Important Compound Classes
Title
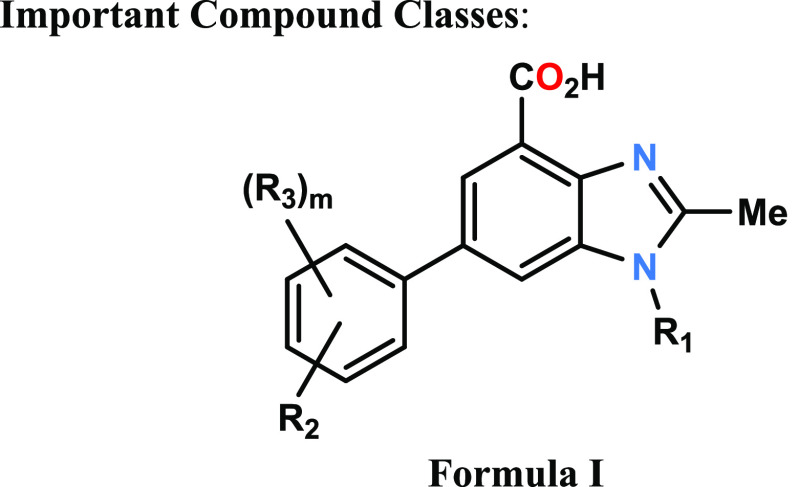
1,4,6-Trisubstituted-2-alkyl-1H-benzo[D]imidazole Derivatives as Dihydroorotate Oxygenase Inhibitors
Patent Application Number
US 2020/0140395 A1
Publication Date
May 7, 2020
Priority Application
IN 201741006586
Priority Date
Feb. 24, 2017
Inventors
Thunuguntla, S. S. R.; Hosahalli, S.; Panigrahi, S. K.; Schwarz, M.; Arlt, M.
Assignee Company
Merck Patent GmbH, Darmstadt (DE)
Disease Area
Autoimmune and inflammatory disorders including multiple sclerosis and rheumatoid arthritis as well as cancer.
Biological Target
Dihydroorotate dehydrogenase (DHODH).
Summary
The invention in this patent application relates to 1,4,6-trisubstituted-2-alkyl-1H-benzo[d]imidazole derivatives represented generally by formula I. These compounds are inhibitors of dihydroorotate dehydrogenase (DHODH) and may potentially be used for the treatment and/or prevention of diseases or disorders associated with the function of DHODH such as autoimmune and inflammatory disorders including multiple sclerosis and rheumatoid arthritis as well as cancer.
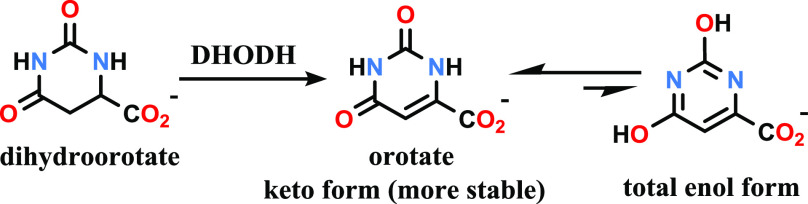
Pyrimidine nucleotides are important to the synthesis of DNA, RNA, and sugar nucleotides. Pyrimidine nucleotides are biosynthesized from l-glutamine, l-aspartic acid, ATP, and bicarbonate via a six-step conserved enzymatic reaction sequence known as the de novo biosynthesis pathway. The fourth step of this synthesis sequence is a rate-limiting oxidation in which dihydroorotate (DHO) is dehydrogenated to orotate (the conjugate base of orotic acid). This oxidation is catalyzed by an essential mitochondrial enzyme known as dihydroorotate oxygenase or dihydroorotate dehydrogenase (DHODH) using the cofactor ubiquinone in the presence of flavin mononucleotide (FMN) as a redox cofactor.
Orotate is 2,6-dioxopyrimidine-4-carboxylate (or uracil-6-carboxylate). This pyrimidine derivative can exist in several tautomeric forms, but the keto form (shown in Scheme 1) is the more stable tautomer.
Scheme 1. Enzymatic Conversion of Dihydroorotate to Orotate.
The enzyme DHODH is expressed in every cell but not known to be overexpressed or mutate in cancer cells,; thus, it was not initially regarded as a therapeutic target for treating cancer. However, studies have shown that the de novo biosynthesis of pyrimidine nucleotides is activated in response to activation of oncogenic pathways such as PI3K, PTEN, and mTOR in proliferating cancer cells. In order for these cells to sustain proliferation, they require high concentrations of pyrimidine nucleotides for the synthesis of DNA and RNA.
These data suggest that reducing the pyrimidine nucleotide concentration is a potentially effective strategy to slow the proliferation of cancer cells. Therefore, because of the key role of DHODH in the biosynthesis of pyrimidine nucleotides, its inhibition has become a viable therapeutic target for the treatment of cancer and autoimmune diseases such as rheumatoid arthritis.
Preclinical studies have provided evidence to show that inhibition of DHODH prevents the growth of many types of cancers. Moreover, it was demonstrated that inhibition of DHODH induces differentiation of acute myeloid leukemia (AML) cells. Pharmacologic inhibition of de novo pyrimidine nucleotide synthesis sensitizes triple-negative breast cancer cells to genotoxic chemotherapy agents and reduces chemotherapy resistance.
Inhibitors of DHODH block the synthesis of pyrimidine-based nucleotides and are known to potentially possess wide applications as chemotherapeutic agents. Studies have determined that the inhibition of DHODH is a promising target for treating transplant rejection, rheumatoid arthritis, psoriasis, as well as autoimmune diseases. Furthermore, DHODH inhibitors may also be useful in the treatment of viral mediated diseases.
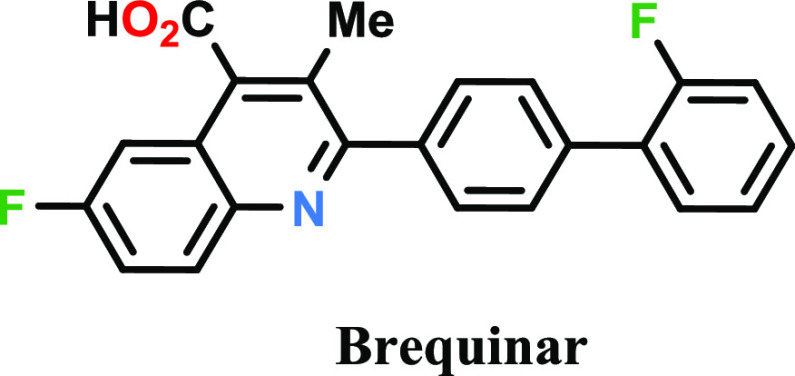
Several known DHODH inhibitors such as brequinar, leflunomide, and teriflunomide have been clinically evaluated but were not approved for use in oncology due to a narrow therapeutic window as a result of lesser potency and/or off-target activities such as their inhibition of kinases.
Brequinar (Figure 1) exhibits anticancer activity toward L1210 murine leukemia and potentiates 5-fluorouracil antitumor activity in a murine model colon 38 tumor by tissue-specific modulation of uridine nucleotide pools.
Figure 1.
Structure of brequinar.
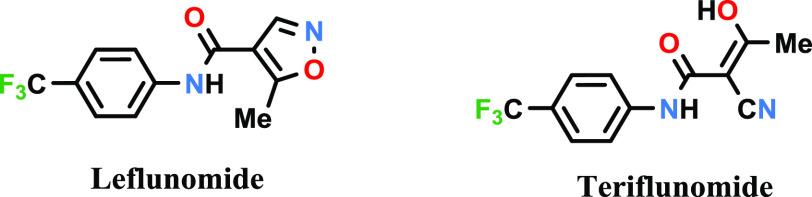
Leflunomide, (Figure 2) has been used for the treatment of rheumatoid arthritis, and it is also under evaluation for use in the treatment of inflammatory bowel disease and chronic allograft rejection. Leflunomide quickly metabolizes in vivo into the active metabolite teriflunomide (Figure 2) through the opening of the isoxazole ring. Teriflunomide was approved in the USA for the treatment of patients with relapsing multiple sclerosis. It is about a 100 times more potent inhibitor of DHODH than leflunomide.
Figure 2.
Structures of leflunomide and teriflunomide.
There is an unmet and crucial need for new effective immunosuppressive therapies to treat the increased number of patients suffering from a wide variety of autoimmune and chronic inflammatory diseases. The compounds of formula I described in this patent application are dihydroorotate dehydrogenase (DHODH) inhibitors and thus may provide useful treatments for autoimmune and inflammatory disorders such as multiple sclerosis, inflammatory bowel disease, rheumatoid arthritis. and other DHODH-associated disorders. These compounds may also potentially be useful for treatment of cancer and leukemia.
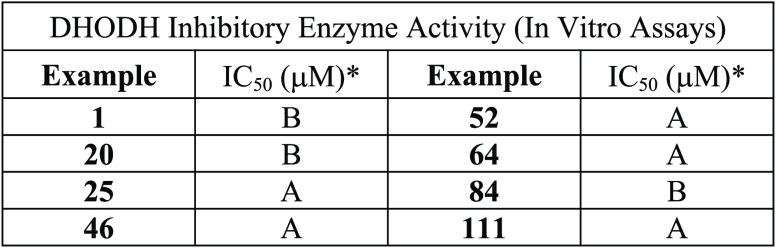
Key Structures
The inventors described
the structures
and methods of synthesis of 156 examples of formula I including the
following representative examples.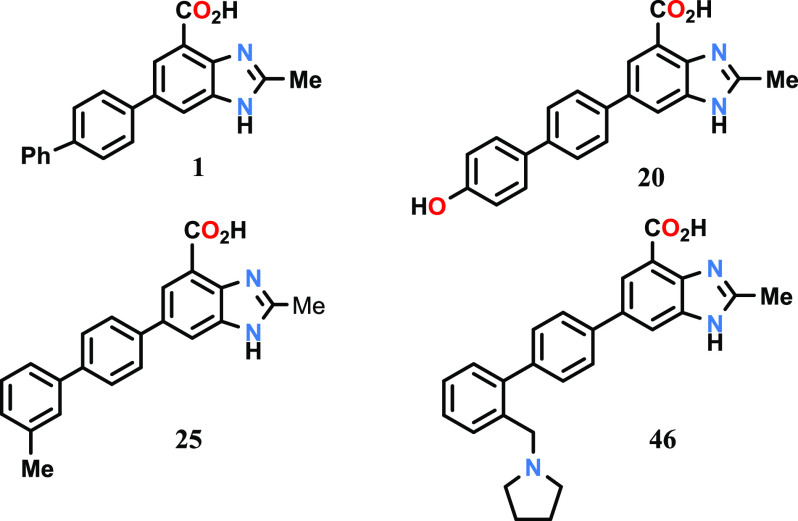
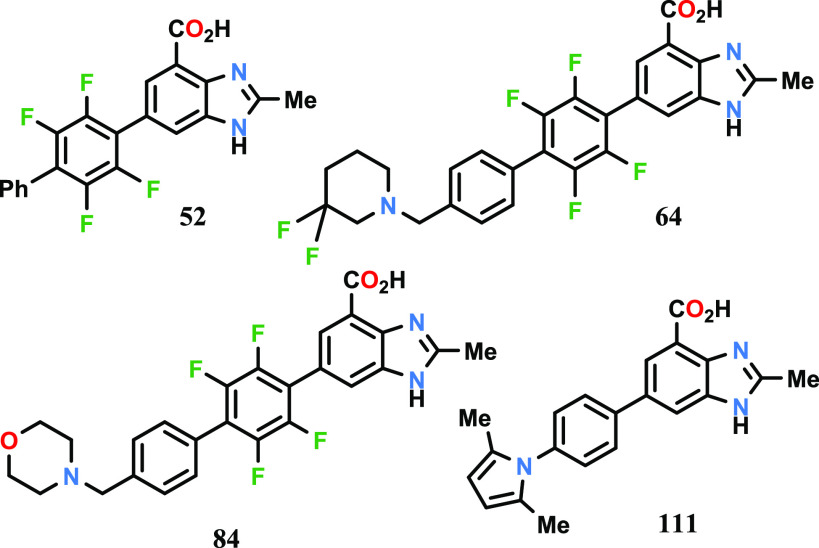
Biological Assay
Measurement of DHODH Inhibitory Enzyme Activity (In Vitro Assay)
Biological Data
The inventors reported the DHODH inhibitory activity data using the above-mentioned assay for 32 compounds of formula I. The IC50 data for the representative examples are listed in the following table.
* A refers to an IC50 value in the range of 0.001 to 0.0099 μM; B refers to IC50 value in the range of 0.01 to 0.099 μM.
Recent Review Articles
-
1.
Boukalova S.; Hubackova S.; Milosevic M.; Ezrova Z.; Neuzil J.; Rohlena J.. Biochim. Biophys. Acta, Mol. Basis Dis. 2020, 1866 ( (6), ), 165759.
-
2.
Kuduk S. D.; Cisar J. S.; Pietsch E. C.. Med. Chem. Rev. 2019, 54, 177–196.
-
3.
Sykes D. B.Expert Opin. Ther. Targets 2018, 22 ( (11), ), 893–898.
-
4.
Loeffler M.; Carrey E. A.; Zameitat E.. Nucleosides, Nucleotides Nucleic Acids 2016, 35 ( (10–12), ), 566–577.
The author declares no competing financial interest.