Abstract
Cognitive estimation is a mental ability applied to solve numerical problems when precise facts are unknown, unavailable or impractical to calculate. It has been associated with several underlying cognitive components, most often with executive functions and semantic memory. Little is known about the neural correlates of cognitive estimation. To address this issue, the present cross-sectional study applied lesion-symptom mapping in a group of 55 patients with left hemineglect due to right-hemisphere stroke. Previous evidence suggests a high prevalence of cognitive estimation impairment in these patients, as they might show a general bias towards large magnitudes. Compared to 55 age- and gender-matched healthy controls, the patient group demonstrated impaired cognitive estimation. However, the expected large magnitude bias was not found. Lesion-symptom mapping related their general estimation impairment predominantly to brain damage in the right anterior temporal lobe. Also critically involved were the right uncinate fasciculus, the anterior commissure and the right inferior frontal gyrus. The main findings of this study emphasize the role of semantic memory in cognitive estimation, with reference to a growing body of neuroscientific literature postulating a transmodal hub for semantic cognition situated in the bilateral anterior temporal lobe. That such semantic hub function may also apply to numerical knowledge is not undisputed. We here propose a critical contribution of the right anterior temporal lobe to at least one aspect of number processing, i.e. the knowledge about real-world numerical magnitudes.
Keywords: cognitive estimation, anterior temporal lobe, lesion-symptom mapping, representational hemineglect, stroke
The anterior temporal lobe is assumed to contain a transmodal hub for semantic cognition. Whether this function applies to numerical knowledge is unclear. Based on lesion-symptom mapping, Pflugshaupt et al. propose that the right anterior temporal lobe critically contributes to the knowledge about real-world magnitudes.
Graphical Abstract
Graphical Abstract.
Introduction
In our everyday lives, we often face challenges that require numerical skills. Typical situations are, for example, the preparation of a meal for a group of friends, the anticipation of the time needed to walk to the nearest bus stop or a shopping stroll during which we ask ourselves whether desired purchases are within budget. For some of these challenges, precise knowledge is known or at least available (e.g. quantities given in a cooking recipe), while others are handled through rough approximation. The latter process is called cognitive estimation. It can be defined as a problem solving strategy applied to answer questions under uncertainty, when exact facts are unknown, unavailable or impractical to calculate.
Cognitive estimation is usually regarded as a complex process based on multiple cognitive components. Many authors have suggested that executive functions such as planning, reasoning or monitoring are involved, and also working memory and semantic memory/knowledge (Brand et al., 2003; Della Sala et al., 2004; Levinoff et al., 2006; D'Aniello et al., 2015b). Others added language comprehension (Shallice and Evans, 1978) and numeracy (Cipolotti et al., 2018) to that list. Emphasizing the role of semantic knowledge, D'Aniello et al. (2015a) hypothesized that cognitive estimation performance might reflect crystallized intelligence as conceptualized by Cattell (1963).
If the latter is true, cognitive estimation ability is expected (i) to rise through childhood, youth and early adulthood, and (ii) to remain relatively stable thereafter until old age, as shown for crystallized intelligence measures such as vocabulary knowledge (McArdle et al., 2002; Li et al., 2004). Interestingly, empirical support can be found for both predictions. On one hand, it was demonstrated that school children perform worse than adults on cognitive estimation tests (CETs) (Silverman and Ashkenazi, 2016) and that estimation performance steadily increases during childhood (Harel et al., 2007). Concerning its development from early to late adulthood, several cross-sectional studies conducted in large groups of participants (N > 100) with wide age ranges found no effect of age on estimation performance (Bullard et al., 2004; Della Sala et al., 2004; Scarpina et al., 2015). The results of another cross-sectional study suggest that cognitive estimation performance might even improve until old age (MacPherson et al., 2014), in sharp contrast to the decline of many intellectual abilities observed between middle and late adulthood (Gauvrit et al., 2017; Cansino et al., 2018).
Various standardized tests to assess cognitive estimation have been published. Possibly the first was the CET introduced by Shallice and Evans (1978), including 15 questions such as ‘how long is the average tie?’. Since then, a number of CET variants (Axelrod and Millis, 1994; Gillespie et al., 2002; MacPherson et al., 2014) and similar assessment tools such as the Biber Cognitive Estimation Test ( Bullard et al., 2004) have been developed. Not only were these tests applied in healthy participants but also in diverse clinical samples (Wagner et al., 2011). Impaired cognitive estimation was demonstrated, for example, in patients with Alzheimer's disease (Della Sala et al., 2004), Korsakoff's syndrome (Brand et al., 2003), vascular dementia (Billino et al., 2008), frontotemporal dementia (Bisbing et al., 2015), right temporal lobe epilepsy (Parente et al., 2013), stroke (Blake et al., 2002), traumatic brain injury (Silverberg et al., 2007), major depressive disorder (Barabassy et al., 2010) and schizophrenia (Roth et al., 2012).
Relatively little is known about the neural correlates of cognitive estimation. Some evidence comes from lesion studies. It has been shown that patients with frontal brain damage perform significantly worse on cognitive estimation tasks than healthy controls (Smith and Milner, 1984; Della Sala et al., 2004; MacPherson et al., 2014) or than patients with more posterior lesions (Shallice and Evans, 1978; Smith and Milner, 1984; Cipolotti et al., 2018), suggesting a dominant role of the frontal lobe in cognitive estimation. As a notable exception, Taylor and O'Carrol (1995) found no difference in estimation performance between frontal and non-frontal lesion groups. To the best of our knowledge, however, cognitive estimation performance has never been investigated in brain-damaged patients with modern lesion-symptom mapping methods that allow researchers to draw statistical inference (Karnath et al., 2019).
The main goal of this study was to fill this gap by applying voxel-based lesion-symptom mapping (VLSM) in a group of patients with left hemineglect due to right-hemisphere stroke. There were both methodological and empirical reasons for the selection of this patient group. With regard to the former, strokes can be reliably demarcated on MRI scans, and they show a sudden onset of behavioural changes that are attributable to the premorbid function of the damaged brain region (de Haan and Karnath, 2018). Furthermore, language impairment that might interfere with the administration of cognitive estimation tasks is typically absent in right-hemisphere stroke (Jordan and Hillis, 2005). Most importantly, it has been shown that left hemineglect patients are biased towards large quantities when numerical intervals have to be mentally bisected (Zorzi et al., 2002, 2006; Rossetti et al., 2004; Priftis et al., 2006; Zamarian et al., 2007) or during timed number comparison (Vuilleumier et al., 2004). These findings were interpreted as a form of representational hemineglect (Zorzi et al., 2006), assuming a ‘mental number line’ that is oriented from left to right. Empirical support for the latter comes from a multitude of studies performed in healthy participants, as reviewed by Winter et al. (2015). The existence of a mental number line implies a tight link between spatial and numerical cognition, an idea that has already been put forward in the 19th century by Galton (1880). Neuroanatomically, this link was attributed to common parietal circuits for attention to external space and internal representations of numbers (Dehaene et al., 2003; Hubbard et al., 2005). Further elaborating on this idea, Walsh (2003) introduced A Theory of Magnitude, proposing that not only space and quantity, but also time may be processed by the same, generalized magnitude system located in the parietal lobe.
These theories and findings suggest that patients with left hemineglect due to right-hemisphere damage might show a general bias towards large magnitudes. We thus had two main hypotheses in mind concerning the cognitive estimation performance of our patient group, i.e. that it is impaired, and that the impairment results from overestimation. In addition, VLSM was expected to reveal critical lesion sites for estimation impairment in two areas: the relatively coarse lesion evidence (Shallice and Evans, 1978; Smith and Milner, 1984; Cipolotti et al., 2018) suggests frontal involvement, while prominent cognitive theories about number processing in the brain (Dehaene et al., 2003; Walsh 2003; Hubbard et al., 2005) point towards the parietal lobe.
Materials and methods
Participants
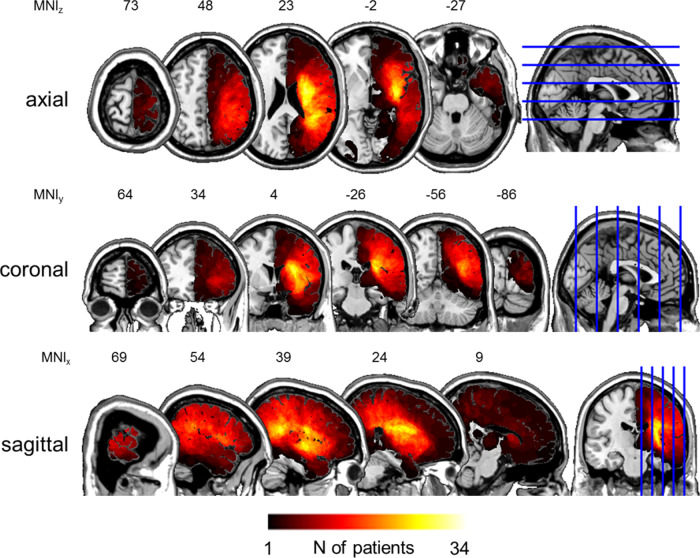
Overall, 55 right-hemisphere first-time stroke patients (23 women, 32 men) participated in the study. They were all recruited at the beginning of an inpatient neurorehabilitation in our clinic. Cognitive estimation performance was tested during the 2nd week after admission, with a mean time post-stroke of 28.45 days (SD = 21.16, range: 10–138). The mean age of the patient sample was 67.84 years (SD = 10.98, range: 43–84), the mean years of education 11.83 years (SD = 3.37, range: 6–20). Fifty-two of them were right-handed, three left-handed. Stroke aetiology was ischaemic in 38 patients and haemorrhagic in 17 patients. Forty-two patients suffered stroke in the territory of the medial cerebral artery, ten patients in two cerebral artery territories (five medial plus anterior, five medial plus posterior) and three patients suffered thalamic stroke. Lesion overlap maps are depicted in Fig. 1.
Figure 1.
Lesion overlap maps. Normalized lesions of all patients (N = 55) in the three principal planes. Lesions were normalized to Montreal Neurological Institute space and superimposed on the ch2 template available in MRIcroN (https://www.nitrc.org/projects/mricron, 17 September 2020, date last accessed). The number of overlapping lesions is colour-coded as a heat map. Voxels with maximum overlap were damaged in N = 34 patients. Note the accumulation of lesion overlap in the territory of the right middle cerebral artery. Horizontal image orientation follows the neurological convention (right on right side).
Apart from a history of right-hemisphere stroke, the main inclusion criterion was the presence of left-sided hemineglect, as indicated by a sum score larger than zero in the Catherine Bergego Scale (Bergego et al., 1995). This rating scale assesses the severity of hemineglect in 10 activities of daily living (e.g. eating, face care, paying attention to people, finding personal belongings), with sum scores ranging from 0 (no neglect) to 30 (severe neglect). Based on data from a group of 83 right-hemisphere stroke patients, Azouvi et al. (2003) have shown that the Catherine Bergego Scale is a valid and reliable tool, and that its sensitivity might outperform that of conventional paper-and-pencil tests. In this study, Catherine Bergego Scale scores were assessed by trained neurorehabilitation nurses during the 1st week after admission. The patient sample showed a mean Catherine Bergego Scale sum score of 15.20 (SD = 7.34, range: 3–29). Excluded from the study were patients with insufficient knowledge of the German language and those contraindicated for MRI due to, for example, claustrophobia or implanted electronic devices.
Fifty-five healthy volunteers (25 women, 30 men) participated in the study as controls, including 53 right-handers and 2 left-handers. Their mean years of education was 13.93 years (SD = 2.68, range: 8–19), their mean age 64.67 years (SD = 9.67, range: 47–90). The latter value was not significantly different from that of patients (independent samples t-test: T = 1.60, df = 108, ptwo-tailed = 0.112). Similarly, gender (Chi-squared test: χ2 = 0.148, df = 1, ptwo-tailed = 0.701) and handedness (Chi-squared test: χ2 = 0.210, df = 1, ptwo-tailed = 0.647) frequencies did not differ significantly between groups.
To objectify the presence of spatial inattention in patients, six conventional hemineglect tests were administered to both groups. Patients performed these tests during the 2nd week after admission, together with the cognitive estimation task described below.
Three of the hemineglect tests measured the horizontal symmetry of spatial attention in peripersonal space: (i) the random shape cancellation task (Weintraub and Mesulam, 1988), where the centre of cancellation as introduced by Rorden and Karnath (2010) was used to evaluate spatial inattention, (ii) the two part picture task (Brunila et al., 2003), for which we calculated a laterality index [LI = (right − left)/(right + left)] to analyse spatial inattention and (iii) a line bisection task requiring participants to mark the perceived midpoint of 15 horizontal lines that systematically varied in length (6, 12 or 18 cm) and position (far left, near left, central, near right or far right). Here the deviation from the true midpoint was used to indicate spatial inattention, expressed as a percentage of the true line half, applying the formula of Schenkenberg et al. (1980).
The other three hemineglect tests measured the horizontal symmetry of spatial attention in representational space: (iv) the representational drawing task of the behavioural inattention test (Wilson et al., 1987), (v) a mental map task requiring participants to imagine the map of Switzerland and to name as many cities as possible during one minute, followed by as many lakes as possible during another minute and (vi) a mental number line bisection (MNLB) task during which participants had to guess the midpoint of 15 orally presented number pairs (number range: 0–50). All of three possible interval lengths (6, 12 or 18) were equally often chosen. Eight number pairs were presented in ascending order, seven in descending order. Identical to the bisection of physical lines, the percentage deviation from the true midpoint was used to examine spatial inattention, applying the same formula (Schenkenberg et al., 1980). With reference to previous studies (Zorzi et al., 2002; Priftis et al., 2006), deviations towards the smaller of the two numbers were interpreted as leftwards, deviations towards the larger number as rightwards. Spatial inattention during the representational drawing and the mental map tasks was examined based on [LI = (right − left)/(right + left)]. This included assigning cities and lakes named during the mental map task to either the left (east) or right (west) half of the map of Switzerland.
Prior to the behavioural examination, all participants gave written informed consent. The study was carried out in accordance with the latest version of the Declaration of Helsinki and approved by the local ethics committee.
Cognitive estimation
The A-version of the Cognitive Estimation Task (CET-A) was translated into German and used to assess cognitive estimation performance in all participants. MacPherson et al. (2014) have developed this task, based on normative data from 184 healthy British volunteers (103 women, 81 men) with a mean age of 48.07 years (SD = 17.51, range: 18–79). It contains nine orally presented items requiring untimed numerical responses to questions related to speed (four items; e.g. What is the average jogging speed?), length (three items; e.g. What is the length of an average man's mountain bike?) and quantity (two items, e.g. How many segments are there in an orange?). We told participants that the precise answer to these questions is normally not known, and that they should make a reasonable guess of what the answer could be.
Responses were scored according to the procedure described in the normative study (MacPherson et al., 2014), applying the same percentile boundaries to categorize estimates. Values between the 20th and 80th percentile of the responses given by the normative sample were considered normal and thus awarded zero points. One (deviation) point was attributed to values between the 10th and the 20th percentile as well as to those between the 80th and the 90th percentile. Two points were scored when values lied between the 5th and the 10th percentile or between the 90th and 95th percentile, and three points were awarded to values below the 5th or above the 95th percentile. Values identical to percentile boundaries were assigned to the less extreme percentile range. Then, the sum of all (deviation) points was calculated (range: 0–27) and adjusted for age, gender and education, applying the revised correction grid of MacPherson et al. (2014; shown in Supplementary Table 6 of the erratum accompanying the publication). The resulting adjusted sum score was used as an indicator of general, non-directional cognitive estimation performance, with larger scores indicating worse performance. Furthermore, and similar to other studies (Shallice and Evans, 1978), we also analysed the frequency of very extreme estimates, i.e. answers awarded with three deviation points.
The hypothesized overestimation of patients was analysed with two statistical approaches. First, we applied Z standardization to all numerical responses, based on item-specific means and standard deviations from the normative sample (MacPherson et al., 2014). This was necessary to make responses from different item categories (speed, length, quantity) comparable. Positive Z-scores denote overestimation—relative to the answers of the normative sample (MacPherson et al., 2014)—while negative Z-scores represent underestimation. The mean Z-score averaging the nine CET-A items was then calculated for every participant as the main indicator for the direction of cognitive estimation (i.e. under- versus overestimation). Second, frequencies of estimates in all of the seven deviation point categories (i.e. <5th, 5th–10th, 10th–20th, 20th–80th, 80th–90th, 90th–95th and >95th percentile) were added across participants and compared between groups. This allowed a more detailed analysis of estimation direction.
Lesion-symptom mapping
High-resolution 3D MRI was acquired in all patients on a Siemens 3T Magnetom Skyra scanner, with a mean time post-stroke of 37.85 days (SD = 33.55, range: 7–165). Two sequences were applied: (i) a fluid-attenuated inversion-recovery/FLAIR sequence (TR/TE = 500/342 ms, voxel size = 0.9 mm × 0.9 mm × 2.0 mm) used for identification and demarcation of lesions, and (ii) a magnetization prepared rapid acquisition gradient echo/MPRAGE sequence (TR/TE = 2420/4.18 ms, voxel size = 1 mm × 1 mm × 1 mm) applied to enhance the quality of normalization. First, lesions were outlined with MRIcroN (https://www.nitrc.org/projects/mricron, 17 September 2020, date last accessed) by a researcher blinded to the behavioural data. The Clinical Toolbox (Rorden et al., 2012; https://www.nitrc.org/projects/clinicaltbx, 17 September 2020, date last accessed) run in SPM8 (https://www.fil.ion.ucl.ac.uk/spm, 17 September 2020, date last accessed) was then used to normalize lesions, applying the unified segmentation and normalization approach (Ashburner and Friston, 2005). Enantiomorphic normalization (Nachev et al., 2008) was chosen to avoid lesion-related distortions. Normalized lesions were statistically analysed with NiiStat (https://www.nitrc.org/projects/niistat/, 17 September 2020, date last accessed), a freely available set of MATLABTM (The MathWorks, Inc, Natick, MA, USA) scripts. Finally, the resulting statistical maps were visualized and further processed in MRIcroN and Affinity PhotoTM (Serif Ltd, Nottingham, UK).
Similar to a previous study (Findlater et al., 2016), we applied two complimentary methods to correlate lesion location with behavioural deficits in patients, i.e. VLSM as well as region of interest-based lesion-symptom mapping (RLSM; labelled sROI by Findlater et al., 2016). VLSM is based on running a statistical test at each voxel to relate its status (lesioned versus non-lesioned) with observed behaviour (de Haan and Karnath, 2018). While delivering excellent spatial resolution, VLSM suffers from relatively low statistical power, due to the large number of statistical tests that have to be corrected for multiple comparisons. As a complement, RLSM has been developed to offer more statistical power, at the cost of less spatial resolution (Findlater et al., 2016). Here, voxels are assigned to regions as defined in brain atlases. The proportion of damage to a given region—rather than damage to single voxels—is then related to patient behaviour, thereby strongly reducing the number of statistical comparisons.
Statistical analysis
Inferential statistical analyses of the cognitive estimation variables combined group comparisons with single-case methods. First, patients and controls were compared with one-tailed, independent samples t-tests to examine the main hypotheses, applying a P threshold of 0.05 and Bonferroni correction for multiple comparisons. These analyses comprised three CET-A variables—i.e. the adjusted sum score, the number of very extreme estimates and the mean Z-score—resulting in a Bonferroni-corrected P threshold of 0.017. Variables yielding significant group differences were further analysed by comparing every patient individually with the entire control group, applying Singlims_ES, a single case method developed by Crawford et al. (2010). On one hand, this allowed descriptive analysis of the frequency of patients with impairment on a given variable. In addition, results from single-case methods were used to define patient subgroups (impaired versus unimpaired) analysed during lesion-symptom mapping as described below. With regard to the frequencies of estimates in the seven CET-A deviation point categories, Chi-square tests were run to examine group differences, applying a Bonferroni-corrected P threshold of 0.007.
VLSM and RLSM analyses were both performed with NiiStat. We generally applied a P threshold of 0.05 and permutation-based (N = 2000) correction for multiple comparisons. As suggested by Rorden et al. (2007), Liebermeister tests were used to compare lesion location in patient subgroups, i.e. those with impaired performance as opposed to patients with normal performance on a given variable. Furthermore, analyses were restricted to the right cerebral hemisphere as well as to voxels damaged in at least five patients to ensure sufficient statistical power. RLSM was based on the AALCAT atlas included in NiiStat. This atlas combines all 116 regions of interest from the AAL atlas (Tzourio-Mazoyer et al., 2002) with 34 white matter region of interest from the tractography atlas first published by Catani and Thiebaut de Schotten (2008).
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Hemineglect
Patients displayed significant left-sided hemineglect in all three peripersonal tasks and in two of the three representational tasks (Table 1). Unexpectedly, the MNLB task revealed a significant bias towards smaller numbers in the patient group. Within the theoretical framework of a left-to-right oriented mental number line, patients thus showed paradoxical right-sided hemineglect on this task (Fig. 2).
Table 1.
Hemineglect tests
| Space | Neglect test (spatial asymmetry index a ) | Patients (N = 55) |
Controls (N = 55) |
Statistics
b
(df = 108) |
|||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | T | P | ||
| Peripersonal | Random shape cancellation (CoC) | 0.380 | 0.369 | 0.000 | 0.005 | 7.645 | <0.001** |
| Peripersonal | Two part picture (LI) | 0.412 | 0.490 | 0.000 | 0.020 | 6.227 | <0.001** |
| Peripersonal | Line bisection (% deviation) | 21.087 | 20.785 | 0.866 | 3.102 | 7.136 | <0.001** |
| Representational | Representational drawing (LI) | 0.070 | 0.136 | −0.002 | 0.032 | 3.811 | <0.001** |
| Representational | Mental map (LI) | 0.021 | 0.387 | −0.104 | 0.202 | 2.134 | 0.035* |
| Representational | MNLB (% deviation) | −4.317 | 3.536 | −1.385 | 1.353 | −5.744 | <0.001** |
Note that for all of these indices, positive values indicate rightwards deviation (eastwards on the mental map, towards the larger number during MNLB), while negative values denote leftwards deviation (westwards on the mental map, towards the smaller number during MNLB).
Independent samples t-tests.
Significant at the P < 0.05 level (one-tailed).
Highly significant at the P < 0.001 level (one-tailed).
CoC, centre of cancellation (as introduced by Rorden and Karnath, 2010).
Figure 2.
Results of the line bisection (A) and MNLB task (B). Bars depict mean values of patients (grey) and controls (light grey), error bars show standard deviations. The deviation from the true midpoint (y-axis) is expressed as a percentage of the true line half, applying the formula of Schenkenberg et al. (1980). Note that positive values represent rightwards deviation in A and deviation towards the larger number in B.
Cognitive estimation
The general cognitive estimation performance of patients was impaired. Relative to controls, they displayed significantly higher adjusted sum scores on the CET-A and made significantly more very extreme estimates (Table 2). Single case analyses yielded that the former of these two impairments was found in 23 (42%), the latter in 26 (47%) of the 55 patients.
Table 2.
Cognitive estimation performance
| CET-A variable | Patients (N = 55) |
Controls (N = 55) |
Statistics
a
(df = 108) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | T | P | |
| Adjusted sum score | 9.64 | 3.89 | 5.84 | 3.43 | 5.431 | <0.001** |
| No. of very extreme estimates | 2.29 | 1.36 | 0.98 | 1.01 | 5.743 | <0.001** |
| Mean Z-score | 0.26 | 0.75 | 0.23 | 0.47 | 0.278 | 0.781 |
Independent samples t-test, applying a Bonferroni-corrected P threshold of 0.017.
Highly significant at the P < 0.001 level (one-tailed).
With regard to the direction of estimation performance, mean Z-scores did not differ significantly between groups (Table 2). Analysing summed frequencies of estimates in each CET-A deviation point category revealed two significant group differences: patients displayed fewer normal estimates (i.e. values between the 20th and 80th percentile, labelled 0 in Fig. 3; χ2 = 30.744, df = 1, P < 0.001) and more very extreme underestimates (i.e. values below the 5th percentile, labelled −3 in Fig. 3; χ2 = 35.779, df = 1, P < 0.001) than controls.
Figure 3.
Frequency distribution of CET-A estimates. Summed frequencies of estimates (55 participants × 9 items = 495 estimates per group) in the seven CET-A deviation point categories. Note that for illustrative purposes, deviation points assigned to underestimates are given a negative sign. Asterisks denote significant group differences, based on Chi-square tests and a Bonferroni-corrected P threshold of 0.007.
Lesion-symptom mapping
Based on adjusted CET-A sum scores of patients as the behaviour variable, VLSM yielded one significant voxel cluster. Fig. 4 depicts that it was found in the right anterior temporal lobe (ATL). Damage to these voxels was associated with impaired general cognitive estimation performance.
Figure 4.

VLSM. Voxels significantly more often damaged in patients with impaired general cognitive estimation performance (N = 23), as opposed to patients with normal estimation performance (N = 32). Adjusted CET-A sum scores were used as the behavioural variable. Depicted in the axial plane is the only significant voxel cluster (shades of red), overlaid on the ch2 template available in MRIcroN (https://www.nitrc.org/projects/mricron, 17 September 2020, date last accessed). The superimposed cut-out in the top left corner shows a magnified view on this cluster. Horizontal image orientation follows the neurological convention (right on right side).
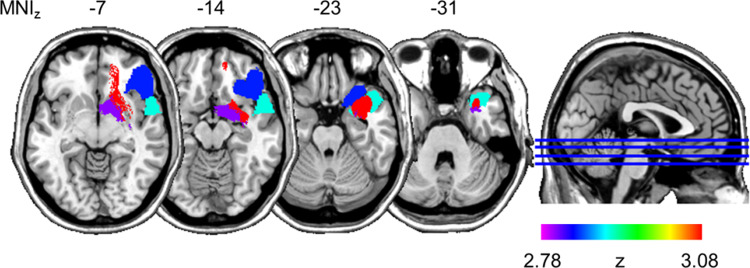
As shown in Fig. 5, RLSM performed with the same behavioural variable revealed four regions that survived significance threshold: the right uncinate fasciculus (Z = 3.077), the right superior temporal pole (Z = 2.878), orbital parts of the right inferior frontal gyrus (Z = 2.834) and the right half of the anterior commissure (Z = 2.804). Impaired general cognitive estimation performance was associated with brain damage in these four regions. With regard to the increased number of very extreme estimates, VLSM and RLSM analyses yielded no significant lesion correlates.
Figure 5.
RLSM. Regions significantly more often damaged in patients with impaired general cognitive estimation performance (N = 23), compared to patients with normal estimation performance (N = 32). Adjusted CET-A sum scores were used as the behavioural variable. Four regions survived significance threshold and were superimposed on the ch2 template available in MRIcroN (https://www.nitrc.org/projects/mricron, 17 September 2020, date last accessed): the right uncinate fasciculus (red), the right superior temporal pole (cyan), orbital parts of the right inferior frontal gyrus (blue) and the right half of the anterior commissure (magenta). Horizontal image orientation follows the neurological convention (right on right side).
Discussion
The first main hypothesis of this study was confirmed. A group of patients with left hemineglect due to right-hemisphere damage demonstrated impaired cognitive estimation performance on the CET-A. Relative to healthy controls, they showed higher adjusted sum scores as well as increased percentages of very extreme estimates. Both findings indicate a general, non-directional estimation impairment in patients. Lesion-symptom mapping related this impairment predominantly to brain damage in the right ATL. VLSM as well as RLSM mapping revealed significant lesion correlates in this region. Furthermore, RLSM identified two major fibre tracts connecting the right ATL with other parts of the brain as significant lesion correlates, i.e. the anterior commissure and the right uncinate fasciculus. The former connects the right ATL with its contralateral counterpart, the latter with right prefrontal areas. What the functional role of the right ATL might be in cognitive estimation will be discussed in detail below.
While the hypothesized parietal involvement was not found, RLSM further disclosed a frontal lesion correlate, as expected on the basis of previous, relatively coarse lesion studies (Shallice and Evans, 1978; Smith and Milner, 1984; Cipolotti et al., 2018). More precisely, brain damage to orbital parts of the right inferior frontal gyrus was associated with poor cognitive estimation in this study. According to recent evidence (Adelhöfer and Beste, 2019; Puglisi et al., 2019), this gyrus is critically involved in inhibitory control, one of several main executive functions (Cristofori et al., 2019). We thus speculate that the functional contribution of the right inferior frontal gyrus to cognitive estimation might be the inhibition of poorly monitored, extreme or even bizarre responses.
The second main hypothesis of this study was not confirmed, as the patient group did not display the expected overestimation. Expressed as normalized Z-scores and averaged across all CET-A items, the mean direction of estimates did not differ significantly between groups. Similarly, the frequency of overestimates was not significantly increased in patients, relative to controls. In fact, patients made significantly more very extreme underestimates than controls. This unexpected preference for smaller magnitudes was also found in the MNLB task. Restated in different terms, while several previous studies demonstrated a bias towards large magnitudes in patients with left hemineglect (Zorzi et al., 2002, 2006; Rossetti et al., 2004; Vuilleumier et al., 2004; Priftis et al., 2006; Zamarian et al., 2007), ours did not.
A first reason for this difference might be that the phenomenon appears task-specific. The vast majority of the positive findings mentioned above have been elicited with MNLB tasks. In contrast, several random number generation tasks (Loetscher and Brugger, 2009; Loetscher et al., 2010) did not reveal a bias towards large magnitudes in patients with left hemineglect, similar to the CET-A applied in this study. And even during MNLB tasks that evoked positive findings, this bias was not consistently shown by patients. For example, it was typically absent when very small numerical intervals (i.e. 3 or 5) had to be bisected (Zorzi et al., 2002; Priftis et al., 2006). Inconsistent results have also been reported for large intervals such as 20 or 40 (Zamarian et al., 2007). Moreover, many positive findings are based on relatively small patient samples comprising less than nine participants (Zorzi et al., 2002, 2006; Rossetti et al., 2004; Vuilleumier et al., 2004; Priftis et al., 2006; Zamarian et al., 2007). Two studies with larger patient groups, however, found no significant shift of perceived midpoints towards large numbers during an MNLB task (Doricchi et al., 2005; Loetscher et al., 2010), and neither did the present study. In sum, the large magnitude bias of patients with left hemineglect seems a rare phenomenon, observed only in a subset of patients during certain conditions of specific tasks.
Nevertheless, our patient sample displayed severely impaired cognitive estimation performance on the CET-A. Instead of the hypothesized bias towards large magnitudes, we assume that their impairment might reflect partial loss of knowledge about real-world numerical magnitudes. In other words, their impairment may have something to do with semantic memory malfunction. That cognitive estimation is associated with semantic memory—also known as semantic knowledge—has been assumed by many authors based on correlational analyses of cognitive test results (Brand et al., 2003; Della Sala et al., 2004; Levinoff et al., 2006; D'Aniello et al., 2015b). Here, we substantiate this view by adding neuroanatomical evidence to it: the present lesion-symptom mapping analysis revealed that the right ATL is the most critical brain region for cognitive estimation, and there is a growing body of neuroscientific literature suggesting that the bilateral ATL contains a transmodal hub for semantic cognition. This hub has been conceptualized as a functional unit performing higher-order generalizations through bidirectional connections with modality-specific areas distributed across the brain (Patterson et al., 2007; Ralph et al., 2017).
To the best of our knowledge, Patterson et al. (2007) have first proposed a semantic hub function of the bilateral ATL, thereby primarily referring to behavioural and structural neuroimaging findings in patients with semantic dementia (SD). This neurodegenerative disorder is characterized by progressive semantic impairment across various modalities (Ralph et al., 2017), associated with relatively focal atrophy centred on the bilateral ATL (Hodges and Patterson, 2007). In more recent years, the notion of a semantic hub in the bilateral ATL has been corroborated in patients as well as healthy participants with a variety of methodological approaches including repetitive transcranial magnetic stimulation (Lambon Ralph et al., 2009; Pobric et al., 2010), intracranial electrode recordings (Abel et al., 2015; Chen et al., 2016), distortion-corrected functional magnetic resonance imaging (Binney et al., 2016; Hoffman and Lambon Ralph, 2018) or functional connectivity analyses (Jackson et al., 2016; Chiou and Lambon Ralph, 2019). There is also supporting evidence from lesion-symptom mapping. In particular, VLSM performed in post-stroke aphasia patients has associated damage to the left ATL with increased frequencies of semantic naming errors (Schwartz et al., 2009; Walker et al., 2011). As a complement, this study revealed lesion-mapping evidence that the right ATL might be critically involved in another aspect of semantic cognition, i.e. the knowledge about real-world numerical magnitudes.
The semantic hub in the bilateral ATL is assumed to process virtually all types of concepts based on sensory, motor, linguistic and affective sources of information (Ralph et al., 2017). However, some patients with SD show relatively preserved number knowledge and calculation skills in combination with otherwise profound semantic impairment, as revealed by several single case studies (Cappelletti et al., 2001; Crutch and Warrington, 2002; Zamarian et al., 2006). This dissociation was interpreted as evidence for conceptual independence and neuroanatomical segregation of numerical knowledge from other semantic memory content (Cappelletti et al., 2001; Zamarian et al., 2006), with the former being predominantly processed in parietal areas (Dehaene et al., 2003) that are typically spared by SD-related atrophy (Rosen et al., 2002). As such, the finding challenges not only the notion of a modality-invariant semantic hub in the bilateral ATL but also our interpretation of the present results, i.e. that the right ATL is associated with magnitude knowledge.
A solution to this seeming contradiction was presented by Julien et al. (2008, 2010). In contrast to the single cases mentioned above, they investigated a group of 14 SD patients with varying degrees of semantic impairment and temporal atrophy asymmetry (i.e. eight patients left-dominant, four patients right-dominant, two patients without asymmetry). Concerning arithmetic skills, the patient group generally performed well on addition and subtraction tasks, while the identification of arithmetic signs and both mental and written multiplication were impaired, irrespective of education and hemispheric side of atrophy (Julien et al., 2008). Similar dissociations between preserved and impaired numerical skills were found in a series of tasks requiring the processing of quantities (Julien et al., 2010). Patients' performance was unimpaired on Piagetian conservation and basic number comparison tasks, and they estimated the number of visually presented items (e.g. playing cards, sweets, matches) as accurately as healthy controls. However, and this is particularly noteworthy in light of the present findings, SD patients displayed impairment on real-world estimation tasks. For example, they performed poorly when the age, weight or height of people presented on photographs had to be estimated. And identical to the CET-A performance of our patient sample, they showed impaired cognitive estimation of real-world quantities that were not visually presented to them, e.g. the number of seats on a bus or the number of countries in the world. These group results indicate that some aspects of number processing are impaired in patients with SD, and that the ATL may contribute to the conceptual understanding of quantity.
If the latter conclusion is true, it has implications for theories about number processing in the brain. The leading model assumption, i.e. the triple-code model (Dehaene, 1992; Dehaene and Cohen, 1995), does not include the ATL as a relevant structure. It postulates that numerical cognition primarily relies on combined activity of three regions that are specialized on specific representations or codes: Number magnitudes are analogically coded in the bilateral parietal lobe, number forms visually in bilateral occipito-temporal areas, and number words verbally in classic language areas of the left hemisphere such as the left inferior frontal gyrus (Dehaene, 1992; Dehaene and Cohen, 1995). Based on a meta-analysis of 53 functional neuroimaging studies performed in healthy adults to examine the neural correlates of diverse number and/or calculation skills—with the notable exception of cognitive estimation—Arsalidou and Taylor (2011) proposed to add further brain areas and functional contributions to that network. For example, the middle and superior frontal gyri might be critically involved in generating strategies to solve multi-step arithmetic tasks and—together with other areas such as the dorsal cingulate gyri or the bilateral cerebellum—in providing working memory resources needed to solve mathematical problems. As mentioned before, we here put forward the hypothesis that the ATL might add yet another contribution to the number processing network in the brain, i.e. the knowledge about real-world numerical magnitudes.
This study is not without limitations. Most importantly, our patient sample comprised participants with right-sided brain damage only. Any conclusion drawn from the lesion-symptom mapping evidence described above is therefore restricted to right-hemisphere function. On the basis of this study alone, it thus remains unclear whether the right or the bilateral ATL critically supports real-world numerical magnitude knowledge. Previous evidence favours the latter assumption, as the left and right ATL show less hemispheric specialization than more posterior temporal areas (Hoffman and Lambon Ralph, 2018), and may together form an integrated, partially redundant system for the representation of concepts (Lambon Ralph et al., 2010).
Another limitation of this study is inherent to all lesion-symptom methods. Different regions of the brain are not equally vulnerable to brain damage. For example, it is known that middle cerebral artery strokes are far more frequent than strokes in other neurovascular territories (Ng et al., 2007). Similarly, different structures within neurovascular territories show considerable variation in their susceptibility to stroke (Sperber and Karnath, 2016). As a consequence, the functional contribution of rarely injured areas is likely to remain unnoticed in studies applying lesion-symptom mapping (Karnath et al., 2019). Brain regions other than the right ATL might thus contribute to real-world magnitude knowledge, and methodological approaches other than lesion-symptom mapping might be needed to detect these regions.
Acknowledgements
We would like to thank the patients and the healthy control participants for contributing to this research project.
Funding
This study was supported by grant No. 320030_169789 of the Swiss National Science Foundation (SNF).
Competing interests
The authors report no competing interests.
Glossary
- ATL =
anterior temporal lobe;
- CET =
cognitive estimation test;
- CET-A =
A-version of the Cognitive Estimation Task;
- MNLB =
mental number line bisection;
- RLSM =
region of interest-based lesion-symptom mapping;
- SD =
semantic dementia;
- VLSM =
voxel-based lesion-symptom mapping
Contributor Information
Tobias Pflugshaupt, Neurocenter, Luzerner Kantonsspital, Luzern, Switzerland.
Daniel Bauer, Neurocenter, Luzerner Kantonsspital, Luzern, Switzerland.
Julia Frey, Neurocenter, Luzerner Kantonsspital, Luzern, Switzerland.
Tim Vanbellingen, Neurocenter, Luzerner Kantonsspital, Luzern, Switzerland; Gerontechnology and Rehabilitation Group, ARTORG Center for Biomedical Engineering, University of Bern, Bern, Switzerland.
Brigitte C Kaufmann, Neurocenter, Luzerner Kantonsspital, Luzern, Switzerland; Gerontechnology and Rehabilitation Group, ARTORG Center for Biomedical Engineering, University of Bern, Bern, Switzerland.
Stephan Bohlhalter, Neurocenter, Luzerner Kantonsspital, Luzern, Switzerland.
Thomas Nyffeler, Neurocenter, Luzerner Kantonsspital, Luzern, Switzerland; Gerontechnology and Rehabilitation Group, ARTORG Center for Biomedical Engineering, University of Bern, Bern, Switzerland.
References
- Abel TJ, Rhone AE, Nourski KV, Kawasaki H, Oya H, Griffiths TD, et al. Direct physiologic evidence of a heteromodal convergence region for proper naming in human left anterior temporal lobe. J Neurosci 2015; 35: 1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelhöfer N, Beste C. Validity expectancies shape the interplay of cueing and task demands during inhibitory control associated with right inferior frontal regions. Brain Struct Funct 2019; 224: 1911–24. [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Taylor MJ. Is 2 + 2=4? Meta-analyses of brain areas needed for numbers and calculations. NeuroImage 2011; 54: 2382–93. [DOI] [PubMed] [Google Scholar]
- Ashburner J Friston KJ.. Unified segmentation. NeuroImage 2005; 26: 839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Axelrod BN, Millis SR. Preliminary standardization of the cognitive estimation test. Assessment 1994; 1: 269–74. [Google Scholar]
- Azouvi P, Olivier S, de Montety G, Samuel C, Louis-Dreyfus A, Tesio L. Behavioral assessment of unilateral neglect: study of the psychometric properties of the Catherine Bergego Scale. Arch Phys Med Rehabil 2003; 84: 51–7. [DOI] [PubMed] [Google Scholar]
- Barabassy A, Beinhoff U, Riepe MW. Cognitive estimation in aged patients with major depressive disorder. Psychiatry Res 2010; 176: 26–9. [DOI] [PubMed] [Google Scholar]
- Bergego C, Azouvi P, Samuel C, Marchal F, Louis-Dreyfus A, Jokic C, et al. Validation d’une échelle d’évaluation fonctionnelle de l’héminégligence dans la vie quotidienne: l’échelle CB. Ann Réadapt Méd Phys 1995; 38: 183–9. [Google Scholar]
- Billino J, Brand M, Roesler A. Cognitive estimation in patients with early subcortical vascular dementia. Int J Geriatr Psychiatry 2008; 23: 982–3. [DOI] [PubMed] [Google Scholar]
- Binney RJ, Hoffman P, Lambon Ralph MA. Mapping the multiple graded contributions of the anterior temporal lobe representational hub to abstract and social concepts: evidence from distortion-corrected fMRI. Cereb Cortex 2016; 26: 4227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbing TA, Olm CA, McMillan CT, Rascovsky K, Baehr L, Ternes K, et al. Estimating frontal and parietal involvement in cognitive estimation: a study of focal neurodegenerative diseases. Front Hum Neurosci 2015; 9:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake H, McKinney M, Treece K, Lee E, Lincoln NB. An evaluation of screening measures for cognitive impairment after stroke. Age Ageing 2002; 31: 451–6. [DOI] [PubMed] [Google Scholar]
- Brand M, Kalbe E, Fujiwara E, Huber M, Markowitsch HJ. Cognitive estimation in patients with probable Alzheimer's disease and alcoholic Korsakoff patients. Neuropsychologia 2003; 41: 575–84. [DOI] [PubMed] [Google Scholar]
- Brunila T, Jalas M, Lindell JA, Tenovuo O, Hämäläinen H. The two part picture in detection of visuospatial neglect. Clin Neuropsychol 2003; 17: 45–53. [DOI] [PubMed] [Google Scholar]
- Bullard SE, Fein D, Gleeson MK, Tischer N, Mapoum RL, Kaplan E. The Biber Cognitive Estimation Test. Arch Clin Neuropsychol 2004; 19: 835–46. [DOI] [PubMed] [Google Scholar]
- Cansino S, Torres-Trejo F, Estrada-Manilla C, Hernández-Ramos E, Martínez-Galindo JG, Gómez-Fernández T, et al. Mediators of episodic memory decay across the adult life span. Sci Rep 2018; 8: 2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Butterworth B, Kopelman M. Spared numerical abilities in a case of semantic dementia. Neuropsychologia 2001; 39: 1224–39. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008; 44: 1105–32. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Theory of fluid and crystallized intelligence: a critical experiment. J Educ Psychol 1963; 54: 1–22. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shimotake A, Matsumoto R, Kunieda T, Kikuchi T, Miyamoto S, et al. The ‘when’ and ‘where’ of semantic coding in the anterior temporal lobe: temporal representational similarity analysis of electrocorticogram data. Cortex 2016; 79: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou R, Lambon Ralph MA. Unveiling the dynamic interplay between the hub- and spoke-components of the brain's semantic system and its impact on human behaviour. NeuroImage 2019; 199: 114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti L, MacPherson SE, Gharooni S, van-Harskamp N, Shallice T, Chan E, et al. Cognitive estimation: performance of patients with focal frontal and posterior lesions. Neuropsychologia 2018; 115: 70–7.28811256 [Google Scholar]
- Crawford JR, Garthwaite PH, Porter S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: rationale, methods, implementations, and proposed reporting standards. Cogn Neuropsychol 2010; 27: 245–60. [DOI] [PubMed] [Google Scholar]
- Cristofori I, Cohen-Zimerman S, Grafman J. Executive functions. Handb Clin Neurol 2019; 163: 197–219. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. Preserved calculation skills in a case of semantic dementia. Cortex 2002; 38: 389–99. [DOI] [PubMed] [Google Scholar]
- D'Aniello GE, Castelnuovo G, Scarpina F. Could cognitive estimation ability be a measure of cognitive reserve? Front Psychol 2015. a; 6: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aniello GE, Scarpina F, Albani G, Castelnuovo G, Mauro A. Disentangling the relationship between cognitive estimation abilities and executive functions: a study on patients with Parkinson's disease. Neurol Sci 2015. b; 36: 1425–9. [DOI] [PubMed] [Google Scholar]
- de Haan B, Karnath HO. A hitchhiker's guide to lesion-behaviour mapping. Neuropsychologia 2018; 115: 5–16. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Varieties of numerical abilities. Cognition 1992; 44: 1–42. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. Towards an anatomical and functional model of number processing. Math Cogn 1995; 1: 83–120. [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol 2003; 20: 487–506. [DOI] [PubMed] [Google Scholar]
- Della Sala S, MacPherson SE, Phillips LH, Sacco L, Spinnler H. The role of semantic knowledge on the cognitive estimation task. Evidence from Alzheimer's disease and healthy adult aging. J Neurol 2004; 251: 156–64. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Guariglia P, Gasparini M, Tomaiuolo F. Dissociation between physical and mental number line bisection in right hemisphere brain damage. Nat Neurosci 2005; 8: 1663–5. [DOI] [PubMed] [Google Scholar]
- Findlater SE, Desai JA, Semrau JA, Kenzie JM, Rorden C, Herter TM, et al. Central perception of position sense involves a distributed neural network—evidence from lesion-behavior analyses. Cortex 2016; 279: 42–56. [DOI] [PubMed] [Google Scholar]
- Galton F. Visualised numerals. Nature 1880; 21: 252–6. [Google Scholar]
- Gauvrit N, Zenil H, Soler-Toscano F, Delahaye JP, Brugger P. Human behavioral complexity peaks at age 25. PLoS Comput Biol 2017; 13: e1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Evans RI, Gardener EA, Bowen A. Performance of older adults on tests of cognitive estimation. J Clin Exp Neuropsychol 2002; 24: 286–93. [DOI] [PubMed] [Google Scholar]
- Harel BT, Cillessen AH, Fein DA, Bullard SE, Aviv A. It takes nine days to iron a shirt: the development of cognitive estimation skills in school age children. Child Neuropsychol 2007; 13: 309–18. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome [Review]. Lancet Neurol 2007; 6: 1004–14. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Lambon Ralph MA. From percept to concept in the ventral temporal lobes: graded hemispheric specialisation based on stimulus and task. Cortex 2018; 101: 107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P, Dehaene S. Interactions between number and space in parietal cortex. Nat Rev Neurosci 2005; 6: 435–48. [DOI] [PubMed] [Google Scholar]
- Jackson RL, Hoffman P, Pobric G, Lambon Ralph MA. The semantic network at work and rest: differential connectivity of anterior temporal lobe subregions. J Neurosci 2016; 36: 1490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LC, Hillis AE. Aphasia and right hemisphere syndromes in stroke. Curr Neurol Neurosci Rep 2005; 5: 458–64. [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Neary D, Snowden JS. Arithmetic knowledge in semantic dementia: is it invariably preserved? Neuropsychologia 2008; 46: 2732–44. [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Neary D, Snowden JS. Understanding quantity in semantic dementia. Cogn Neuropsychol 2010; 27: 3–29. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Sperber C, Rorden C. Reprint of: mapping human brain lesions and their functional consequences. NeuroImage 2019; 190: 4–13. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain 2010; 133: 3243–55. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex 2009; 19: 832–8. [DOI] [PubMed] [Google Scholar]
- Levinoff EJ, Phillips NA, Verret L, Babins L, Kelner N, Akerib V, et al. Cognitive estimation impairment in Alzheimer disease and mild cognitive impairment. Neuropsychology 2006; 20: 123–32. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol Sci 2004; 15: 155–63. [DOI] [PubMed] [Google Scholar]
- Loetscher T, Brugger P. Random number generation in neglect patients reveals enhanced response stereotypy, but no neglect in number space. Neuropsychologia 2009; 47: 276–9. [DOI] [PubMed] [Google Scholar]
- Loetscher T, Nicholls ME, Towse JN, Bradshaw JL, Brugger P. Lucky numbers: spatial neglect affects physical, but not representational, choices in a lotto task. Cortex 2010; 46: 685–90. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Wagner GP, Murphy P, Bozzali M, Cipolotti L, Shallice T. Bringing the cognitive estimation task into the 21st century: normative data on two new parallel forms. PLoS One 2014; 9: e92554 Erratum in: PLoS One; 9: e104483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Dev Psychol 2002; 38: 115–42. [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. NeuroImage 2008; 39: 1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke 2007; 38: 2309–14. [DOI] [PubMed] [Google Scholar]
- Parente A, Manfredi V, Villani F, Franceschetti S, Giovagnoli AR. Investigating higher-order cognitive functions in temporal lobe epilepsy: cognitive estimation. Epilepsy Behav 2013; 29: 330–6. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007; 8: 976–87. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Category-specific versus category-general semantic impairment induced by transcranial magnetic stimulation. Curr Biol 2010; 20: 964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priftis K, Zorzi M, Meneghello F, Marenzi R, Umiltà C. Explicit versus implicit processing of representational space in neglect: dissociations in accessing the mental number line. J Cogn Neurosci 2006; 18: 680–8. [DOI] [PubMed] [Google Scholar]
- Puglisi G, Howells H, Sciortino T, Leonetti A, Rossi M, Conti Nibali M, et al. Frontal pathways in cognitive control: direct evidence from intraoperative stimulation and diffusion tractography. Brain 2019; 142: 2451–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci 2017; 18: 42–55. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. NeuroImage 2012; 61: 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. A simple measure of neglect severity. Neuropsychologia 2010; 48: 2758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 2007; 19: 1081–8. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002; 58: 198–208. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Jacquin-Courtois S, Rode G, Ota H, Michel C, Boisson D. Does action make the link between number and space representation? Visuo-manual adaptation improves number bisection in unilateral neglect. Psychol Sci 2004; 15: 426–30. [DOI] [PubMed] [Google Scholar]
- Roth RM, Pixley HS, Kruck CL, Garlinghouse MA, Giancola PR, Flashman LA. Performance on the cognitive estimation test in schizophrenia. Appl Neuropsychol Adult 2012; 19: 141–6. [DOI] [PubMed] [Google Scholar]
- Scarpina F, D’Aniello GE, Mauro A, Castelnuovo G, MacPherson SE. How many segments are there in an orange: normative data for the new cognitive estimation task in an Italian population. Neurol Sci 2015; 36: 1889–95. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology 1980; 30: 509–17. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain 2009; 132: 3411–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Evans ME. The involvement of the frontal lobes in cognitive estimation. Cortex 1978; 14: 294–303. [DOI] [PubMed] [Google Scholar]
- Silverberg ND, Hanks RA, McKay C. Cognitive estimation in traumatic brain injury. J Int Neuropsychol Soc 2007; 13: 898–902. [DOI] [PubMed] [Google Scholar]
- Silverman S, Ashkenazi S. Deconstructing the cognitive estimation task: a developmental examination and intra-task contrast. Sci Rep 2016; 6: 39316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Milner B. Differential effects of frontal-lobe lesions on cognitive estimation and spatial memory. Neuropsychologia 1984; 22: 697–705. [DOI] [PubMed] [Google Scholar]
- Sperber C, Karnath HO. Topography of acute stroke in a sample of 439 right brain damaged patients. NeuroImage Clin 2016; 10: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, O'Carroll R. Cognitive estimation in neurological disorders. Br J Clin Psychol 1995; 34: 223–8. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002; 15: 273–89. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Ortigue S, Brugger P. The number space and neglect. Cortex 2004; 40: 399–410. [DOI] [PubMed] [Google Scholar]
- Wagner GP, MacPherson SE, Parente MA, Trentini CM. Cognitive estimation abilities in healthy and clinical populations: the use of the cognitive estimation test. Neurol Sci 2011; 32: 203–10. [DOI] [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher A, Dell GS, et al. Support for anterior temporal involvement in semantic error production in aphasia: new evidence from VLSM. Brain Lang 2011; 117: 110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V. A Theory of Magnitude: common cortical metrics of time, space and quantity. Trends Cogn Sci 2003; 7: 483–8. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM. Visual hemispatial inattention: stimulus parameters and exploratory strategies. J Neurol Neurosurg Psychiatry 1988; 51: 1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Halligan PW, Behavioural inattention test. Titchfield, UK: Thames Valley Test Company; 1987. [Google Scholar]
- Winter B, Matlock T, Shaki S, Fischer MH. Mental number space in three dimensions [Review]. Neurosci Biobehav Rev 2015; 57: 209–19. [DOI] [PubMed] [Google Scholar]
- Zamarian L, Egger C, Delazer M. The mental representation of ordered sequences in visual neglect. Cortex 2007; 43: 542–50. [DOI] [PubMed] [Google Scholar]
- Zamarian L, Karner E, Benke T, Donnemiller E, Delazer M. Knowing 7 × 8, but not the meaning of ‘elephant’: evidence for the dissociation between numerical and non-numerical semantic knowledge. Neuropsychologia 2006; 44: 1708–23. [DOI] [PubMed] [Google Scholar]
- Zorzi M, Priftis K, Meneghello F, Marenzi R, Umiltà C. The spatial representation of numerical and non-numerical sequences: evidence from neglect. Neuropsychologia 2006; 44: 1061–7. [DOI] [PubMed] [Google Scholar]
- Zorzi M, Priftis K, Umiltà C. Brain damage: neglect disrupts the mental number line. Nature 2002; 417: 138–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.