapy according to the Food and Drug Administration (FDA) and EMA and were treated as add-on therapy with either weight-adapted reslizumab intravenously or mepolizumab subcutaneously once every 4 weeks. Antibody initiation and switching were performed by the treating physician on an individual basis and solely on clinical grounds. Reasons for switching to anti-IL-5Rα therapy (benralizumab) were documented and included the following options: adverse effects, no reduction of exacerbation rate, no reduction of OCS dosage, no improvement in physical fitness, persistent severe obstruction in pulmonary function testing (PFT), inadequate treatment response according to patient, persistence of symptoms, loss of effectiveness or persistence of nasal polyps. All patients fulfilled criteria for add-on anti-IL-5Rα treatment. None of the patients used self-administration at home. All patients were treated with benralizumab for 4–6 months as suggested by German national asthma guidelines21 before treatment response was evaluated. In the absence of official recommendations, the length of interval between discontinuation of anti-IL5 and start of anti-IL5Rα therapy was decided individually by the treating physician, as were adjustments of asthma medication and OCS therapy during antibody treatment.
Routine Follow-Up
Routine follow-up included spirometry or body plethysmography standardized to ERS/ATS guidelines, measurement of exhaled nitric oxide (eNO), capillary blood gas analysis, and laboratory testing (differential blood count, IgE), if indicated. Structured questionnaires assessing for asthma control (Asthma Control Test – ACT), exacerbation rate and changes in medication were also completed at each follow-up visit. The ACT cut-off for GINA-defined uncontrolled asthma is ≤19; the recommendation for patients with severe asthma is ≤16.22 Exacerbations were defined as worsening of asthma symptoms requiring OCS for at least three days or an increase in the OCS dose. Moreover, patients were asked whether their subjective condition under antibody therapy had improved, worsened or was unchanged (categorial answer). For their answer, which was based on subjective judgement, patients were asked to consider asthma-related symptoms, quality of life (QoL) and improvement of subjective physical fitness, measured as flight of stairs or a distance a patient is able to walk until a break is needed.
Data Collection
Pulmonary function tests (PFT) including eNO measurement and capillary blood gas analysis were performed at three different time points: 1st “baseline (BL)” 3 months prior to treatment start with anti-IL-5 therapy, 2nd time-point (T1) during anti-IL-5 therapy (after a minimum of 4 months of anti-IL-5 therapy) and 3rd time-point (T2) during anti-IL-5Rα therapy (after a minimum of 4 months of treatment). All PFTs were performed under continued stable inhaled therapy.
Information concerning number of exacerbations (actual number and annualized to follow-up duration), ACT and change in patients’ subjective condition were assessed at the same time points. Laboratory testing (eosinophilic granulocytes count and IgE) was not performed at each attendance; therefore, laboratory findings from within the last 12 months prior to treatment with anti-IL-5 antibodies were included. Information concerning the year of first diagnosis, smoking status, allergies, nasal polyposis and side effects of oral corticosteroids (osteoporosis, diabetes, cataract, weight gain, skin alteration) were assessed prior to start of anti-IL-5 treatment.
Statistical Analysis
Statistical analyses and figure preparation were performed using RStudio version 1.2.5019 (RStudio Inc, USA) and STATA version 16 (State Corp LP, USA). Categorical variables are stated as numbers (n) and percentages (%). Depending on distribution, continuous variables are shown as mean ± standard deviation (SD) or median with interquartile ranges (IQR) unless indicated otherwise. For group comparisons, Fisher’s exact test, Chi-squared test, two-sided paired t-test or Mann–Whitney-U-test were used, as appropriate. All reported p-values are two-sided. P-values < 0.05 were considered statistically significant.
Results
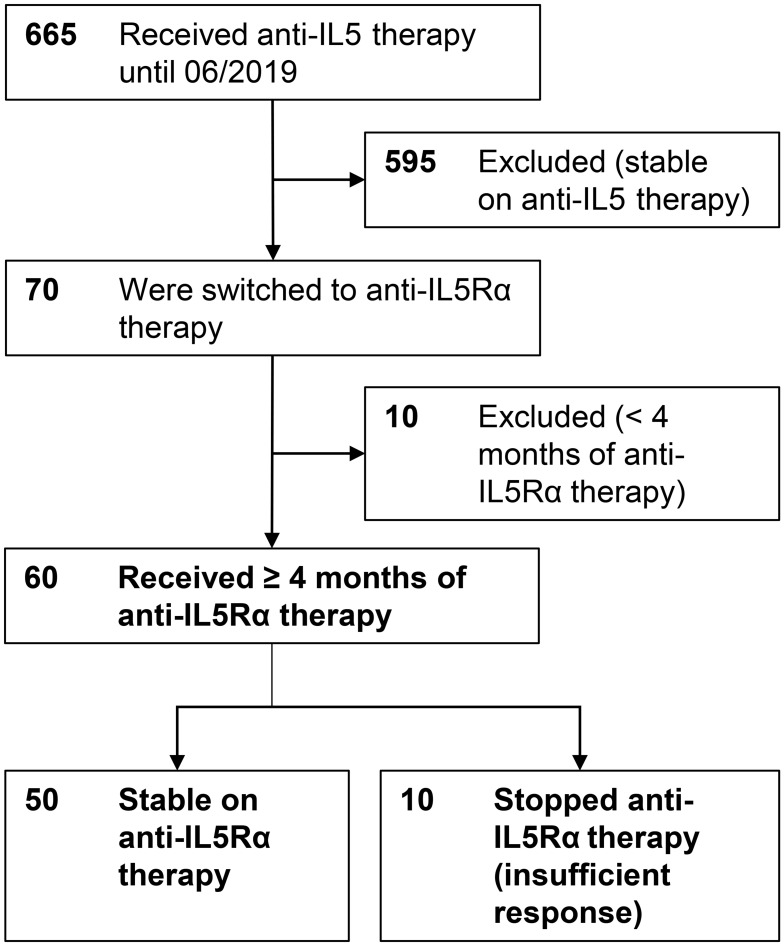
In total, 665 patients with severe eosinophilic asthma received anti-IL-5 therapy (mepolizumab or reslizumab) in the participating centres until June 2019. Seventy patients (10.5%) were switched to anti-IL-5Rα treatment and data from 60 patients (9%) were analyzed. Ten patients had only recently switched to anti-IL-5Rα treatment and had not attended the first follow-up at 4 months and were therefore excluded from the analysis (Figure 1).
Figure 1.
Flow chart of study population, number of patients in bold letters.
Median age was 54 years [IQR 47; 59] and the most frequent comorbidities were chronic rhinosinusitis with nasal polyps (CRSwNP, 45%), allergic rhinitis (32%) and aspirin intolerance (17%, Table 1).
Table 1.
Patient Characteristics of All Patients Receiving Anti-IL-5Rα Therapy
| Patient Characteristics | All (n=60) |
|---|---|
| Male, n (%) | 29 (48) |
| Age, median (IQR) | 54 (47; 59) |
| Body mass index, median (IQR) | 27 (23; 31) |
| Receiving daily OCS, n (%) | 41 (68) |
| OCS dose, mg/d (IQR) | 10 (5;15) |
ICS dose
|
38 (63) 22 (37) |
| Primary anti-IL-5 therapy, n (%) | |
| Mepolizumab | 48 (80) |
| Reslizumab | 12 (20) |
| Former smokers, n (%) | 24 (40) |
| Comorbidities, n (%) | |
| Chronic rhinosinusitis | 34 (56) |
| Chronic rhinosinusitis with nasal polyps (CRSwNP)a | 27 (45) |
| Chronic rhinosinusitis without nasal polyps (CRSsNP)a | 7 (12) |
| Allergic rhinitis | 20 (33) |
| Allergic rhinitis with CRSwNP | 9 (15) |
| Allergic rhinitis with CRSsNP | 4 (7) |
| Atopic dermatitis | 2 (3) |
| COPDb | 6 (10) |
| Aspirin intolerance | 10 (17) |
| Diabetes mellitus | 6 (10) |
| Osteoporosis | 8 (13) |
Notes: aDiagnoses of CRSwNP or CRSsNP were self-reported by the patient and were not regularly verified by an ENT specialist; bCOPD was diagnosed by the presence of fixed obstruction in the context of a significant smoking history and lung function. No minimum number of pack years was determined.
Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps; COPD, chronic obstructive pulmonary disease; OCS, oral corticosteroids.
Anti-IL-5 Therapy
The median duration of the previous anti-IL-5 therapy was 8 months [IQR 5; 15, range 4–30]. The median time between stop of anti-IL-5 therapy and start of anti-IL-5Rα therapy was 1 month [IQR 1; 3], with an interval of more than 3 months in 8 patients (median of 8 months [IQR 7; 9]).
Reasons for discontinuation of anti-IL-5 therapy are displayed in detail in Table 2, most frequently named were “inadequate treatment response”, “persistent impairment of pulmonary function tests”, “persistent impairment of physical fitness” and “continued need for OCS”. In summary, inadequate treatment response was the main reason for switching antibody medication in 50 patients (83%) while 10 patients (17%) stopped the anti-IL-5 treatment due to adverse events (Table 2).
Table 2.
Reasons for Treatment Switch from Anti-IL-5 to Anti-IL-5Rα Therapy. Multiple Answers per Patient Were Possible
| Reasons to Stop Anti-IL-5 Treatment and Switch to Anti-IL-5R Therapy | n (% of All 60 Patients) |
|---|---|
| Ongoing exacerbations | 22 (37) |
| Ongoing OCS use, no dose reduction possible | 24 (40) |
| Persistent impairment of pulmonary function | 22 (37) |
| Persistent Impairment of physical fitness | 26 (43) |
| Inadequate treatment response | 40 (67) |
| Loss of Effectiveness (long term therapy or too long intervals) | 10 (17) |
| No effect on nasal polyps | 3 (5) |
| Adverse effects – total | 10 (17) |
| Diarrhoea | 1 (2) |
| Headache | 2 (3) |
| Injection site reaction | 3 (5) |
| Muscle pain | 2 (3) |
| Joint pain | 1 (2) |
Abbreviation: OCS, oral corticosteroids.
Anti-IL-5Rα Therapy
Until June 2019 the median time of anti-IL-5Rα therapy in all patients was 5 months [IQR 4; 6]. In 10 patients (17%) benralizumab treatment was stopped after 4 months due to inadequate response (n=6, 10%), adverse effects (n=3, 5%) and self-initiated discontinuation by the patient (n=1, 2%; Table 3). Due to further symptom progress under anti-IL-5Rα treatment, two of these patients were switched back to the initial anti-IL-5 therapy and another two were switched to anti-IL-4R (dupilumab). The other 6 patients had not received any further antibody therapy at the time of data collection.
Table 3.
Reasons for Stopping Anti-IL-5Rα Therapy in 10 Patients. Multiple Answers per Patient Were Possible
| Reasons for Stopping Anti-IL-5Rα Therapy | n (% of All 60 Patients) |
|---|---|
| Inadequate treatment response | 4 (7) |
| Loss of Effectiveness (long term therapy or too long intervals) | 2 (3) |
| No effect on nasal polyps | 1 (2) |
| Adverse effects – total | 3 (5) |
| Weight loss | 1 (2) |
| Coronary artery spasm during therapy with benralizumab and sumatriptan | 1 (2) |
| Recurrence of chronic urticaria | 1 (2) |
Clinical Parameters Under Anti-IL-5 and Anti-IL-5Rα Therapy
Comparisons between all three timepoints were performed and are displayed in Table 4 and Figure 2.
Table 4.
Outcome Parameters at BL (Baseline), T1 (Visit 1 Under Anti-IL-5 Therapy), T2 (Visit 2 Under Anti-IL-5Rα Therapy)
| BL | T1 | T2 | pBL-T1 | pT1-T2 | pBL-T2 | |
|---|---|---|---|---|---|---|
| ACT, points | 10 (8;13) | 16 (10;19) | 19 (14;22) | <0.001 | 0.005 | <0.001 |
| Pulmonary function tests, median (IQR) | ||||||
| FEV1% of predicted | 61 (41;74) | 61 (43;79) | 68 (49;87) | 0.1202 | 0.011 | 0.003 |
| FEV1 in mL | 1900 (1335; 2535) | 1970 (1260; 2495) | 1995 (1400; 2900) | 0.180 | 0.0556 | 0.0175 |
| FVC % of predicted | 79 (65;94) | 84 (68;100) | 85 (71;101) | 0.118 | 0.563 | 0.002 |
| RV % of predicted | 160 (133;182) | 153 (121;171) | 134 (113;163) | 0.4432 | 0.077 | 0.042 |
| TLC % of predicted | 109 (85;121) | 108 (94;123) | 110 (95;119) | 0.6518 | 0.1693 | 0.6518 |
| MEF25–75% of predicted | 33 (19;56) | 34 (16;53) | 47 (24;64) | 0.3 | 0.01 | 0.002 |
| eNO, ppb | 44 (22;85) | 41 (23;78) | 43 (25;66) | 0.2777 | 0.9842 | 0.4921 |
| Blood gases, median (IQR) | ||||||
| pO2, mmHg | 70 (65;85) | 71 (63;75) | 73 (65;86) | 0.511 | 0.2552 | 0.629 |
| pCO2, mmHg | 37 (34;39) | 37 (34;40) | 37 (34;39) | 0.7109 | 0.1958 | 0.2216 |
| Laboratory, median (IQR) | ||||||
| Blood eosinophils absolute (cells/µL) | 600 (400;1170) | 84 (27;152) | 7 (0;10) | <0.001 | <0.001 | <0.001 |
| IgE, IE/mL | 175 (63;402) | 101 (25;257) | 123 (25;277) | 0.790 | 0.061 | 0.091 |
| Number of exacerbations 12 months prior to and under therapy, mean (±SD) | 4.02 (±3.6) | 1.47 (±1.8) | 0.5 (± 1.3) | n/a | n/a | n/a |
| Number of annualized exacerbations, mean (±SD) | 4.02 (±3.6) | 1.88 (±2.2) | 1.1 (±3.5) |
<0.001 | <0.092 | <0.001 |
| Change of subjective condition under therapy, n (%) | n/a | n/a | n/a | |||
| Worsened | 4 (7) | 9 (15) | ||||
| Unchanged | 21 (35) | 15 (25 | ||||
| Improved | 33 (55) | 33 (55) | ||||
| Receiving daily OCS, n (%) | 41 (68) | 32 (53) | 19 (32) | <0.001 | 0.001 | <0.001 |
| OCS dose, mg/d | 10 (5;15) | 5 (2.5;10) | 0 (0;5) | <0.001 | 0.002 | <0.001 |
Note: For comparisons, Fisher’s exact test, Chi-squared test, Mann–Whitney U-test or two-sided paired t-test were used as appropriate.
Abbreviations: ACT, asthma control test; eNO, exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MEF, mean expiratory flow; n/a, not applicable; OCS, oral corticosteroids; RV, residual volume; TLC, total lung capacity.
Figure 2.
Change (delta) from baseline to anti-IL-5 (T1) and anti-IL-5Rα (T2) therapy of asthma control (A), pulmonary function tests (C–E), capillary oxygenation (B) and blood eosinophil (eos) counts (F).
Abbreviations: ACT, asthma control test; FEV1, forced expiratory volume in 1 second; RV, residual volume; eos, eosinophils.
Oral Corticosteroids (OCS)
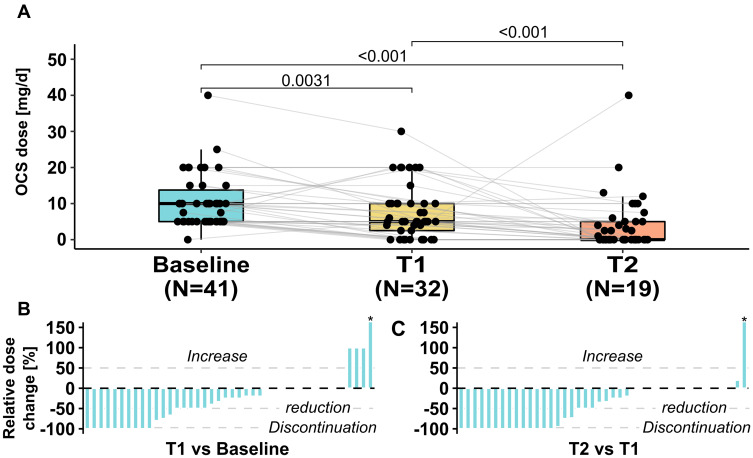
At BL, 41 patients (68%) were taking OCS with a median dose of 10 mg/d [IQR 5; 15]. At T1 under anti-IL-5 therapy with a median duration of 8 months, the median OCS dose was reduced to 5 mg/d [IQR 2.5; 10] with 9 patients having discontinued OCS (p=0.003). At T2 following 4–6 months of anti-IL-5Rα therapy, the median dose was further reduced to 0 mg/d [IQR 0; 5] with 13 additional patients having discontinued OCS (p<0.001), while 19 patients (32%) still received OCS (Table 3 and Figure 3).
Figure 3.
(A) Boxplots with individual OCS doses at baseline, under anti-IL5 (T1) and anti-IL5Rα (T2) therapy. The relative dose change of each patient receiving OCS is shown as waterfall plots in (B and C). Patients who never received OCS at any time are not considered in the graph or median dose calculations. *One patient at T1 and T2 newly received OCS and relative dose change is arbitrarily displayed as 150%.
Abbreviation: OCS, oral corticosteroids.
Exacerbations
Patients reported a mean number of 4.02 exacerbations (±3.6) in the 12 months prior to start of anti-IL-5 therapy. Annualized exacerbation rates were calculated with a mean of 1.88 (±2.2) exacerbations per year under anti-IL-5 and 1.1 (±3.5) under anti-IL-5Rα therapy (p = 0.092). Notably, in 22 patients (37%) ongoing exacerbations were the reason for therapy switch; of these patients, 8 patients (36%) developed another exacerbation during anti-IL-5Rα therapy over a median time of 5 months [IQR 4; 6]. In 2 patients (9%) ongoing exacerbations led to discontinuation of anti-IL-5Rα therapy.
Inhaled Asthma Treatment
At baseline, all 60 patients (100%) were on high or medium ICS/LABA, with 38 patients (63%) on high-dose ICS and 22 patients (37%) on medium dose. Under anti-IL-5 treatment 1 patient (2%) reduced ICS from high-dose to medium-dose and 5 patients (8%) increased the dose from medium to high-dose, resulting in 43 patients on high-dose ICS. Fifty-four patients (90%) remained on stable ICS. Under anti-IL-5R therapy 8 patients (13%) reduced ICS from high-dose to medium-dose and 4 patients (7%) increased the dose from medium to high-dose resulting in 39 patients on high-dose ICS. Forty-eight patients (80%) remained on stable ICS. Forty-five patients were on additional LAMA therapy; there were also no significant changes between the time-points.
Asthma Control (ACT)
At BL the ACT was 10 points [IQR 8; 13] and improved to 16 [IQR 10; 19] at T1. The ACT score further improved significantly to 19 points [IQR 14; 22] at T2 under anti-IL-5Rα treatment when compared to visit 1 (p = 0.005) or baseline (p < 0.001).
Patient Subjective Assessment
Thirty-three patients (55%) reported an improvement of subjective condition under anti-IL-5 therapy at T1 with 33 patients (55%) reporting further improvement under anti-IL-5Rα therapy at T2. Twenty-one patients (35%) at T1 and 15 patients (25%) at T2 reported no changes in the subjective condition, and 4 patients (7%) at T1 and 9 patients (15%) at T2 reporting worsened subjective condition. Among patients who felt no change (n=21) or worsened (n=4) under anti-IL-5 therapy, 12 (48%) stated improved subjective condition under anti-IL-5Rα therapy.
PFTs and Capillary Blood Gas Analysis
FEV1 did not change significantly between T1 (61% of predicted [IQR 43; 79]) and BL (61% of predicted [IQR 41; 74], p = 0.1202), but was significantly improved at T2 (68% of predicted [IQR 49; 87]) compared to baseline and to T1 (p= 0.003 and p=0.011, Table 4). FEV1 increased from 1900 mL [IQR 1335; 2535] at BL to 1995 mL [IQR 1400; 2900] at T2 (p=0.018). Likewise, mean expiratory flow (MEF25–75) increased significantly at T2, but not at T1. Residual volume (RV) was significantly lower at T2 compared to BL (p=0.04).
Forced vital capacity (FVC) was significantly improved at T1 and T2 compared to BL (pT1-BL = 0.01, pT2-BL <0.002), and remained unchanged between T1 and T2. The paO2 improved between T1 and T2, but not statistically significant (p = 0.256), the paCO2 remained unchanged under therapy.
Biomarkers
Blood eosinophils showed a decrease from 600 cells/µL [IQR 400; 1170] at baseline to 84 cells/mL ([IQR 27; 152] p = 0.006) at T1 and 7 cells/µL at T2 ([IQR 0; 10] p < 0.001). Of note, timepoints of blood samples collection in relation to antibody application varied within the study population. No significant changes in IgE or FeNO were observed (Figure 2).
Discussion
We investigated the switch from anti-IL-5 to anti-IL-5Rα therapy in patients with severe eosinophilic asthma and inadequate treatment response or intolerability to the former therapy. Key findings of this study were an improvement of PFTs, subjective condition and asthma control, as well as reduction in OCS under anti-IL-5Rα therapy.
Out of 665 patients treated with anti-IL-5 antibodies in all participating centers only 70 patients were switched to anti-IL-5Rα therapy (10.5%). While in the majority of patients anti-IL-5 therapy is well-tolerated and effective, a small number of patients show an inadequate response to anti-IL-5 therapy despite adequate patient selection.
In our study, a switch to anti-IL-5Rα therapy in patients with inadequate response to previous anti-IL-5 therapy led to clinical stabilization despite a higher disease severity in our real-life cohort compared to cohorts in former randomized trials: patients reported more frequent exacerbations within the past year (4.0 versus 2.5 to 3.6) and were more symptomatic at baseline.9,23–25 In our study, FEV1 improved significantly under anti-IL-5 therapy compared to BL with another significant improvement after switch to anti-IL-5Rα therapy. However, with a median increase of 95 mL from baseline to anti-IL-5Rα therapy, the improvement found in the present cohort does not entirely reach the results from the Sirocco or Calima RCTs, probably explained by more severe disease in our cohort.
One of the main reasons for labeling patients’ treatment response as inadequate and stopping anti-IL-5 therapy in our cohort was ongoing OCS dependency, highlighting the reduction of OCS doses as a main treatment target. In our cohort, a significant decrease in the median OCS dose under anti-IL-5 therapy was observed and OCS could be discontinued in 22% of patients. Notably, inadequate treatment response to anti-IL-5 therapy leading to treatment switch did not mean there was no therapeutic effect at all, eg, asthma control and continuous OCS use were significantly improved compared to baseline. However, this therapeutic effect was judged insufficient by the treating physician and/or the patient leading to an individualized decision to switch treatment on clinical grounds, with no standardized criteria for switching therapies. Remarkably, a further significant reduction of the median OCS dose could be achieved under anti-IL-5Rα therapy, allowing another 13 patients (of 41 patients on OCS at that time) to stop their systemic OCS treatment.
Switch to anti-IL-5Rα therapy also led to significant improvement in asthma control, assessed by the ACT score. Unlike PFTs and OCS dosages, the ACT represents a subjective but widely used and clinically highly relevant criterion.26 The ACT cut-off for GINA-defined uncontrolled asthma is ≤19. After prospective evaluation in a cohort of patients with severe asthma, Korn et al recommended reducing the ACT cut-off for uncontrolled asthma in severe asthma to 16.22 In our cohort, the median ACT improved from 16 points under anti-IL-5 therapy to 19 points under anti-IL-5Rα treatment, reaching the area of controlled asthma and demonstrating the impact of benralizumab on disease severity and symptoms.
Concerning the exacerbation rate, a further improvement after 4–6 months of anti-IL-5Rα treatment compared to anti-IL-5 therapy could be observed and out of 22 patients in which anti-IL-5 treatment was stopped due to ongoing exacerbations only 2 patients discontinued anti-IL-5Rα therapy due to exacerbations. But these results are limited on the one hand by the possible influence on exacerbations under anti-IL-5Rα therapy by the previous anti-IL-5 therapy and on the other hand by the short interval of assessment.27 However, it has been previously shown, that the response to anti-IL-5 at 16 weeks predicts the response at 52 weeks, including exacerbations, suggesting that this early time-point is also useful for analysis.28
Anti-IL-5Rα therapy showed significant efficacy in most patients formerly treated with anti-IL-5 therapy, but nevertheless 10 out of 60 patients did not respond to anti-IL-5Rα treatment and 2 out of the 10 were switched back to the former anti-IL-5 treatment.
Both anti-IL-5 and anti-IL-5Rα therapies lead to clinical improvement via interfering with the eosinophilic inflammation but differ in their mode of action. By interfering with IL-5, mepolizumab reduces but does not deplete the number of mature and late immature eosinophils in blood, bone marrow and airways, whereas the number of early immature eosinophils remains unaffected.29 Unlike mepolizumab and reslizumab, benralizumab directly induces antibody-dependent cell-mediated cytotoxicity leading to depletion of eosinophils, their progenitor cells in blood, bone marrow and airway tissue as well as other IL-5 receptor bearing cells such as basophils.30
These mechanisms might explain why the outcome improved under anti-IL-5Rα, when it was inadequate under anti-IL-5. Notably, in our study the blood eosinophil count was further reduced after switching from anti-IL-5 to anti-IL-5Rα therapy.
Mukherjee et al recently found that suboptimal response to anti-IL-5 was mostly found in OCS dependent patients who had underlying airway autoimmune phenomena.12 These autoimmune phenomena even increased in some patients after initiation of mepolizumab, but not reslizumab therapy and the authors suggested that complement activation via the IgG1-antibody mepolizumab could be the reason for this, while the IgG4 antibody reslizumab cannot activate C1q. In our study, we did not differentiate between mepolizumab and reslizumab because the resulting numbers would be too small for meaningful analyses. As benralizumab is also an IgG4 antibody, it is also possible that such mechanisms played a role in our patients.
Although our initial screening indicated that the vast majority of patients respond to initial anti-IL-5 therapy, 10.5% had an unsatisfactory response. A recent study found that for anti-IL-5 therapy daily prednisone requirement, sinus disease, and late-onset asthma diagnoses were the strongest predictors of sub-optimal response.12 For benralizumab on the other hand enhanced treatment effects were found in patients with higher eosinophil counts, OCS use, nasal polyposis and a baseline FVC < 65%.31
Our study was not designed to establish baseline predictors of anti-IL-5 treatment failure but to provide data for the practical approach of switching treatment to anti-IL-5Rα if response to anti-IL-5 therapy was insufficient. Current guidelines recommend switching biological treatments if outcome under current therapy is insufficient,32 but data to support this is scarce. Here, we show that treatment switch from anti-IL5 to anti-IL-5Rα is useful for the majority of these patients.
Limitations
There are important limitations of this analysis, mainly inherent by its retrospective design. This especially limits conclusion about exacerbation rates which seems to drop but estimation is prone to error due to short follow-up periods especially under anti-IL-5Rα therapy. Furthermore, influence on the reduced exacerbation rate under anti-IL-5Rα therapy by the previous anti-IL-5 therapy has to be taken into consideration. Further, important limitations are the moderate number of patients and absent standardization of treatment discontinuation and treatment switch. Results of peripheral eosinophils and derivable conclusions from eosinophil data are limited as timepoints of blood sample collection in relation to antibody application varied and were not standardized. Lastly, OCS sparing effects and observed changes in ACT and FEV1 after a median of 4–6 months of anti-IL-5Rα-therapy may be partially subject to placebo effect, as a control group is lacking.
Conclusion
Switching to anti-IL-5Rα therapy in patients with severe eosinophilic asthma who stopped anti-IL-5 treatment due to inadequate treatment response or intolerance was feasible and associated with improved pulmonary function, asthma control and reduction of OCS use, providing a promising therapeutic option.
Funding Statement
There is no funding to report.
Abbreviations
ACT, asthma control test; COPD, chronic obstructive pulmonary disease; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps; eNO, exhaled nitric oxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; ICS, inhaled corticosteroids; IL-5, interleukin 5; IL-5Rα, interleukin 5 receptor alpha; LABA, long acting beta agonist; LAMA, long acting muscarinic antagonist; MEF, mean expiratory flow; OCS, oral corticosteroids; PFT, pulmonary function testing; QoL, quality of life; RV, residual volume; SEA, severe eosinophilic asthma; TLC, total lung capacity.
Disclosure
Katrin Milger reports personal fees from GSK, AstraZeneca, Novartis, and Sanofi-Aventis, outside the submitted work. Stephanie Korn reports grants, personal fees from AstraZeneca, GlaxoSmithKline, Novartis, and Sanofi, and personal fees from Teva, outside the submitted work. Roland Buhl reports personal fees from AstraZeneca, Chiesi, Cipla, Sanofi, and Teva, grants, personal fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Roche, outside the submitted work. Juergen Behr reports personal fees from Novartis, Astra Zeneca, Boehringer-Ingelheim, and Roche, outside the submitted work. Tobias Welte reports grants from German Ministry of Research and Education (BMBF), during the conduct of the study; and personal fees from AstraZeneca, GSK, Novartis, and Sanofi-Aventis, outside the submitted work. Hendrik Suhling reports personal fee from GSK, AstraZeneca, Novartis and SanofiAvensis outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S [DOI] [PubMed] [Google Scholar]
- 2.Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197(1):22–37. doi: 10.1164/rccm.201611-2232PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–1039. doi: 10.1056/NEJM199010113231505 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22. doi: 10.1038/nri3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin Pharmacokinet. 2011;50(4):215–227. doi: 10.2165/11584340-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 6.Ghazi A, Trikha A, Calhoun WJ. Benralizumab–a humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity–a novel approach for the treatment of asthma. Expert Opin Biol Ther. 2012;12(1):113–118. doi: 10.1517/14712598.2012.642359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 8.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 9.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 10.Pelaia C, Busceti MT, Solinas S, Terracciano R, Pelaia G. Real-life evaluation of the clinical, functional, and hematological effects of mepolizumab in patients with severe eosinophilic asthma: results of a single-centre observational study. Pulm Pharmacol Ther. 2018;53:1–5. doi: 10.1016/j.pupt.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 11.Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med. 2018;18(1):119. doi: 10.1186/s12890-018-0689-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee M, Forero DF, Tran S, et al. Sub-optimal treatment response to anti-IL-5 monoclonal antibodies in severe eosinophilic asthmatics with airway autoimmune phenomena. Eur Respir J. 2020;56(4):2000117. doi: 10.1183/13993003.00117-2020 [DOI] [PubMed] [Google Scholar]
- 13.Poznanski SM, Mukherjee M, Zhao N, et al. Asthma exacerbations on benralizumab are largely non-eosinophilic. Allergy. 2020. doi: 10.1111/all.14514 [DOI] [PubMed] [Google Scholar]
- 14.Bourdin A, Husereau D, Molinari N, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018;52(5):1801393. doi: 10.1183/13993003.01393-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabon Y, Molinari N, Marin G, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017;47(1):129–138. doi: 10.1111/cea.12853 [DOI] [PubMed] [Google Scholar]
- 16.Busse W, Chupp G, Nagase H, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200.e20. doi: 10.1016/j.jaci.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 17.Henriksen DP, Bodtger U, Sidenius K, et al. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma - a systematic review and meta-analysis. Eur Clin Respir J. 2018;5(1):1536097. doi: 10.1080/20018525.2018.1536097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casale TB, Pacou M, Mesana L, Farge G, Sun SX, Castro M. Reslizumab compared with benralizumab in patients with eosinophilic asthma: a systematic literature review and network meta-analysis. J Allergy Clin Immunol Pract. 2019;7(1):122–130.e1. doi: 10.1016/j.jaip.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 19.Mauger D, Apter AJ. Indirect treatment comparisons and biologics. J Allergy Clin Immunol Pract. 2019;7(1):131–133. doi: 10.1016/j.jaip.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 20.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 21.Buhl R, Bals R, Baur X, et al. S2k-Leitlinie zur Diagnostik und Therapie von Patienten mit Asthma. Pneumologie. 2017;71(12):849–919. doi: 10.1055/s-0043-119504 [DOI] [PubMed] [Google Scholar]
- 22.Korn S, Both J, Jung M, Hübner M, Taube C, Buhl R. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107(6):474–479. doi: 10.1016/j.anai.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 24.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 25.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 26.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 27.Gerhardsson de Verdier M, Gustafson P, McCrae C, Edsbäcker S, Johnston N. Seasonal and geographic variations in the incidence of asthma exacerbations in the United States. J Asthma. 2017;54(8):818–824. doi: 10.1080/02770903.2016.1277538 [DOI] [PubMed] [Google Scholar]
- 28.Bateman ED, Djukanović R, Castro M, et al. Predicting responders to reslizumab after 16 weeks of treatment using an algorithm derived from clinical studies of patients with severe eosinophilic asthma. Am J Respir Crit Care Med. 2019;199(4):489–495. doi: 10.1164/rccm.201708-1668OC [DOI] [PubMed] [Google Scholar]
- 29.Menzies-Gow A, Flood-Page P, Sehmi R, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111(4):714–719. doi: 10.1067/mai.2003.1382 [DOI] [PubMed] [Google Scholar]
- 30.Matucci A, Maggi E, Vultaggio A. Eosinophils, the IL-5/IL-5Rα axis, and the biologic effects of benralizumab in severe asthma. Respir Med. 2019;160:105819. doi: 10.1016/j.rmed.2019.105819 [DOI] [PubMed] [Google Scholar]
- 31.Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52(4):1800936. doi: 10.1183/13993003.00936-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agache I, Akdis C, Akdis M, et al. EAACI biologicals guidelines - recommendations for severe asthma. Allergy. 2020. doi: 10.1111/all.14268 [DOI] [PubMed] [Google Scholar]