Key Points
Question
In statin-treated patients with high cardiovascular risk, high triglycerides, and low HDL cholesterol levels, does adding a carboxylic acid formulation of omega-3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid) to background therapy improve cardiovascular outcomes?
Findings
In this randomized clinical trial of 13 078 patients that was stopped early, daily supplementation with omega-3 fatty acids, compared with corn oil, resulted in no significant difference in a composite outcome of major adverse cardiovascular events (hazard ratio, 0.99).
Meaning
These findings do not support use of this omega-3 fatty acid formulation to reduce major adverse cardiovascular events in patients with high cardiovascular risk.
Abstract
Importance
It remains uncertain whether the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) reduce cardiovascular risk.
Objective
To determine the effects on cardiovascular outcomes of a carboxylic acid formulation of EPA and DHA (omega-3 CA) with documented favorable effects on lipid and inflammatory markers in patients with atherogenic dyslipidemia and high cardiovascular risk.
Design, Setting, and Participants
A double-blind, randomized, multicenter trial (enrollment October 30, 2014, to June 14, 2017; study termination January 8, 2020; last patient visit May 14, 2020) comparing omega-3 CA with corn oil in statin-treated participants with high cardiovascular risk, hypertriglyceridemia, and low levels of high-density lipoprotein cholesterol (HDL-C). A total of 13 078 patients were randomized at 675 academic and community hospitals in 22 countries in North America, Europe, South America, Asia, Australia, New Zealand, and South Africa.
Interventions
Participants were randomized to receive 4 g/d of omega-3 CA (n = 6539) or corn oil, which was intended to serve as an inert comparator (n = 6539), in addition to usual background therapies, including statins.
Main Outcomes and Measures
The primary efficacy measure was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina requiring hospitalization.
Results
When 1384 patients had experienced a primary end point event (of a planned 1600 events), the trial was prematurely halted based on an interim analysis that indicated a low probability of clinical benefit of omega-3 CA vs the corn oil comparator. Among the 13 078 treated patients (mean [SD] age, 62.5 [9.0] years; 35% women; 70% with diabetes; median low-density lipoprotein [LDL] cholesterol level, 75.0 mg/dL; median triglycerides level, 240 mg/dL; median HDL-C level, 36 mg/dL; and median high-sensitivity C-reactive protein level, 2.1 mg/L), 12 633 (96.6%) completed the trial with ascertainment of primary end point status. The primary end point occurred in 785 patients (12.0%) treated with omega-3 CA vs 795 (12.2%) treated with corn oil (hazard ratio, 0.99 [95% CI, 0.90-1.09]; P = .84). A greater rate of gastrointestinal adverse events was observed in the omega-3 CA group (24.7%) compared with corn oil–treated patients (14.7%).
Conclusions and Relevance
Among statin-treated patients at high cardiovascular risk, the addition of omega-3 CA, compared with corn oil, to usual background therapies resulted in no significant difference in a composite outcome of major adverse cardiovascular events. These findings do not support use of this omega-3 fatty acid formulation to reduce major adverse cardiovascular events in high-risk patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT02104817
This randomized trial examines the effects on cardiovascular outcomes of a carboxylic acid formulation of EPA and DHA (omega-3 CA) with documented favorable effects on lipid and inflammatory markers in patients with atherogenic dyslipidemia and high cardiovascular risk.
Introduction
Considerable interest has focused on the potential cardiovascular benefits of omega-3 fatty acids. Observational studies have demonstrated an inverse association between dietary consumption of either fatty fish or omega-3 fatty acids and incident cardiovascular events1,2 and that circulating concentrations of eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) inversely correlate with cardiovascular risk.3,4 Omega-3 fatty acid supplementation exerts favorable effects on lipoprotein metabolism and inflammatory, oxidative, thrombotic, vascular, and arrhythmogenic factors implicated in cardiovascular disease.5,6 One study, prior to routine clinical use of statins, demonstrated cardiovascular benefit with 1 g/d of an EPA and DHA supplement,7 but subsequent larger trials failed to replicate these findings.8,9
Most trials recruited a broad cohort of patients and administered low doses of omega-3 fatty acids that did not produce substantial increases in EPA or DHA concentrations. Recent trials have studied higher dosages of omega-3 fatty acids, reporting a cardiovascular benefit in 2 trials of purified EPA.10,11 However, other recent trials studying lower doses of omega-3 fatty acids in a broader range of patients failed to demonstrate significant reductions of total cardiovascular events.12,13
Omega-3 CA (Epanova; AstraZeneca) is a carboxylic acid formulation of omega-3 fatty acids (EPA and DHA) that does not require hydrolysis by pancreatic lipase during intestinal absorption, eliminating the need for consumption with a high-fat meal, resulting in greater bioavailability compared with standard omega-3 ethyl ester formulations. Administration of 4 g/d of omega-3 CA produces similar increases in plasma EPA levels as doses of purified EPA approved for clinical use, and also increases DHA concentrations.14,15 Initial trials of omega-3 CA demonstrated dose-dependent lowering of plasma triglyceride levels up to 31%.14,15
This trial, the Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH), evaluated the effects of omega-3 CA on clinical outcomes in patients at high cardiovascular risk.
Methods
Study Organization and Oversight
The trial was coordinated by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research). The protocol was developed by members of the independent academic executive steering committee in conjunction with the sponsor. The study protocol and statistical analysis plan are available in Supplement 1 and Supplement 2. The study design was approved by responsible regulatory agencies and ethics committees or institutional review boards at each site prior to commencing patient enrollment. All potential patients provided written informed consent prior to study entry. IQVIA provided operational management of sites and collected the data. A data monitoring committee (DMC) that was independent from the executive steering committee and sponsor monitored the trial and performed analyses of unblinded data supported by an independent data analysis center at Statistics Collaborative Inc.
Study Population
Details of the study design have been published previously.16 Adult patients (≥18 years) considered at high risk for a future cardiovascular event were eligible to participate. High cardiovascular risk was defined as (1) the presence of established atherosclerotic cardiovascular disease involving the coronary, peripheral, carotid, or aortic territories (secondary prevention); (2) type 1 or 2 diabetes with age 40 years or older for men and 50 years or older for women with at least 1 additional risk factor including chronic smoking, hypertension, high-sensitivity C-reactive protein (hs-CRP) level of 2 mg/L or higher, or moderately increased albuminuria; or (3) high-risk primary prevention patients aged at least 50 years for men or at least 60 years for women with at least 1 additional risk factor, including a family history of premature coronary artery disease, chronic smoking, hs-CRP level of 2 mg/L or higher, impaired kidney function, or coronary calcium score greater than 300 Agatston units.
At least 50% of randomized patients were required to satisfy criteria for secondary cardiovascular prevention. All eligible patients were also required to be treated with a statin for at least 4 weeks; have a low-density lipoprotein (LDL) cholesterol level lower than 100 mg/dL or be treated with maximally tolerated statin therapy; and have atherogenic dyslipidemia, defined as triglyceride levels of 180 to less than 500 mg/dL and high-density lipoprotein (HDL) cholesterol levels lower than 42 mg/dL for men or lower than 47 mg/dL for women. Patients were excluded from enrollment if they had a prior ischemic cardiovascular event within the preceding 30 days or consumed more than one capsule (1 g) per day of omega-3 dietary supplements or any prescription medication containing EPA or DHA. Use of fibrates or weight loss drugs was also prohibited. Patient race and ethnicity were reported by participants using an open-ended question to account for ethnic variability in baseline systemic omega-3 fatty acid concentrations.
Study Procedures
The protocol specified that enrolled patients receive treatment with a stable dose of statin therapy for at least 4 weeks and lifestyle advice for the prevention of cardiovascular disease. Patients who met all inclusion criteria and volunteered to participate were randomized in a 1:1 ratio to treatment with omega-3 CA, 4 g/d, or a matching corn oil comparator for a maximum duration of 5 years (Figure 1). Randomization was performed using a computer-generated random number with a blocking size of 6. Corn oil was selected because it was considered an inert comparator without effects on biochemical parameters associated with cardiovascular risk.17,18 Patients reported for study visits at 3, 6, and 12 months following randomization and then every 6 months thereafter. Additional telephone calls were made on a 3-month basis commencing at month 9. A visit for assessment of any adverse events was performed 3 weeks after the last dose of study medication. Plasma and red blood cell concentrations of EPA and DHA were determined by OmegaQuant.
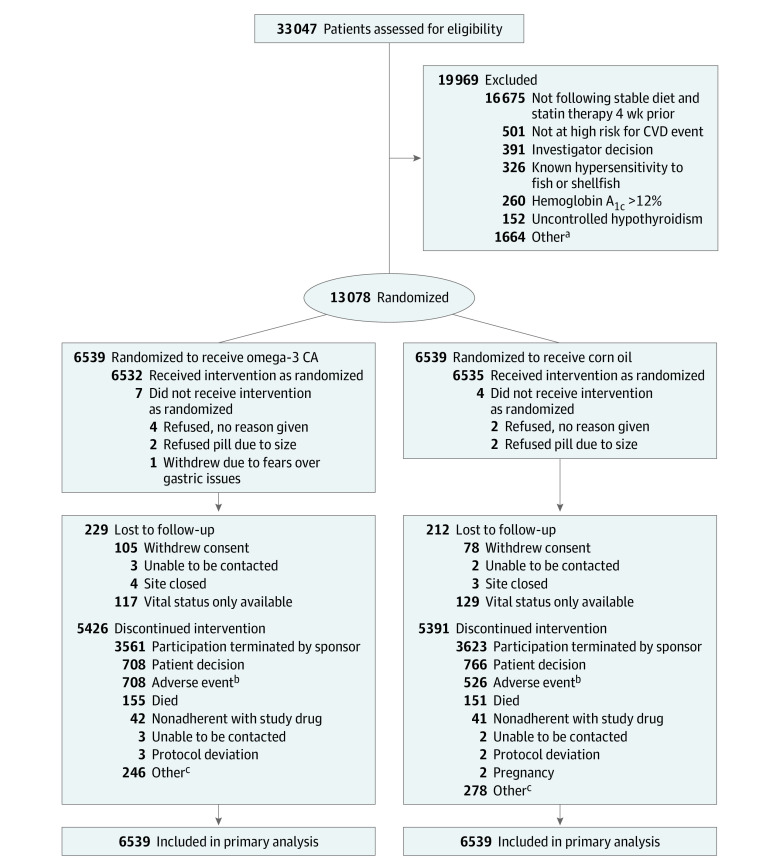
Figure 1. Recruitment, Randomization, and Patient Flow in the STRENGTH Clinical Trial .
CA indicates carboxylic acid formulation; CVD, cardiovascular disease.
aOther reasons for not meeting inclusion/exclusion criteria include not meeting age requirement; elevated liver enzymes; use of fibrates, bile acid sequestrants, or niacin within 4 weeks of randomization; not following a stable diet; poorly controlled hypertension; and occurrence of myocardial infarction or coronary bypass graft surgery within 30 days of randomization.
bAdverse events leading to study drug discontinuation by system organ class (omega-3 CA/corn oil; multiple events are possible): gastrointestinal (403/202), neoplasms (81/78), cardiac (39/46), nervous system (36/42), infections (32/30), skin (24/20), kidney/urinary (16/25), investigations (21/14), metabolic disorders (18/17), musculoskeletal (14/18), hepatobiliary (13/14), injury (11/13), vascular (13/11), respiratory (13/10), and psychiatric (11/7).
cOther reasons abstracted from free text (omega-3 CA/corn oil): investigator decision (22/22), patient decision (26/33), potential lost to follow-up (113/129), reached end point (18/18), moved (31/36), social reasons (7/13), comorbid condition (11/8), pill burden (5/10), study terminated (9/4), and site closed (4/5).
Study End Points
The primary efficacy end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and hospitalization for unstable angina. Secondary efficacy end points included the following: (1) composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and hospitalization for unstable angina in patients with established cardiovascular disease at baseline, (2) composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke in the whole cohort and in patients with established cardiovascular disease at baseline, (3) composite of cardiac death, nonfatal myocardial infarction, coronary revascularization, and hospitalization for unstable angina in the whole cohort and in patients with established cardiovascular disease at baseline, (4) cardiovascular death in the whole cohort and in patients with established cardiovascular disease at baseline, and (5) all-cause death in the whole cohort and in patients with established cardiovascular disease at baseline.
The primary end point and key secondary end points (1) through (5) were evaluated in a hierarchical manner (see eAppendix in Supplement 3). Prespecified tertiary efficacy end points included new-onset atrial fibrillation, thrombotic events, and new-onset heart failure. Changes in lipid levels, inflammatory markers, and levels of both EPA and DHA were also prespecified efficacy parameters. A post hoc analysis investigated the association between both plasma and red blood cell concentrations of EPA and DHA with cardiovascular event rates. All investigator-reported primary and secondary events, as well as heart failure events, were adjudicated by a core laboratory at C5Research.
Sample Size Calculation and Power
The primary efficacy analysis was based on time to first occurrence of any positively adjudicated primary end point including all randomized patients regardless of treatment adherence. Time-to-event analysis was calculated from randomization date to the date of the event, or censored at the last known follow-up for each patient. The trial was designed to enroll 13 000 patients and study completion required positive adjudication of 1600 primary events to provide 90% power to detect a 15% reduction in relative risk in the omega-3 CA group. A 15% reduction in the risk of cardiovascular events was selected because it was deemed the minimally important difference of clinical significance by consensus among the trial executive committee.
Assuming a 4% annual primary end point event rate in the corn oil group, a trial duration of 4.5 years was projected. Interim analyses for superiority or futility were specified at 50% and 75% of the required primary end point events. A group sequential design was used with superiority boundaries for both interim analyses set at an absolute value for a z score of 3.719 and futility boundaries set at a z score of 0.3085 at the first interim analysis and 1.2375 for the second interim analysis.
Statistical Analysis
The full analysis set included all patients according to their randomization group. A safety analysis population was defined as any patient who took at least 1 dose of study drug. The efficacy objectives were evaluated in all randomized patients using analysis of time from randomization to the first event. Censoring rules are described in Supplement 1. Estimates of hazard ratios (HRs) and 95% CIs for omega-3 CA compared with corn oil were calculated using Cox proportional hazards models with covariates for established cardiovascular disease at baseline (yes/no) and region. The proportionality assumption was assessed by including a time-dependent covariate (treatment × time interaction) to the model. Biochemical parameters are presented as median with first (Q1) and third (Q3) quartiles.
The differences in percentage change from baseline between the omega-3 CA and corn oil groups were estimated from an analysis of covariance model (ANCOVA) with treatment group as a main effect and natural log of the baseline as a covariate. The dependent variable was calculated as the natural log of the ratio of the follow-up visit to the baseline visit: log[100 × log(follow-up/baseline)]. The least-squares estimates for differences between treatment groups were then back-transformed from the log scale and expressed as the geometric mean ratio. A sensitivity analysis was conducted using multiple imputation methods to assess the effect of missing biomarker data.
Significance testing was performed using 2-sided tests (α= .05). Primary and key secondary efficacy end points were evaluated sequentially to control the type I error rate. Other end points were not adjusted for multiplicity, and findings for analyses of these end points should be interpreted as exploratory. The statistical analysis plan (Supplement 1) prespecified that a hierarchical testing strategy was to be used, and that once an end point was not statistically significant at an α of .05, all subsequent comparisons will be considered exploratory and nominal P values will be reported. Subgroup analyses of the primary end point were conducted as prespecified, with any potential difference determined by the presence of a nominally significant P value on formal interaction testing.
All analyses were conducted using SAS version 9.4. Additional analytic methods are described in the study protocol and statistical analysis plan (Supplement 1 and Supplement 2).
Early Trial Termination
On January 8, 2020, when 1384 primary end points had been recorded in 13 078 randomized patients, the independent DMC recommended termination of the trial due to a low probability of demonstrating a clinical benefit of omega-3 CA compared with corn oil. This decision was based on the data crossing the futility boundary prespecified in the group sequential monitoring plan in conjunction with an increased risk of atrial fibrillation (oral communication, DMC chair Mark Pfeffer, MD, PhD, to executive committee chair Steven E. Nissen, MD, August 2020). The executive steering committee and sponsor accepted this recommendation and terminated the trial on this date, and patients were recommended to stop study medication. End-of-study visits were scheduled for all patients, with the last patient visit completed by May 14, 2020. The executive steering committee and others involved in the conduct of the trial remained blinded to treatment allocation and results until the conclusion of the trial and finalization of the database.
Study drug was stopped as soon as feasible following the termination of the trial. Because the study was terminated during the early phases of the coronavirus disease 2019 (COVID-19) pandemic, the end-of-treatment visit was permitted to be completed by telephone to allow the study to close in a timely and orderly manner, with the least possible effect on study integrity.
Results
Study Population
A total of 33 047 patients were assessed for eligibility; after exclusions, 13 078 patients were enrolled at 675 sites in 22 countries in North America, Europe, South America, Asia, Australia, New Zealand, and South Africa between October 30, 2014, and June 14, 2017, and entered into the primary analysis. The disposition of patients during the study is summarized in Figure 1. At study closure the median patient follow-up was 42.0 months (interquartile range [IQR], 37.5-48.3). Patients were treated with study drug for a median of 38.2 months (IQR, 30.5-44.9).
Vital status was recorded in 99.8% of patients and 96.6% of patients had complete follow-up for assessment of the primary end point. Baseline characteristics of patients at randomization were similar in the 2 treatment groups (Table 1). Patients (mean age, 62.5 years; men, 65%; White race, 82%) demonstrated a high rate of cardiovascular risk factors, including diabetes (70%) and established atherosclerotic disease (56%), in both groups. All patients were treated with statins (50% high-intensity) at randomization. A high rate of use of other evidence-based preventive therapies was observed in both groups.
Table 1. Patient Characteristics and Medication Use in a Trial of Omega-3 Fatty Acids to Reduce Major Adverse Cardiovascular Events.
| No. (%) | ||
|---|---|---|
| Omega-3 CA (n = 6539) | Corn oil (n = 6539) | |
| Age, mean (SD), y | 62.5 (9.0) | 62.5 (9.0) |
| Sex | ||
| Male | 4250 (65.0) | 4260 (65.1) |
| Female | 2289 (35.0) | 2279 (34.9) |
| Body mass index, mean (SD) | 32.2 (5.7) | 32.2 (5.6) |
| Race | ||
| White | 5341 (81.7) | 5382 (82.3) |
| Asian | 698 (10.7) | 657 (10.0) |
| Black | 180 (2.8) | 166 (2.5) |
| Othera | 320 (4.9) | 334 (5.1) |
| Ethnicity: Hispanic or Latino | 264/4647 (5.7) | 268/4675 (5.7) |
| Comorbidities | ||
| Established CVD at baseline | 3638 (55.6) | 3678 (56.2) |
| Coronary disease | 3009 (46.0) | 3026 (46.3) |
| Cerebrovascular disease | 536 (8.2) | 512 (7.8) |
| Peripheral vascular disease | 227 (3.5) | 257 (3.9) |
| Aortic disease | 214 (3.3) | 244 (3.7) |
| Diabetes at baselineb | 4608 (70.5) | 4562 (69.8) |
| Hypertension | 5732 (87.7) | 5688 (87.0) |
| eGFR,c mean (SD), mL/min/1.73 m2 | 77.2 (19.9) | 77.5 (19.7) |
| Medication use | ||
| RAAS blockers | 5315 (81.3) | 5310 (81.2) |
| Antiplatelet agents | 4623 (70.7) | 4700 (71.9) |
| β-Blockers | 4347 (66.5) | 4348 (66.5) |
| High-intensity statin | 3255 (49.8) | 3273 (50.1) |
| Other statin | 3284 (50.2) | 3266 (49.9) |
| Ezetimibe | 234 (3.6) | 245 (3.7) |
Abbreviations: CA, carboxylic acid formulation; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; RAAS, renin-angiotensin aldosterone system.
The “other” category included American Indian or Alaska Native; Native Hawaiian or other Pacific Islander; multiple races; and unknown.
Diabetes on or before the first dose of study medication, defined by patient self-report, chart review, or use of diabetes medications.
Estimated glomerular filtration rate was estimated using the CKD-EPI formula: eGFR = 141 × min(SCr/κ, 1)α × max(SCr /κ, 1) − 1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black]; where k = 0.7 for females or 0.9 for males and α = −0.329 for females or −0.411 for males.
Clinical End Points
At the completion of the study, 1580 patients had experienced an adjudicated first primary end point event. The primary end point of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization occurred in 785 patients (12.0%) treated with omega-3 CA and 795 (12.2%) treated with corn oil (HR, 0.99 [95% CI, 0.90-1.09]; P = .84) (Table 2, Figure 2).
Table 2. Incidence of Adjudicated Clinical Events in a Trial of Omega-3 Fatty Acids to Reduce Major Adverse Cardiovascular Eventsa.
| Omega-3 CA (n = 6539) | Corn oil (n = 6539) | Omega 3 CA vs corn oil | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-years | IR per 100 person-years | No. of patients with events (% of total) | Person-years | IR per 100 person-years | No. of patients with events (% of total) | Difference in IR (95% CI) | HR (95% CI) | P value | |||
| Primary end point | |||||||||||
| MACEb | 21 908 | 3.58 | 785 (12.0) | 21 920 | 3.63 | 795 (12.2) | −0.04 (−0.40 to 0.31) | 0.99 (0.90-1.09) | .84 | ||
| Components of the composite outcome | |||||||||||
| Cardiovascular death | 23 500 | 0.97 | 228 (3.5) | 23 575 | 0.90 | 211 (3.2) | 0.07 (−0.10 to 0.25) | 1.09 (0.90-1.31) | .37 | ||
| Nonfatal MI | 22 650 | 0.96 | 218 (3.3) | 22 725 | 0.99 | 226 (3.5) | −0.03 (−0.21 to 0.15) | 0.97 (0.81-1.17) | .77 | ||
| Nonfatal stroke | 22 786 | 0.62 | 142 (2.2) | 22 871 | 0.55 | 125 (1.9) | 0.08 (−0.06 to 0.22) | 1.14 (0.90-1.45) | .28 | ||
| Revascularization | 22 236 | 1.86 | 414 (6.3) | 22 270 | 1.98 | 441 (6.7) | −0.12 (−0.38 to 0.14) | 0.94 (0.83-1.08) | .41 | ||
| Hospitalization for unstable angina | 22 854 | 0.38 | 87 (1.3) | 22 895 | 0.45 | 104 (1.6) | −0.07 (−0.19 to 0.04) | 0.84 (0.63-1.12) | .23 | ||
| Secondary end pointsc | |||||||||||
| MACE in patients with established CVD at baseline | 11 695 | 4.87 | 569 (15.6) | 11 751 | 5.19 | 610 (16.6) | −0.32 (−0.90 to 0.25) | 0.94 (0.84-1.05) | .27 | ||
| Cardiovascular eventsd | 22 425 | 2.41 | 541 (8.3) | 22 507 | 2.30 | 517 (7.9) | 0.11 (−0.17 to 0.40) | 1.05 (0.93-1.19) | .40 | ||
| Cardiovascular events in patients with established CVD at baseline | 12 091 | 3.17 | 383 (10.5) | 12 223 | 3.15 | 385 (10.5) | 0.02 (−0.43 to 0.46) | 1.01 (0.87-1.16) | .94 | ||
| Coronary eventse | 22 121 | 2.51 | 556 (8.5) | 22 127 | 2.78 | 616 (9.4) | −0.27 (−0.57 to 0.03) | 0.91 (0.81-1.02) | .09 | ||
| Coronary events in patients with established CVD at baseline | 11 826 | 3.53 | 417 (11.5) | 11 892 | 4.15 | 493 (13.4) | −0.62 (−1.12 to −0.12) | 0.85 (0.75-0.97) | .02 | ||
| Cardiovascular death in patients with established CVD at baseline | 12 722 | 1.19 | 152 (4.2) | 12 927 | 1.07 | 138 (3.8) | 0.13 (−0.13 to 0.39) | 1.12 (0.89-1.41) | .34 | ||
| All-cause death | 23 500 | 1.59 | 373 (5.7) | 23 575 | 1.41 | 333 (5.1) | 0.17 (−0.05 to 0.40) | 1.13 (0.97-1.31) | .11 | ||
| All-cause death in patients with established CVD at baseline | 12 722 | 1.84 | 234 (6.4) | 12 927 | 1.56 | 202 (5.5) | 0.28 (−0.04 to 0.60) | 1.18 (0.97-1.42) | .09 | ||
| Tertiary end points | |||||||||||
| Heart failure event: hospitalization or urgent outpatient visit for heart failure | 22 830 | 0.62 | 142 (2.2) | 22 899 | 0.56 | 128 (2.0) | 0.06 (−0.01 to 0.20) | 1.12 (0.88-1.42) | .35 | ||
| Atrial fibrillationf | 22 740 | 0.63 | 144 (2.2) | 22 916 | 0.38 | 86 (1.3) | 0.26 (0.13 to 0.39) | 1.69 (1.29-2.21) | <.001 | ||
| Stent thrombosisf | 23 009 | 0.05 | 12 (0.18) | 23 063 | 0.07 | 17 (0.26) | −0.02 (−0.07 to 0.02) | 0.71 (0.34-1.48) | .36 | ||
| Venous thromboembolism or pulmonary embolismf | 22 987 | 0.12 | 27 (0.41) | 23 061 | 0.07 | 17 (0.26) | 0.04 (−0.01 to 0.10) | 1.62 (0.88-2.97) | .12 | ||
Abbreviations: CA, carboxylic acid formulation; CVD, cardiovascular disease; IR, incidence rate; MACE, major adverse cardiovascular events; MI, myocardial infarction.
P values were generated from the Wald test using a Cox proportional hazards model containing factors for randomized treatment group, established cardiovascular disease at baseline, and region. Estimates for the subgroup of patients with established cardiovascular disease at baseline adjusted only for treatment group and region. The proportionality assumptions were met for all adjudicated end points.
MACE: first occurrence of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, emergent/elective coronary revascularization, and hospitalization for unstable angina.
The statistical analysis plan (Supplement 1) prespecified that a hierarchical testing strategy was to be used and that once an end point was not statistically significant at α = .05 all subsequent comparisons would be considered exploratory and nominal P values reported.
Cardiovascular event: defined as the first occurrence of any of the components of cardiovascular events, including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
Coronary events: defined as the first occurrence of any of the components of coronary events, including cardiac death, nonfatal myocardial infarction, emergent/elective coronary revascularization, or hospitalization for unstable angina.
Not adjudicated.
Figure 2. Time to First Incidence of Any Component of the Primary Composite End Point and Time to Core MACE.
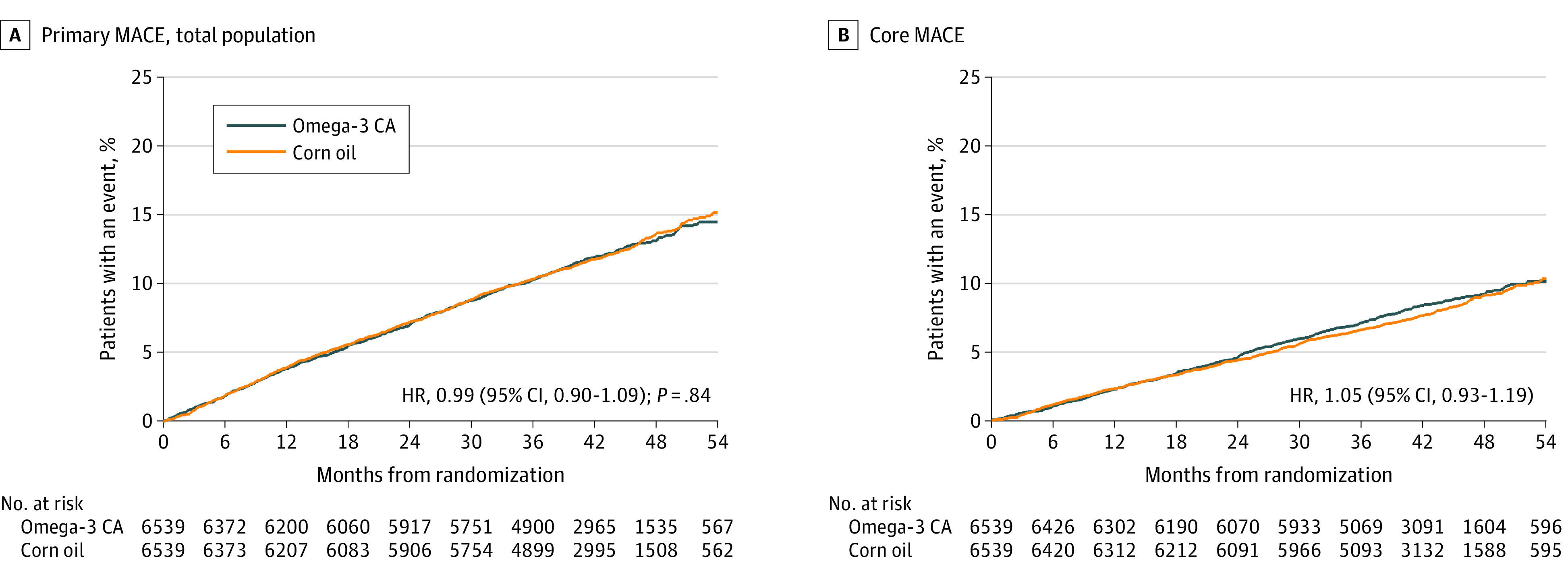
A, The primary composite end point consisted of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and hospitalization for unstable angina. Median (Q1-Q3) observation time was 41.3 (36.0-47.5) months for patients receiving omega-3 CA and 41.4 (35.9-47.4) months for patients receiving corn oil. B, Core major adverse cardiovascular events (MACE) included cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. Median (Q1-Q3) observation time of 41.5 (36.6-47.8) months for patients receiving omega-3 CA and 41.6 (36.8-47.4) months for patients receiving corn oil.
Similarly, the secondary end point of cardiovascular death, myocardial infarction, or stroke occurred in 541 patients (8.3%) treated with omega-3 CA and 517 (7.9%) treated with corn oil (HR, 1.05 [95% CI, 0.93-1.19]; nominal P = .40). An additional secondary end point—cardiac death, myocardial infarction, coronary revascularization, or hospitalization for unstable angina—occurred in 556 patients (8.5%) treated with omega-3 CA and 616 (9.4%) treated with corn oil (HR, 0.91 [95% CI, 0.81-1.02]; nominal P = .09).
There were no significant differences between the treatment groups with regard to the risk of individual components of the primary end point (Table 2). Survival curves for the primary end point in patients with and without established cardiovascular disease are shown in eFigure 1 in Supplement 3.
Prespecified subgroup analyses (Figure 3) revealed an HR for the primary end point of 0.94 (95% CI, 0.84-1.05) in the secondary prevention population and 1.16 (95% CI, 0.95-1.41) in the primary prevention population, with a nominal interaction P value for these 2 subgroups of .07. There were numerically fewer cardiovascular events in the omega-3 CA group among patients treated with ezetimibe (nominal interaction P = .008). There was a nominally significant reduction in the risk of cardiac death, myocardial infarction, coronary revascularization, and hospitalization for unstable angina in patients with established cardiovascular disease at baseline, although this finding was unadjusted for multiplicity (Table 2). All-cause mortality occurred in 373 patients (5.7%) in the omega-3 CA group and 333 (5.1%) in the corn oil group (nominal P = .11).
Figure 3. Effect of Omega-3 CA on the Primary Composite Cardiovascular End Point in Prespecified Subgroups.
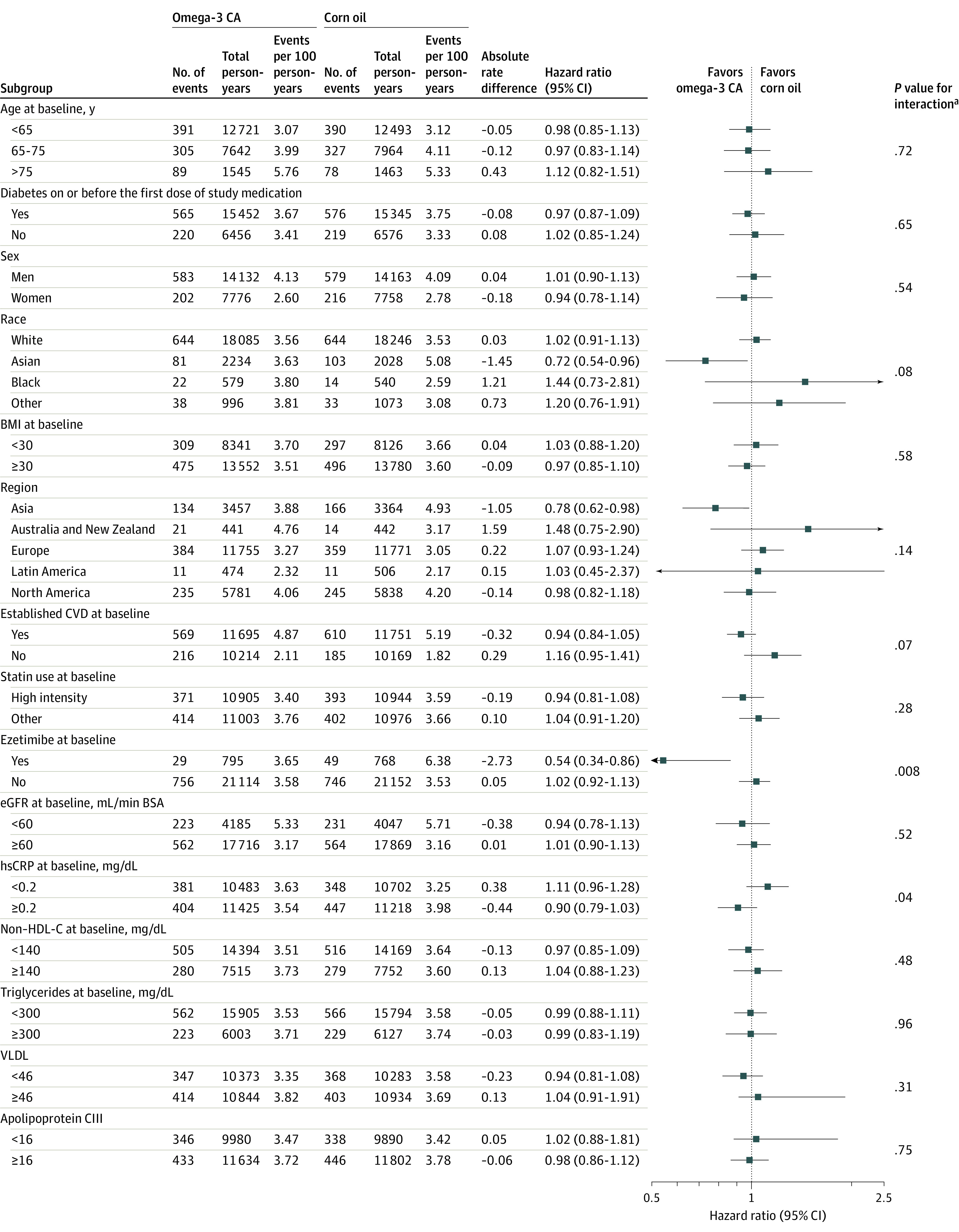
BSA, body surface area; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; and VLDL, very low-density lipoprotein. SI conversion factors are in Table 4.
aP value estimated using a Cox proportional hazards model with factors for treatment, established cardiovascular status at baseline, region, subgroup (only if not one of the covariates), and treatment × subgroup interaction in the model.
With regard to prespecified tertiary end points, an increased rate of investigator-reported new-onset atrial fibrillation was observed in the omega-3 CA group (2.2% vs 1.3%; HR, 1.69 [95% CI, 1.29-2.21]; nominal P < .001) compared with corn oil (number needed to harm, 114) (eFigure 2 in Supplement 3). There were no significant differences between the groups with regard to new-onset heart failure (2.2% vs 2.0%; HR, 1.12 [95% CI, 0.88-1.42]; nominal P = .35) or venous thromboembolic events (0.41% vs 0.26%; HR, 1.62 [95% CI, 0.88-2.97]; nominal P = .12).
In a post hoc exploratory analysis, no association was observed between either plasma or red blood cell EPA or DHA concentrations after 12 months of treatment and subsequent cardiovascular event rates (Table 3).
Table 3. Primary Event Rate by Tertile of Percent Change in Fatty Acid From Baseline to Month 12a.
| Tertile 1b | Tertile 2 | Tertile 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| No./No. (%) | Person-years | No./No. (%) | Person-years | HR (95% CI) | No./No. (%) | Person-years | HR (95% CI) | |
| Plasma EPA | ||||||||
| Omega-3 CA (<144%, 144% to 435%, >435%) | 180/1725 (10.4) | 5933 | 191/1725 (11.1) | 6035 | 1.04 (0.85-1.28) | 204/1725 (11.8) | 5951 | 1.13 (0.92-1.38) |
| Corn oil (<−28%, −28% to 10%, >10%) | 195/1735 (11.2) | 6005 | 194/1736 (11.2) | 6059 | 0.99 (0.81-1.20) | 185/1736 (10.7) | 5956 | 0.96 (0.78-1.17) |
| RBC EPA | ||||||||
| Omega-3 CA (<117%, 117% to 450%, >450%) | 185/1716 (10.8) | 5887 | 182/1717 (10.6) | 6011 | 0.96 (0.78-1.18) | 202/1716 (11.8) | 5945 | 1.08 (0.89-1.32) |
| Corn oil (<−20%, −20% to 3%, >3%) | 193/1728 (11.2) | 5980 | 194/1728 (11.2) | 6015 | 0.99 (0.82-1.22) | 186/1729 (10.8) | 5945 | 0.97 (0.79-1.19) |
| Plasma DHA | ||||||||
| Omega-3 CA (<16%, 16%-68%, >68%) | 178/1725 (10.3) | 5992 | 192/1725 (11.1) | 5989 | 1.08 (0.88-1.32) | 205/1725 (11.9) | 5937 | 1.16 (0.95-1.42) |
| Corn oil (<−17%, −17% to 6%, >6%) | 198/1735 (11.4) | 6067 | 187/1736 (10.8) | 6052 | 0.95 (0.78-1.16) | 189/1736 (10.9) | 5901 | 0.98 (0.80-1.20) |
| RBC DHA | ||||||||
| Omega-3 CA (<12%, 12%-41%, >41%) | 186/1716 (10.8) | 5960 | 189/1717 (11.0) | 5949 | 1.02 (0.83-1.25) | 194/1716 (11.3) | 5935 | 1.05 (0.86-1.28) |
| Corn oil (<−9%, −9% to 3%, >3%) | 191/1728 (11.1) | 6024 | 191/1729 (11.1) | 6052 | 1.00 (0.82-1.22) | 191/1728 (11.1) | 5864 | 1.03 (0.84-1.26) |
Abbreviations: CA, carboxylic acid formulation; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HR, hazard ratio; RBC, red blood cell.
Primary cardiovascular end point rate and hazard ratio estimated using a Cox proportional hazards regression model in patients treated with omega-3 CA and corn oil, according to tertiles of percentage change in either plasma or red blood cell EPA and DHA concentrations in a post hoc exploratory analysis. Tertile range for percentage change provided for individual fatty acid species.
The first tertile is the reference category.
Biochemical Parameters
Prespecified exploratory biochemical parameters at baseline, follow-up, and their percentage change during the course of the study are summarized in Table 4. At randomization, median levels of LDL cholesterol were 75 mg/dL; HDL cholesterol, 36 mg/dL; triglycerides, 240 mg/dL; and hs-CRP, 2.1 mg/L. During the course of the study, greater reductions in triglycerides (−19.0% vs −0.9%; geometric mean ratio [GMR], 0.82 [95% CI, 0.81-0.83]; P < .001), non-HDL cholesterol (−6.1% vs −1.1%; GMR, 0.95 [95% CI, 0.94-0.96]; P < .001), and hs-CRP (−20.0% vs −6.3%; GMR, 0.89 [95% CI, 0.84-0.95]; P < .001) were observed in the omega-3 CA treatment group compared with corn oil group, respectively.
Table 4. Baseline, Follow-up, and Percentage Change in Biochemical Measures.
| Median (Q1-Q3) | Between groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Omega-3 CA | Corn oil | |||||||
| Baseline (n = 6539) | 12-mo Follow-up (n = 5821)a | % Change (n = 5821) | Baseline (n = 6539) | 12-mo Follow-up (n = 5907)a | % Change (n = 5907) | Geometric mean ratiob | P valueb | |
| Total cholesterol, mg/dL | 160.0 (139.0 to 188.0) | 154.0 (131.0 to 185.0) | −3.4 (−14.6 to 9.0) | 160.0 (138.0 to 188.0) | 161.0 (137.0 to 191.0) | 0 (−10.9 to 12.5) | 0.97 (0.96 to 0.97) | <.001 |
| LDL cholesterol, mg/dL | 75.0 (56.0 to 99.0) | 76.0 (56.0 to 102.0) | 1.2 (−18.2 to 25.7) | 75.0 (56.0 to 99.0) | 75.0 (55.0 to 100.0) | −1.1 (−19.7 to 21.8) | 1.03 (1.01 to 1.04) | <.001 |
| HDL cholesterol, mg/dL | 36.0 (31.0 to 40.0) | 37.0 (32.0 to 43.0) | 5.0 (−4.9 to 15.8) | 36.0 (31.0 to 40.0) | 37.0 (32.0 to 42.0) | 3.2 (−5.7 to 14.3) | 1.01 (1.00 to 1.02) | .002 |
| Triglycerides, mg/dL | 239.0 (192.0 to 307.0) | 191.0 (146.0 to 255.0) | −19.0 (−39.2 to 6.4) | 240.0 (191.0 to 309.0) | 235.0 (178.0 to 315.0) | −0.9 (−25.2 to 27.8) | 0.82 (0.81 to 0.83) | <.001 |
| Non-HDL cholesterol, mg/dL | 125.0 (104.0 to 152.0) | 116.0 (94.0 to 146.0) | −6.1 (−20.3 to 9.6) | 125.0 (103.0 to 152.0) | 123.0 (100.0 to 152.0) | −1.1 (−14.9 to 14.5) | 0.95 (0.94 to 0.96) | <.001 |
| Apolipoprotein B, mg/dL | 56.2 (43.8 to 72.3) | 54.9 (43.8 to 69.7) | −2.0 (−24.5 to 27.6) | 55.6 (43.6 to 71.7) | 55.3 (44.3 to 69.4) | −1.0 (−23.5 to 27.1) | 0.99 (0.98 to 1.01) | .34 |
| Apolipoprotein CIII, mg/dL | 17.0 (14.0 to 21.0) | 16.0 (13.0 to 20.0) | −7.0 (−25.0 to 15.0) | 17.0 (14.0 to 21.0) | 18.0 (14.0 to 23.0) | 5.9 (−14.3 to 30.0) | 0.88 (0.87 to 0.89) | <.001 |
| hs-CRP, mg/La | 2.1 (1.1 to 4.2) | 1.7 (0.8 to 3.6) | −20.0 (−53.2 to 36.5) | 2.1 (1.1 to 4.2) | 1.8 (0.9 to 4.0) | −6.3 (−45.3 to 55.9) | 0.89 (0.84 to 0.95) | <.001 |
| EPA, μg/mL | ||||||||
| Plasma | 21.0 (12.7 to 33.9) | 89.6 (46.7 to 131.5) | 268.8 (85.7 to 549.1) | 21.3 (13.3 to 33.7) | 19.0 (11.6 to 30.7) | −10.5 (−36.9 to 26.3) | 3.75 (3.65 to 3.86) | <.001 |
| RBC | 0.60 (0.39 to 0.96) | 2.81 (1.50 to 3.96) | 298.6 (112.9 to 558.0) | 0.61 (0.40 to 0.95) | 0.55 (0.36 to 0.86) | −8.7 (−26.2 to 11.1) | 4.02 (3.92 to 4.12) | <.001 |
| DHA, μg/mL | ||||||||
| Plasma | 61.9 (46.3 to 83.8) | 90.7 (71.4 to 114.0) | 39.7 (5.4 to 86.1) | 62.5 (46.9 to 84.4) | 58.1 (43.4 to 79.9) | −6.9 (−23.7 to 13.8) | 1.50 (1.48 to 1.52) | <.001 |
| RBC | 5.0 (3.9 to 6.2) | 6.6 (5.7 to 7.3) | 23.9 (5.7 to 52.0) | 5.0 (3.9 to 6.1) | 4.8 (3.8 to 6.0) | −3.3 (−11.9 to 6.3) | 1.33 (1.32 to 1.34) | <.001 |
Abbreviations: CA, carboxylic acid formulation; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
SI conversion factors: To convert cholesterol to mmol/L, multiply values by 0.0259; to convert triglycerides to mmol/L, multiply values by 0.0113.
All follow-up measures were at 12 months, except for hs-CRP, which was measured at 60 months (n = 1499 in corn oil placebo and n = 1467 in omega-3 CA).
Geometric mean ratios >1.0 represent an x-fold increase in omega-3 CA compared with corn oil, while values <1.0 represent an x-fold decrease. P values were generated from the analysis of covariance model.
LDL cholesterol levels increased in the omega-3 CA group but not in the corn oil group (1.2% vs −1.1%; GMR, 1.03 [95% CI, 1.01-1.04]; P < .001), while greater increases in HDL cholesterol were observed in the omega-3 CA group (5.0% vs 3.2%; GMR, 1.01 [95% CI, 1.00-1.02]; P = .002). Apolipoprotein CIII levels decreased in the omega-3 CA group but not in the corn oil group (−7.0% vs 5.9%; GMR, 0.88 [95% CI, 0.87-0.89]; P < .001). In contrast, no significant difference was observed with regard to percentage change in apolipoprotein B levels (−2.0% vs −1.0%; GMR, 0.99 [95% CI, 0.98-1.01]; P = .34) between the omega-3 and corn oil treatment groups, respectively.
Administration of omega-3 CA resulted in greater increases in concentrations of EPA as compared with corn oil (Table 4). Concentrations of DHA in plasma and in red blood cells were also increased by omega-3 CA administration, compared with corn oil (Table 4).
Adverse Events
The number of adverse events and serious adverse events are summarized in Table 5. Drug-related adverse events were more commonly observed in the omega-3 CA group than the comparator group (22.2% vs 12.9%, respectively). Discontinuation of study drug treatment (10.8% vs 8.0%) and dose reduction (12.0% vs 6.1%) for adverse events occurred more frequently in patients treated with omega-3 CA compared with those treated with corn oil. There were more gastrointestinal adverse events in the omega-3 CA group (24.7%) compared with corn oil–treated patients (14.7%).
Table 5. Key Adverse Events in the Safety Populationa.
| No. (%) | ||
|---|---|---|
| Omega-3 CA (n = 6532) | Corn oil (n = 6535) | |
| Drug-related adverse event | 1451 (22.2) | 843 (12.9) |
| Adverse event leading to drug discontinuation | 708 (10.8) | 525 (8.0) |
| Gastrointestinal disordersb | 1616 (24.7) | 959 (14.7) |
| Diarrhea | 780 (11.9) | 323 (4.9) |
| Nausea | 207 (3.2) | 113 (1.7) |
| Dyspepsia | 90 (1.4) | 42 (0.6) |
| Abdominal discomfort | 87 (1.3) | 36 (0.6) |
| New onset of diabetesc | 286/1929 (14.8) | 280/1975 (14.2) |
| Syncope | 35 (0.5) | 17 (0.3) |
| Any bleeding event | 322 (4.9) | 322 (4.9) |
| TIMI criteria major bleeding event | 52 (0.8) | 46 (0.7) |
Abbreviations: CA, carboxylic acid formulation; TIMI, Thrombolysis in Myocardial Infarction.
The safety population includes all randomized patients who received at least one dose of study drug.
Gastrointestinal disorders reported by the patient.
In those without diabetes on or before first dose of study medication.
Discussion
In this randomized clinical trial, administration of omega-3 CA did not result in a significant reduction in the composite end point of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or hospitalization for unstable angina compared with use of corn oil. The findings of this trial contribute to a large body of clinical research that has investigated whether administration of omega-3 fatty acids has a role in the prevention of cardiovascular disease. The origins of this research were based on observations that dietary consumption of fatty fish or omega-3 fatty acids were associated with lower rates of incident cardiovascular events in large cohort studies.1,2,19,20 The potential value of omega-3 fatty acids was supported by epidemiological studies demonstrating an inverse relationship between circulating concentrations of omega-3 fatty acids and cardiovascular risk.3,4 Preclinical studies demonstrated favorable effects of EPA and DHA on lipoprotein metabolism and a range of other biological factors implicated in atherosclerosis,5 but several large clinical trials failed to demonstrate any cardiovascular benefit with administration of low doses of omega-3 fatty acids.8,12,13 Despite these findings, over-the-counter use of low-dose omega-3 fatty acids is widespread.21,22
Two large clinical trials have suggested potential benefit of purified formulations of EPA alone. The Japan EPA Lipid Intervention Study (JELIS), an open-label trial that administered EPA, 1.8 g/d, in combination with a statin for a median of 4.6 years in 18 645 Japanese patients with hypercholesterolemia, resulted in fewer major coronary events compared with statin therapy alone (2.8% vs 3.5%; HR, 0.81 [95% CI, 0.69-0.95]).10 The JELIS trial was not conducted using contemporary standards of care: patients were enrolled with mean LDL-C levels of 180 mg/dL, but treated with very low doses of statins (pravastatin 10 mg or simvastatin 5 mg), and elective revascularization was included in a broad composite clinical end point.
The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) trial reported that administration of EPA, 4 g/d, compared with mineral oil for a median duration of 4.9 years in 8179 statin-treated patients with a fasting triglyceride level between 135 and 499 mg/dL (median, 216 mg/dL) resulted in fewer cardiovascular events (17.2% vs 22.0%; HR, 0.75 [95% CI, 0.68-0.83]).11 Concerns emerged in the scientific community and during hearings by the US Food and Drug Administration (FDA) whether mineral oil represented a neutral comparator, particularly in the context of a greater than 30% increase in CRP in the mineral oil treatment group.23 Additional analyses of both EPA studies suggested an inverse association between plasma EPA concentration during treatment and the rate of cardiovascular events.24,25
The current trial similarly administered a 4-g dose of omega-3 fatty acids in high-risk patients with evidence of atherogenic dyslipidemia treated with a statin. In contrast to the trials of purified EPA, this trial administered an omega-3 CA formulation composed of both EPA and DHA. While the administered EPA content of omega-3 CA was less than that dispensed with icosapent, the carboxylic acid formulation has greater bioavailability, permitting substantial elevations in EPA concentrations, confirmed in phase 2 studies.14,15 Although the achieved EPA levels in plasma and red blood cells were higher with icosapent in REDUCE-IT compared with this trial,11 it is uncertain whether these differences would be sufficient to explain the completely different results observed. This uncertainty is heightened by the observation of no significant reduction in the risk of cardiovascular events in those patients with greater, compared with those with lesser, increases in EPA levels in the current trial. Furthermore, triglyceride levels were reduced 18% in both trials after 12 months, which also suggests similar biochemical effects of these treatments. It remains unknown whether administration of omega-3 fatty acids in a carboxylic acid formulation, as opposed to an ethyl ester, might have differential cardiovascular effects.
This trial was stopped prematurely when it became apparent that the probability of clinical benefit was likely to be low and there was evidence of risk, including a higher, albeit small, incidence of investigator-reported atrial fibrillation in the omega-3 CA treatment group. A number of potential factors may have contributed to the differences in outcomes of these clinical trials. While the duration of follow-up was longer in both studies of purified EPA, there was no separation of event curves in this trial in patients treated for a median of more than 3 years (Figure 2). There were differences in the patient populations, with this trial recruiting a greater percentage of patients with diabetes and somewhat fewer with clinically manifest cardiovascular disease. Although the study was terminated prematurely, the number of adjudicated primary end point events was consistent with the original sample size assumptions (1580 vs 1600 events), reducing concerns that the trial may have been underpowered. A high level of follow-up of patients was achieved despite the challenges imposed by closing a large, multinational clinical trial during the COVID-19 pandemic.
A possible explanation for the different outcomes relates to the comparators used. The decision was made with the design of this trial to administer corn oil because it was considered to be a neutral comparator with the least effects on a range of biochemical parameters associated with cardiovascular risk.17,18 In contrast, the cardiovascular effects of icosapent were compared with mineral oil, with adverse effects, compared with baseline, on apolipoprotein B, LDL cholesterol, and hs-CRP levels.11 These effects were not observed with the corn oil group in this trial, highlighting differences between the comparator used in the studies. Given that these parameters are well-established risk factors associated with differences in cardiovascular event rates in clinical trials,26,27,28 the adverse biochemical effects in the mineral oil group may have contributed to the apparent cardiovascular benefit observed with icosapent. However, the FDA subsequently awarded a label claim for cardiovascular event reduction for icosapent ethyl based on analyses that concluded that the effects of mineral oil could not entirely explain the observed differences in outcome.
The omega-3 fatty acid formulations differed in terms of their composition. While cardiovascular benefit has been reported with administration of purified formulations of EPA, omega-3 CA is a combination of EPA and DHA, with the potential to achieve similar tissue EPA concentrations. Theoretically, the lack of cardiovascular benefit with omega-3 CA could reflect adverse effects from coadministration of DHA. Although preclinical studies have reported potentially differential biological effects of EPA and DHA in studies of endothelial cells and vascular reactivity,29,30,31 DHA has not demonstrated an adverse effect on atherosclerosis32,33 and DHA levels have been reported to associate with cardiovascular protection.34 Furthermore, while the increases in plasma and red blood cell concentrations of EPA were substantial, the percentage increases in DHA concentrations were modest (Table 2) and did not correlate with event rates (Table 3). Accordingly, it seems unlikely that the DHA component of the omega-3 CA formulation caused harm.
Administration of omega-3 CA was associated with a greater rate of both gastrointestinal adverse events and study drug discontinuation (Table 5). Investigator-reported new-onset atrial fibrillation was more common in patients receiving omega-3 CA, a finding also reported with purified EPA administration in REDUCE-IT (5.3% vs 3.9% with icosapent vs mineral oil).11 These are potentially important findings that must be considered in the context of the possibility that the observed benefit of purified EPA may have been related to an increase in event rates in the mineral oil placebo treatment group. Accordingly, there is some uncertainty whether there is net benefit or harm with administration of any omega-3 fatty acid formulation. Given that 2 large clinical trials have now demonstrated a higher incidence rate, albeit small, of atrial fibrillation with high-dose omega-3 fatty acid administration, the mechanisms underscoring this observation require additional investigation. In contrast, it was reassuring to observe no excess bleeding with omega-3 CA, despite the high rate of use of background antiplatelet agents in the study.
Limitations
This study has several limitations. First, all patients were at high risk of future cardiovascular events, and background statin therapy was required. Whether benefits might be observed in a lower-risk primary prevention population remains uncertain. Second, this trial evaluated the effect of administration of 4-g/d of a combination of EPA and DHA in fixed proportion. While different doses and proportions were not evaluated, elevations in plasma concentrations of both EPA and DHA were achieved, yet no cardiovascular benefit was observed. Third, no large clinical trial has evaluated the effect of purified DHA at any dose on cardiovascular outcomes.
Conclusions
Among statin-treated patients at high cardiovascular risk, the addition of omega-3 CA, compared with corn oil, to usual background therapies resulted in no significant difference in a composite outcome of major adverse cardiovascular events. These findings do not support use of this omega-3 fatty acid formulation to reduce major adverse cardiovascular events in high-risk patients.
Statistical Analysis Plan
Study Protocol
eFigure 1. Kaplan-Meier Curves for Primary Endpoint MACE in patients with and without established CVD
eFigure 2. Kaplan-Meier Curves for Investigator Reported Atrial Fibrillation
eMethods.
Listing of All Committees, DSMB, and Investigators
Data Sharing Statement
References
- 1.Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) . ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176(8):1155-1166. doi: 10.1001/jamainternmed.2016.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayedi A, Shab-Bidar S. Fish consumption and the risk of chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Adv Nutr. 2020;11(5):1123-1133. doi: 10.1093/advances/nmaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Lemaitre RN, King IB, et al. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med. 2013;158(7):515-525. doi: 10.7326/0003-4819-158-7-201304020-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virtanen JK, Laukkanen JA, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids, mercury, and risk of sudden cardiac death in men: a prospective population-based study. PLoS One. 2012;7(7):e41046. doi: 10.1371/journal.pone.0041046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54(7):585-594. doi: 10.1016/j.jacc.2009.02.084 [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047-2067. doi: 10.1016/j.jacc.2011.06.063 [DOI] [PubMed] [Google Scholar]
- 7.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354(9177):447-455. doi: 10.1016/S0140-6736(99)07072-5 [DOI] [PubMed] [Google Scholar]
- 8.Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration . Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225-234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch J, Gerstein HC, Dagenais GR, et al. ; ORIGIN Trial Investigators . n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309-318. doi: 10.1056/NEJMoa1203859 [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Yokoyama M, Origasa H, et al. ; JELIS Investigators, Japan . Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200(1):135-140. doi: 10.1016/j.atherosclerosis.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 12.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group . Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540-1550. doi: 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastelein JJ, Maki KC, Susekov A, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8(1):94-106. doi: 10.1016/j.jacl.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Maki KC, Orloff DG, Nicholls SJ, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther. 2013;35(9):1400-11.e1, 3. doi: 10.1016/j.clinthera.2013.07.420 [DOI] [PubMed] [Google Scholar]
- 16.Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41(10):1281-1288. doi: 10.1002/clc.23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degirolamo C, Rudel LL. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr Atheroscler Rep. 2010;12(6):391-396. doi: 10.1007/s11883-010-0133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. doi: 10.1371/journal.pmed.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33(12):2657-2661. doi: 10.1093/ajcn/33.12.2657 [DOI] [PubMed] [Google Scholar]
- 20.Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200(2):177-182. doi: 10.3181/00379727-200-43413 [DOI] [PubMed] [Google Scholar]
- 21.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015;(79):1-16. [PMC free article] [PubMed] [Google Scholar]
- 22.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016;316(14):1464-1474. doi: 10.1001/jama.2016.14403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FDA briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting. November 14, 2019. Accessed October 28, 2020. https://www.fda.gov/media/132477/download
- 24.Itakura H, Yokoyama M, Matsuzaki M, et al. ; JELIS Investigators . Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18(2):99-107. doi: 10.5551/jat.5876 [DOI] [PubMed] [Google Scholar]
- 25.Reduction of cardiovascular events with icosapent ethyl-intervention trial (REDUCE-IT). American College of Cardiology. June 15, 2020. Accessed August 2, 2020. https://www.acc.org/latest-in-cardiology/clinical-trials/2018/11/08/22/48/reduce-it
- 26.Mihaylova B, Emberson J, Blackwell L, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590. doi: 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thanassoulis G, Williams K, Ye K, et al. Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: a meta-analysis of randomized trials. J Am Heart Assoc. 2014;3(2):e000759. doi: 10.1161/JAHA.113.000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Cannon CP, Morrow D, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators . C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20-28. doi: 10.1056/NEJMoa042378 [DOI] [PubMed] [Google Scholar]
- 29.Lee SA, Kim HJ, Chang KC, et al. DHA and EPA down-regulate COX-2 expression through suppression of NF-kappaB activity in LPS-treated human umbilical vein endothelial cells. Korean J Physiol Pharmacol. 2009;13(4):301-307. doi: 10.4196/kjpp.2009.13.4.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Li D, Chen J, Roberts GJ, Saldeen T, Mehta JL. EPA and DHA attenuate ox-LDL-induced expression of adhesion molecules in human coronary artery endothelial cells via protein kinase B pathway. J Mol Cell Cardiol. 2003;35(7):769-775. doi: 10.1016/S0022-2828(03)00120-2 [DOI] [PubMed] [Google Scholar]
- 31.Limbu R, Cottrell GS, McNeish AJ. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS One. 2018;13(2):e0192484. doi: 10.1371/journal.pone.0192484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Hu Q, Wu H, et al. Protective role of n6/n3 PUFA supplementation with varying DHA/EPA ratios against atherosclerosis in mice. J Nutr Biochem. 2016;32:171-180. doi: 10.1016/j.jnutbio.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142(3):614S-625S. doi: 10.3945/jn.111.149633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39(1):212-220. doi: 10.1016/j.ypmed.2004.02.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Analysis Plan
Study Protocol
eFigure 1. Kaplan-Meier Curves for Primary Endpoint MACE in patients with and without established CVD
eFigure 2. Kaplan-Meier Curves for Investigator Reported Atrial Fibrillation
eMethods.
Listing of All Committees, DSMB, and Investigators
Data Sharing Statement