Abstract
Coronavirus disease 2019 (COVID-19) is a viral infection that appeared in December 2019. The risk of infection seems to be increased in chronic inflammatory rheumatic diseases due to both immune disturbances related to the disease and treatment. In this case report, we describe the clinical features of 5 rheumatic immune disease patients with the concomitant presence of COVID-19. Among these patients, 3 had rheumatoid arthritis and 2 had systemic lupus erythematosus. Patients' age ranged between 38 and 63 years. Only one patient (SLE) had a severe subtype of COVID-19. All the patients were cured of COVID-19 and were subsequently discharged.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a viral infection that appeared in December 2019 [1]. Its clinical manifestations depend on the degree of inflammatory response, which is linked to the host and to the virus [2]. The risk of infection seems to be increased in chronic inflammatory rheumatic diseases due to the immune disturbances associated, with the disease on one hand, and to the treatment on the other hand, mainly corticosteroids, biotherapies, and immunosuppressive treatments [3]. The health emergency imposed by this disease and the new knowledge it reveals make it necessary to specify the particularities of COVID-19 in patients with chronic inflammatory rheumatism. Few data are available on this subject.
2. Case Series
At date of May 31, 2020, the country (Burkina Faso) had 884 confirmed cases of Coronavirus disease 2019 since the pandemic began, which included 322 women and 562 men, and represented a sex ratio of 1.74 [4]. The diagnosis of COVID-19 was made by a positive real-time PCR testing for SARS-CoV-2 in oro and/or oropharyngeal sample. The national protocol for treatment of COVID-19 is made of hydroxychloroquine (200 mg three times daily for 10 days) and azithromycin (500 mg for Day 1 and 250 mg for Day 2 to Day 5) [5]. Early in the course of COVID-19, patients with chronic inflammatory rheumatic disease were told about the disease and barrier measures; they were also told to stop taking methotrexate or salazopyrin if ever they were a COVID-19 confirmed case. Five patients with chronic inflammatory rheumatic disease (3 rheumatoid arthritis (RA) and 2 systemic lupus erythematosus (SLE)) were among the 884 COVID-19 confirmed cases. Clinical and biological characteristics of the 5 patients are reported in Table 1.
Table 1.
Clinical characteristics and laboratory test of 5 patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Rheumatic diseases | RA | RA | RA | SLE | SLE |
| Clinical characteristics | |||||
| Cough | Yes | Yes | Yes | No | Yes |
| Fever | Yes | Yes | Yes | No | Yes |
| Rhinorrhea | No | No | Yes | No | No |
| Anosmia | No | No | Yes | No | No |
| Ageusia | No | No | Yes | No | No |
| Nasal obstruction | No | No | Yes | No | No |
| Arthralgia and myalgia | Yes | No | No | Yes | No |
| Headache | Yes | Yes | Yes | Yes | Yes |
| Asthenia | Yes | Yes | Yes | No | No |
| Vomiting | No | No | No | No | No |
| Diarrhea | No | No | No | No | No |
| Temperature | 38.7 | 36.9 | 38.9 | 37.1 | 38.4 |
| Blood pressure | 130/80 | 150/100 | 120/75 | 152/110 | 98/69 |
| Oxygen saturation | 98 | 97 | 98 | 95 | 78 |
| Respiratory frequency | Normal | Normal | Normal | Normal | 28 |
| Heart rate | Normal | 100 | Normal | Normal | 130 |
| Clinical subtype | Moderate | Moderate | Moderate | Moderate | Severe |
|
| |||||
| Laboratory parameters | |||||
| Hemoglobin (g/dL) | 12.5 | 12.3 | 13.7 | 13.2 | 12.4 |
| White blood cells (mm3) | 6800 | 6100 | 9030 | 5560 | 19910 |
| Segment neutrophiles (mm3) | 4864 | 4026 | 7040 | 2520 | 18180 |
| Lymphocytes (mm3) | 1515 | 1769 | 1250 | 2580 | 1040 |
| Plaquets (mm3) | 271000 | 254000 | 324000 | 172000 | 333000 |
| C-reactive protein (mg/l) | 190.29 | 26 | 171.34 | 10.05 | 376.28 |
| AST (IU/L) | 30 | 22 | 36 | 10.63 | 49.91 |
| ALT (IU/L) | 31 | 25 | 41 | 13.06 | 47.02 |
| Urea (mmol/L) | 3.47 | 3.75 | 3.68 | 1.71 | 6.85 |
| Creatinine (micromoles/L) | 69.92 | 84 | 76.8 | 64.5 | 137.5 |
| Sodium (mmol/L) | 146 | 146 | 133 | 144 | 125 |
| Potassium (mmol/L) | 4.25 | 4.33 | 3.3 | 4.1 | 2.7 |
| Calcemia (mmol/L) | 2.22 | 2.28 | 2.42 | 2.58 | 2.4 |
RA, rheumatoid arthritis; SLE, systemic erythematosus lupus; AST, aspartate transaminase; ALT, alanine transaminase.
2.1. Patient 1
A 42-year-old woman was followed since 2017 for RA. She was treated by methotrexate (20 mg weekly), folinic acid (15 mg weekly), and methylprednisone (4 mg daily). Her disease was stable (DAS-28 CRP at 2.75). In March 2020, she developed a dry cough, severe asthenia, and fever. After 2 weeks of self-medication (antihistamines, antimalarials, antibiotics, phytomedication), she was tested positive for COVID-19 in April 2020. She then stopped taking methotrexate and replaced it by hydroxychloroquine (200 mg three times daily for 10 days) without azithromycin. The circumstances of her contamination could not be specified. She was confined at home without further treatment and was declared cured 2 weeks later after two negative controls. The evolution was made of persistence of a dry cough after the end of her confinement. A chest CT scan was then performed and showed massive bilateral interstitial pneumonitis (Figure 1(a)). To date, she is doing well without any lung disease symptoms (Figure 1(b)).
Figure 1.
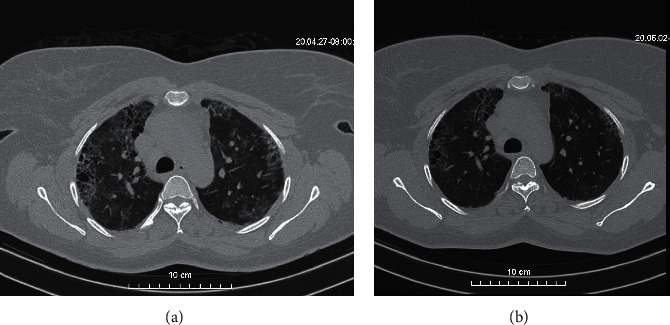
(a) Chest computed tomography (CT) scans (transverse plane) of the patient 1: frosted glass area extended central and bilateral upper lobar peripheral and fibrosis. (b) Chest computerized tomography (CT) scans (transverse plane) control of the patient 1: good evolution with almost disappearance of the frosted glass areas.
2.2. Patient 2
She was a 57-year-old woman with hypertension, asthma, and sleep apnea, irregularly followed in rheumatology department since January 2016 for RA. Because of digestive intolerance to methotrexate, her treatment had been changed to hydroxychloroquine (200 mg twice a day) which she admitted to be taking irregularly.
In March 2020, she attended a consultation for a dry cough that had been progressing for a few days. That cough was accompanied by asthenia, dizziness, and fever. Due to the persistence of the symptomatology despite treatment with antitussive and antibiotics, a chest CT scan was performed and showed bilateral ground glass alveolo-interstitial pneumonitis referring to COVID-19 (Figure 2(a)). A nasopharyngeal sample confirmed Coronavirus 2019 infection. She was hospitalized and treated by hydroxychloroquine and azithromicin, intravenous corticosteroids, and salbutamol. Her condition did not require admission to intensive care unit. She was declared cured after two COVID-19 negative controls and was discharged from hospital in April 2020. A follow-up chest CT scan was performed and showed an almost complete regression of the parenchymal signs of COVID-19 (Figure 2(b)).
Figure 2.
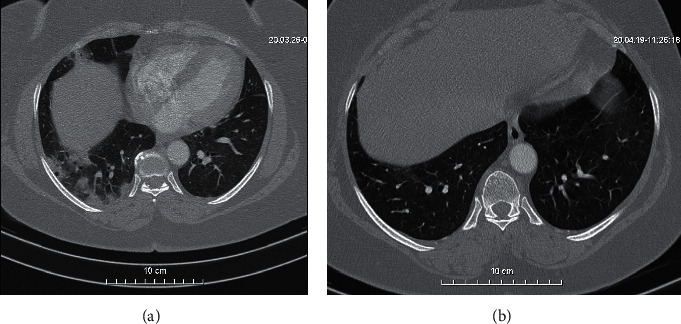
(a) Chest computed tomography (CT) scans (transverse plane) of the patient 2: multifocal nodular condensations with halo sign (peripheral frosted glass) under right posterobasal and anterobasal pleura. (b) Chest computed tomography (CT) scans (transverse plane) control of the patient 2: favorable evolution with disappearance of nodular condensations under right posterobasal pleura.
2.3. Patient 3
He was a 63-year-old man followed for RA for five years on methotrexate (15 mg per week) and folinic acid (10 mg weekly) and who also had heart disease and keratitis.
Four days earlier, due to deep asthenia, flu syndrome secondary to contact with a colleague who has been tested positive for COVID-19, a nasal swab was taken. The result available on March 20, 2020, confirmed a COVID-19 infection. Treatment with methotrexate was suspended. He was hospitalized and treated by hydroxychloroquine and azithromicin. His condition did not require admission into intensive care unit. Two weeks later, two successive tests were negative, confirming his recovery.
2.4. Patient 4
She was a 38-year-old woman with SLE (involving skin and joint) for three years, treated by hydroxychloroquine (200 mg twice daily). In March, she attended a consultation for headaches and arthromyalgia. Because of the persistence of the symptomatology, a nasopharyngeal swab was taken and confirmed the diagnosis of COVID-19. The evolution was favorable with treatment by hydroxychloroquine and azithromycin according to the national protocol.
2.5. Patient 5
She was a 42-year-old woman, followed for about 10 years for SLE with chronic renal failure and nephrotic syndrome and treated by monthly boluses of cyclophosphamide relayed by Azathioprine. In March 2020, she attended a medical consultation for respiratory distress syndrome. Nasopharyngeal swabbing confirmed the diagnosis of COVID-19. Due to the severity of the symptomatology, she was admitted to intensive care unit and treated by intravenous corticosteroids, orotracheal intubation, and oxygen therapy and was discharged from hospital.
3. Discussion
Since the advent of COVID-19, one of the concerns of rheumatologists has been the risk of intensive care unit admission and excess mortality in patients with chronic inflammatory rheumatic disease [6–8]. Few series have been published [9–12]. The incidence of COVID-19 during chronic inflammatory rheumatic disease has not been studied in our series; however, it does not seem to be different from that of the general population [10, 12]. We report a series of five patients (3 RA and 2 SLE) infected by COVID-19.
In patients with RA, the clinical feature of COVID-19 was moderate with recovery of the disease without admission to intensive care. Four of the five patients of Cheng et al. series, although a higher mean age, also had a favorable outcome without admission to the intensive care unit [9]. While waiting for larger series or a meta-analysis, we have the impression that rheumatoid arthritis is not a risk factor for admission to intensive care or for mortality during COVID-19 regardless of the background treatment (csDMARD, bDMARD, and tsDMARD). Patients 1 and 2 had a CT scan showing bilateral ground glass interstitial pneumonitis that regressed after healing as reported by Song et al. in their case report [11].
Few series have been reported on SLE cases with COVID-19. Of the two patients with SLE in our series, patient 5 had a severe subtype of COVID-19 that required orotracheal intubation. In a series by Wallace et al. about 5 SLE, four patients (80%) were hospitalized for COVID-19; three (60%) required invasive ventilation; and one (20%) died of the disease [13]. According to Sawalha et al., patients with SLE are at risk of developing severe COVID-19 due to overexpression of the angiotensin-converting enzyme receptor [14]. It should be noted, however, that patient 5 had significant comorbidities such as heart disease, chronic renal failure, and nephrotic syndrome known to be risk factors for admission to intensive care unit [15].
4. Conclusion
Infection with Sars-Cov 2019 does not appear to be a poor prognosis in patients with rheumatoid arthritis. Patients with SLE would be at risk of severe COVID-19, especially if the patient has mutivisceral SLE damage. However, definitive conclusions must await the results of registries or meta-analyses before any definitive conclusions can be drawn.
Acknowledgments
The authors thank Dr. Désiré Sankara and Dr. Donald Bayala who realized and commented the chest CT scans.
Ethical Approval
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration.
Consent
Written informed consent was obtained from the patients.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarzi-Puttini P., Giorgi V., Sirotti S., et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clinical and Experimental Rheumatology. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 3.Doran M. F., Crowson C. S., Pond G. R., O'Fallon W. M., Gabriel S. E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis & Rheumatism. 2002;46(9):2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 4.Service d’information du gouvernement du Burkina Faso. 2020. https://www.sig.gov.bf/infos-covid-19.
- 5.Ministère de la santé. Ouagadougou, Burkina Faso: Ministère de la Santé; 2020. Arrêté 2020-119/MS/CAB portant adoption du protocole national de prise en charge des cas confirmés de COVID-19 intégrant l’hydroxychloroquine et/ou la chloroquine phosphate. [Google Scholar]
- 6.Favalli E. G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmunity Reviews. 2020;19(5):p. 102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawalha A. H., Manzi S. Coronavirus disease-2019: implication for the care and management of patients with systemic lupus erythematosus. European Journal of Rheumatology. 2020;7(Suppl 2) doi: 10.5152/eurjrheum.2020.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouédraogo D.-D., Tiendrébéogo W. J. S., Kaboré F., Ntsiba H. COVID-19, chronic inflammatory rheumatic disease and anti-rheumatic treatments. Clinical Rheumatology. 2020;39(7):2069–2075. doi: 10.1007/s10067-020-05189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng C., Li C., Zhao T., et al. COVID-19 with rheumatic diseases: a report of 5 cases. Clinical Rheumatology. 2020;39(7):2025–2029. doi: 10.1007/s10067-020-05160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monti S., Balduzzi S., Delvino P., Bellis E., Quadrelli V. S., Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Annals of the Rheumatic Diseases. 2020;79(5):p. 667. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song J., Kang S., Choi S. W., et al. Coronavirus disease 19 (COVID-19) complicated with pneumonia in a patient with rheumatoid arthritis receiving conventional disease-modifying antirheumatic drugs. Rheumatology International. 2020;40(6):991–995. doi: 10.1007/s00296-020-04584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quartuccio L., Valent F., Pasut E., Tascini C., De Vita S. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine. 2020;87(5):439–443. doi: 10.1016/j.jbspin.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace B., Washer L., Marder W., Kahlenberg J. M. Patients with lupus with COVID-19: university of Michigan experience. Annals of the Rheumatic Diseases. In press. [DOI] [PMC free article] [PubMed]
- 14.Sawalha A. H., Zhao M., Coit P., Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clinical Immunology. 2020;215:p. 108410. doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


