Abstract

Decades of research efforts have conclusively provided overwhelming evidence that the cellular prion protein (PrPC) plays a central role in prion diseases, a set of fatal and incurable neurodegenerative disorders for which no therapy is yet available. In this Viewpoint, we provide an overview of the drug discovery strategies in the field, highlighting the current therapeutic hypotheses targeting, whether directly or indirectly, PrPC as well as the antiprion agents closest to clinical application.
Keywords: Prion protein, prion diseases, PrPC, PrPSc, drug discovery, antiprion agents
Prion diseases are rare, progressive, and incurable neurological disorders that naturally afflict both humans and animals, with the former including Creutzfeldt–Jakob disease, fatal familial insomnia, and Gerstmann–Sträussler–Scheinker disease.
Decades of research efforts have determined that these disorders share a common molecular mechanism, which is the conformational conversion of the GPI-anchored, properly folded cellular prion protein (PrPC) into an infectious misfolded self-replicating form (PrP scrapie, PrPSc) that accumulates in the brain of affected individuals.1 While the key role of PrP for prion pathology is thus established, the physiological function of this cell membrane protein is not yet clarified.
The strong scientific evidence confirming PrP as the central player suggests that any therapeutic strategy for prion diseases potentially able to affect PrPC and/or PrPSc, through direct or indirect mechanisms, is worthy of investigation.
Against this backdrop, early attempts to identify therapeutic agents for these disorders have primarily targeted PrPSc. However, several data indicate that PrPSc is a challenging target because of its high aggregation propensity, poorly understood structure, and ability to exist in different structural conformations (known as prion strains) that can evade therapeutic intervention via drug-resistance.
Unlike the unsuccessful modalities against PrPSc, compelling proofs of principle support the hypothesis that targeting the other partner of the misfolding process, i.e. PrPC, would be therapeutically effective against prion diseases.2
Indeed, increasing evidence suggests that PrPC plays a dual role in the pathogenesis of prion diseases by acting as a substrate for PrPSc formation and propagation, and by acting as a transducer of its neurotoxicity.1 Additionally, PrPC has been proposed to play an important role in other neurological disorders such as Alzheimer’s and Parkinson’s diseases, serving as a neuronal receptor for α-synuclein and β-amyloid proteins, respectively.3,4
Over the years, several PrPC-directed drug discovery approaches have thus been pursued to lower/remove the cell-specific substrate needed for prion conversion. Comprehensive reviews of some of these approaches have been published previously.2,5,6 Conversely, here we provide a focused overview of single representative examples for each therapeutic hypothesis directed against PrPC or against signaling pathways in which the protein is involved (Figure 1).
Figure 1.

PrPC-directed drug discovery strategies.
A first strategy consists of lowering the PrP gene expression. Three options have mainly been explored so far: RNA interference (RNAi) compounds, knockout options, and antisense oligonucleotides (ASOs). The latter represents the most recent advance in the prion disease field and offers great promise given their potential modality of lowering the levels of a single target protein in the brain and the ongoing clinical trials in other neurological diseases (e.g., amyotrophic lateral sclerosis, Parkinson’s disease, and Alzheimer’s disease).7,8 Indeed, although ASOs do not efficiently cross the blood–brain barrier (BBB) due to their size and charge, these molecules can achieve broad brain distribution by intrathecal delivery.
As a general background, ASOs are small sequences (typically 16–22 bases in length) of single-stranded DNA-like molecules, able to recognize specific complementary RNA structures. The ASOs initially developed for clinical use had as an important limitation the susceptibility to rapid cellular degradation by nucleases, and for this reason chemical modifications were subsequently introduced to improve their therapeutic usefulness.8 In the context of a PrP-lowering approach, these macromolecules are able to modulate gene expression as they selectively bind PrP mRNA, forming a hybrid ASO-mRNA, which induces degradation of mRNA by the ribonuclease H1. It is noteworthy that a recent study reported that ASOs designed to target and degrade PrP mRNA showed efficacy against prion disease in vivo as they were able to extend the survival of prion-infected mice.9 These and other promising findings have paved the way for ongoing preclinical development of PrP mRNA-targeting ASOs,7 which thus currently appear to be the closest to a possible clinical application.
A second sound approach
involves the targeting followed by the
stabilization of PrPC. As the conversion of PrPC to PrPSc is the key molecular event in the pathogenesis
of prion diseases, a molecule able to lock the protein in its native
conformation might in principle prevent the misfolding process, with
a detrimental effect on the production, aggregation, and replication
of the infectious forms.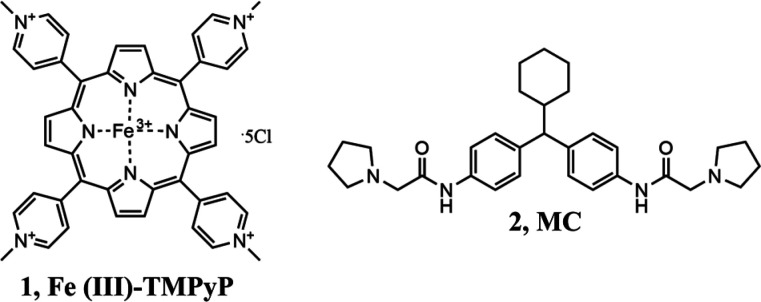
The underlying goal can be achieved with direct high-affinity PrPC binders, e.g. antibodies, aptamers, and small molecules, capable of acting as pharmacological chaperones. In this context, many chemical classes have been described to exert antiprion effects with a PrPC-direct mechanism of action.2,5,6 Two representative examples of such a strategy include the cationic tetrapyrrole Fe(III)-TMPyP ([Fe(III) meso-tetra (N-methyl-4-pyridyl) porphine], 1; Kd for human PrPC of 4.5 ± 2 μM)10 and medical chaperone (MC, 2; Kd for human PrPC of 3.2 ± 5.8 μM),11 which today appear as the most effective molecules targeting PrPC. Compound 1 was shown to bind PrPC by employing multiple biophysical techniques and to produce a dual effect of blocking prion replication and inhibiting PrPC-mediated neurotoxicity.10 Although the therapeutic potential of this antiprion agent is hampered by several factors (e.g., poor pharmacokinetic properties), its value lies in having provided a proof-of-principle for targeting PrPC pharmacologically. For what concerns 2, a recent multidisciplinary study described this compound as a molecular chaperone showing high affinity for PrPC and efficacy in cell-based assays.11 Preliminary data on animal models and toxicity studies underline the therapeutic potential of this agent, that however still requires further optimization and characterization to be considered as a valid clinical candidate.11,12 It is worth noting that, beyond the examples just mentioned, the vast majority of molecules claimed as PrPC binders had low binding specificity, inconsistencies between ligand binding affinity and in vitro active concentrations, and lack of in vivo efficacy. In this context, very recently an emblematic work by Reidenbach et al.13 clearly demonstrated that PrPC is a challenging target for small-molecule ligands. The absence of obvious biologically relevant binding sites and a large amount of negative screening data ultimately lend further support to the idea that PrPC may simply belong to the class of proteins classified as “undruggable” by canonical drug discovery protocols.
In the last years, one of the alternative
or complementary approaches
proposed to block PrPSc-induced neurodegeneration is the
targeting of the PrPC-mediated neurotoxic pathway. Indeed,
several lines of evidence suggest an important role of the cell-surface
PrPC in transducing neurotoxic signals, possibly as a result
of the specific binding interaction between PrPC and PrPSc,1 leading to neurotoxic consequences,
including synaptic dysfunction.14 It is
increasingly evident that treatments that inhibit prion replication
without affecting neurotoxicity could be effective in the first stage
of the prion disease progression characterized by high PrPSc infectivity, with less efficacy at symptomatic stages.9 Conversely, the identification of non-PrP proteins
that affect downstream neurotoxic sequelae has enormous therapeutic
implications since these macromolecules represent valuable targets
for antiprion drugs even in the presence of clinical symptoms.14 In line with this framework, several actors
of the neurotoxic PrP-mediated pathway have been discovered,15 such as neuronal cytoskeleton proteins and glutamate
receptors. Of note, p38 MAPK was identified as a key player of this
toxic pathway.16 Pharmacological kinase
inhibition by VX-745 (3) and genetic suppression
of p38 MAPK prevented PrPSc-induced synaptic abnormalities,
nominating this kinase as a new promising antiprion target to be further
validated in additional in vitro models.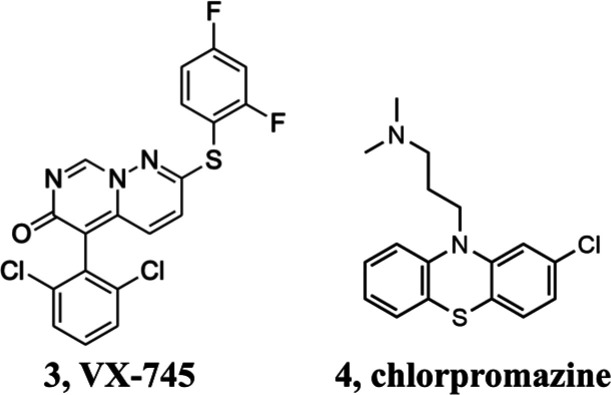
Of note, thanks to the ability of compound 3 to achieve high concentrations in the central nervous system (CNS), this p38 MAPK inhibitor is currently investigated in clinical studies for neurological diseases such as dementia and Huntington’s and Alzheimer’s diseases.17
A fourth strategy currently explored to prevent both prion replication and toxicity is the relocalization of PrPC from the cell surface to intracellular compartments.18 Indeed, since PrPSc needs cell membrane PrPC molecules for its propagation, removal of the protein substrate can negatively affect both prion infectivity and activation of PrPC-mediated neurotoxic signaling.19 A number of compounds have been described as able to redirect PrPC, including chlorpromazine (4). This molecule was initially thought to operate as PrPC binder, but recent studies clarified that it acts on the clathrin-mediated endocytosis altering the PrPC cell surface distribution similarly to what has also been observed for specific inhibitors targeting of the GTPase activity of dynamins.20 Unfortunately, the effects on the relocalization of PrPC were observed at concentrations only slightly lower than those causing cytotoxicity, and this behavior could reflect a possible intrinsic propensity of 4 and dynamin inhibitors to induce the intracellular rerouting of multiple surface proteins, thereby affecting cell viability. Although at present no compound acting with this mode of action appears as an ideal antiprion drug candidate, from a therapeutic point the relocalization of PrPC represents a valuable option worthy of further investigation.
Lastly, drug discovery strategies targeting protein degradation, e.g. monomeric/monovalent protein degraders, hydrophobic tagging-induced degradation, and proteolysis-targeting chimera (PROTAC), represent a rapidly evolving field to selectively reduce the levels of a protein target associated with a disease. Although in principle protein degraders may offer some advantages over classical inhibitors (e.g., relatively high selectivity, strong degradation power at relatively low concentration, and ability to induce the degradation of multiple target molecules),21 some concerns remain to be addressed. Indeed, the aforementioned protein degrader modalities target protein native structures, and thus may have the same limitations of standard pharmacological approaches if applied to challenge targets such as PrPC.
To overcome this hurdle, we have recently reported the hypothesis
of pharmacologically modulating the expression of a specific protein
at the post-translational level by targeting folding intermediates.
This protein-degrader paradigm, named Pharmacological Protein Inactivation
by Folding Intermediate Targeting (PPI-FIT), was tested on PrPC as proof of concept, leading to the identification of four
different small molecules (5, SM875 as representative)
capable of lowering the levels of the PrPC by promoting
its selective degradation.22,23
In light of these findings, while the validity of this innovative approach is currently under intensive investigation on different protein targets, yet the collected results lead to the first example of PrPC small-molecule degraders which as such deserve more in-depth studies.
The described antiprion compounds are only a representative example of the hundreds of molecules tested in several assays in the past three decades. However, despite the massive drug discovery efforts, to date no effective therapeutic against prion diseases has advanced to an approved drug.
The lack of available treatment, combined with the challenging early diagnosis and the extremely rapid progression of prion diseases (average time from disease onset to death is six months), underscores that further work is needed to improve the landscape of potentially effective therapeutics, identify novel targetable mechanisms and/or strategies, as well as develop innovative screening technologies for human prions diseases.
For what concerns the first point, based on the present knowledge, some key aspects to be considered in the search of promising candidates for clinical trials include proofs of principle of their in vivo strain-independent efficacy and wide brain distribution. The ideal therapeutic agent for prion diseases should be thus effective against multiple prion strains and able to reach an adequate concentration in the brain thanks to its ability to either cross the BBB or be successfully delivered directly into the CNS tissues. In this context, to date the PrP-lowering ASOs appear to be the most promising antiprion agents. Indeed, although the potential of ASOs against prion disease has been known for decades, the recent data collected on this class of non-small molecules have been so encouraging to pave the way for ongoing preclinical studies,7−9 which if successful would lead these compounds to advance clinically in the foreseeable future.
It is also worth noting that, since PrPC has been proposed to have implications in several neurological disorders, a PrPC-directed approved drug would be even more valuable as it may offer independent therapeutic options against other brain disorders, such as Alzheimer’s and Parkinson’s diseases.
Glossary
Abbreviations
- ASOs
antisense oligonucleotides
- ER
endoplasmic reticulum
- MC
medical chaperone
- mRNA
messenger RNA
- PPI-FIT
Pharmacological Protein Inactivation by Folding Intermediate Targeting
- PROTAC
proteolysis-targeting chimera
- PrPC
cellular prion protein
- PrPSc
scrapie prion protein
- RNAi
RNA interference
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The work was supported by a grant from Fondazione Telethon to EB (TCP14009) and by a fellowship from Fondazione Telethon to GS. EB is an Assistant Telethon Scientist at the Dulbecco Telethon Institute.
Views expressed in this editorial are those of the author and not necessarily the views of the ACS.
The authors declare the following competing financial interest(s): AA has direct involvement in the ongoing research at Sibylla Biotech SRL, a spin-off company of University of Perugia, University of Trento, and INFN (National Institute for Nuclear Physics). The company exploits the PPI-FIT technology for drug discovery in a wide variety of human pathologies, except prion diseases. GS, EB and MLB are co-founders and shareholders of the company (www.sibyllabiotech.it).
References
- Sigurdson C. J.; Bartz J. C.; Glatzel M. Cellular and Molecular Mechanisms of Prion Disease. Annu. Rev. Pathol.: Mech. Dis. 2019, 14 (October), 497–516. 10.1146/annurev-pathmechdis-012418-013109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colini Baldeschi A.; Vanni S.; Zattoni M.; Legname G. Novel Regulators of PrPC Expression as Potential Therapeutic Targets in Prion Diseases. Expert Opin. Ther. Targets 2020, 24 (8), 759–776. 10.1080/14728222.2020.1782384. [DOI] [PubMed] [Google Scholar]
- Ferreira D. G.; Temido-Ferreira M.; Miranda H. V.; Batalha V. L.; Coelho J. E.; Szegö É. M.; Marques-Morgado I.; Vaz S. H.; Rhee J. S.; Schmitz M.; Zerr I.; Lopes L. V.; Outeiro T. F. α-Synuclein Interacts with PrP C to Induce Cognitive Impairment through MGluR5 and NMDAR2B. Nat. Neurosci. 2017, 20 (11), 1569–1579. 10.1038/nn.4648. [DOI] [PubMed] [Google Scholar]
- Laurén J.; Gimbel D. A.; Nygaard H. B.; Gilbert J. W.; Strittmatter S. M. Cellular Prion Protein Mediates Impairment of Synaptic Plasticity by Amyloid-B Oligomers. Nature 2009, 457 (7233), 1128–1132. 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca M. L. M. L.; Iraci N.; Biggi S.; Cecchetti V.; Biasini E. Pharmacological Agents Targeting the Cellular Prion Protein. Pathogens 2018, 7 (1), 27. 10.3390/pathogens7010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustazza C.; Sbriccoli M.; Minosi P.; Raggia C.. Small Molecules with Anti Prion Activity. Curr. Med. Chem. 2019, 26. 10.2174/0929867326666190927121744. [DOI] [PubMed] [Google Scholar]
- Raymond G. J.; Zhao H. T.; Race B.; Raymond L. D.; Williams K.; Swayze E. E.; Graffam S.; Le J.; Caron T.; Stathopoulos J.; O’Keefe R.; Lubke L. L.; Reidenbach A. G.; Kraus A.; Schreiber S. L.; Mazur C.; Cabin D. E.; Carroll J. B.; Minikel E. V.; Kordasiewicz H.; Caughey B.; Vallabh S. M.. Antisense Oligonucleotides Extend Survival of Prion-Infected Mice. JCI Insight 2019, 4 ( (16), ). 10.1172/jci.insight.131175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj A.; Rader D. J. Antisense Oligonucleotides for Atherosclerotic Disease. Nat. Med. 2020, 26 (4), 471–472. 10.1038/s41591-020-0835-2. [DOI] [PubMed] [Google Scholar]
- Vallabh S. M.; Minikel E. V.; Schreiber S. L.; Lander E. S. Towards a Treatment for Genetic Prion Disease: Trials and Biomarkers. Lancet Neurol. 2020, 19 (4), 361–368. 10.1016/S1474-4422(19)30403-X. [DOI] [PubMed] [Google Scholar]
- Massignan T.; Cimini S.; Stincardini C.; Cerovic M.; Vanni I.; Elezgarai S. R.; Moreno J.; Stravalaci M.; Negro A.; Sangiovanni V.; Restelli E.; Riccardi G.; Gobbi M.; Castilla J.; Borsello T.; Nonno R.; Biasini E. A Cationic Tetrapyrrole Inhibits Toxic Activities of the Cellular Prion Protein. Sci. Rep. 2016, 6 (1), 1–14. 10.1038/srep23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K.; Kamatari Y. O.; Ono F.; Shibata H.; Fuse T.; Elhelaly A. E.; Fukuoka M.; Kimura T.; Hosokawa-Muto J.; Ishikawa T.; Tobiume M.; Takeuchi Y.; Matsuyama Y.; Ishibashi D.; Nishida N.; Kuwata K. A Designer Molecular Chaperone against Transmissible Spongiform Encephalopathy Slows Disease Progression in Mice and Macaques. Nat. Biomed. Eng. 2019, 3 (3), 206–219. 10.1038/s41551-019-0349-8. [DOI] [PubMed] [Google Scholar]
- Hosokawa-Muto J.; Kimura T.; Kuwata K. Oral Toxicity Study of an Antiprion Compound N,N’-[(Cyclohexylmethylene)Di-4,1-Phenylene]Bis[2-(1-Pyrrolidinyl)Acetamide] in Rats and Cynomolgus Monkeys. Fundam. Toxicol. Sci. 2019, 6 (5), 187–195. 10.2131/fts.6.187. [DOI] [Google Scholar]
- Reidenbach A. G.; Mesleh M. F.; Casalena D.; Vallabh S. M.; Dahlin J. L.; Leed A. J.; Chan A. I.; Usanov D. L.; Yehl J. B.; Lemke C. T.; Campbell A. J.; Shah R. N.; Shrestha O. K.; Sacher J. R.; Rangel V. L.; Moroco J. A.; Sathappa M.; Nonato M. C.; Nguyen K. T.; Wright S. K.; Liu D. R.; Wagner F. F.; Kaushik V. K.; Auld D. S.; Schreiber S. L.; Minikel E. V.. Multimodal Small-Molecule Screening for Human Prion Protein Binders. J. Biol. Chem. 2020, 10.1074/jbc.ra120.014905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer R. C.; Harris D. A. Identification of Anti-Prion Drugs and Targets Using Toxicity-Based Assays. Curr. Opin. Pharmacol. 2019, 44, 20–27. 10.1016/j.coph.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEGG PATHWAY database . https://www.genome.jp/kegg-bin/show_pathway?hsa05020+H00061 (accessed 2020-09-25).
- Fang C.; Wu B.; Le N. T. T.; Imberdis T.; Mercer R. C. C.; Harris D. A. Prions Activate a P38 MAPK Synaptotoxic Signaling Pathway. PLoS Pathog. 2018, 14 (9), 1–32. 10.1371/journal.ppat.1007283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov , https://clinicaltrials.gov (accessed 2020-10-09).
- Filesi I.; Cardinale A.; Mattei S.; Biocca S. Selective Re-Routing of Prion Protein to Proteasomes and Alteration of Its Vesicular Secretion Prevent PrPSc Formation. J. Neurochem. 2007, 101 (6), 1516–1526. 10.1111/j.1471-4159.2006.04439.x. [DOI] [PubMed] [Google Scholar]
- Mader M.; de Dios A.; Shih C.; Bonjouklian R.; Li T.; White W.; López de Uralde B.; Sánchez-Martinez C.; del Prado M.; Jaramillo C.; de Diego E.; Martín Cabrejas L. M.; Dominguez C.; Montero C.; Shepherd T.; Dally R.; Toth J. E.; Chatterjee A.; Pleite S.; Blanco-Urgoiti J.; Perez L.; Barberis M.; Lorite M. J.; Jambrina E.; Nevill C. R.; Lee P. a; Schultz R. C.; Wolos J. a; Li L. C.; Campbell R. M.; Anderson B. D. Imidazolyl Benzimidazoles and Imidazo[4,5-b]Pyridines as Potent P38alpha MAP Kinase Inhibitors with Excellent in Vivo Antiinflammatory Properties. Bioorg. Med. Chem. Lett. 2008, 18 (1), 179–183. 10.1016/j.bmcl.2007.10.106. [DOI] [PubMed] [Google Scholar]
- Stincardini C.; Massignan T.; Biggi S.; Elezgarai S. R.; Sangiovanni V.; Vanni I.; Pancher M.; Adami V.; Moreno J.; Stravalaci M.; Maietta G.; Gobbi M.; Negro A.; Requena J. R.; Castilla J.; Nonno R.; Biasini E.. An Antipsychotic Drug Exerts Anti-Prion Effects by Altering the Localization of the Cellular Prion Protein. PLoS One 2017, 12 ( (8), ). 10.1371/journal.pone.0182589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Fei Y.; Lu B. Emerging New Concepts of Degrader Technologies. Trends Pharmacol. Sci. 2020, 41 (7), 464–474. 10.1016/j.tips.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini E.; Barreca M. L.; Faccioli P.. Small Molecules Inducing the Degradation of the Cellular Prion Protein. Pat. Appl. IT 102020000006517, 2020.
- Spagnolli G.; Massignan T.; Astolfi A.; Biggi S.; Brunelli P.; Libergoli Mi.; Ianeselli A.; Orioli S.; Boldrini A.; Terruzzi L.; Maietta G.; Rigoli M.; Lorenzo N. L.; Fernandez L. C.; Tosatto L.; Linsenmeier L.; Vignoli B.; Petris G.; Gasparotto D.; Pennuto M.; Guella G.; Canossa M.; Altmeppen H. C.; Lolli G.; Biressi S.; Pastor M. M.; Requena J. R. J.; Mancini I.; Barreca M. L.; Faccioli P.; Biasini E.. Pharmacological Protein Inactivation by Targeting Folding Intermediates. bioRxiv 2020, 10.1101/2020.03.31.018069. [DOI] [Google Scholar]


