Abstract
Visible light-mediated photocatalysis, which relies on the ability of photocatalysts to absorb low-energy visible light and engage in single-electron transfer (SET) or energy transfer (ET) processes with organic substrates, has emerged as one of the fastest growing fields in organic synthesis. This catalytic platform enables a highly selective approach to promote radical-based organic transformations which unlocks unique reaction pathways. Due to the extremely mild conditions of these transformations and compatibility in aqueous environments, photocatalysis has emerged as an enabling technology in drug discovery. Photocatalysis is uniquely positioned for application in pharmaceutical development because of its demonstrated potential for broad functional group tolerance, biocompatibility, site-specific selectivity, and operational simplicity. This review will highlight the recent advances of visible-light photocatalysis through its application in peptide functionalization, protein bioconjugation, Csp3–Csp2 cross-coupling, late-stage functionalization, isotopic labeling, DNA-encoded library technology (DELT), and microenvironment mapping (μMap).
Keywords: Visible-light photocatalysis, Csp3−Csp2 cross-coupling, late-stage functionalization, isotopic labeling, DNA-encoded library technology (DELT), microenvironment mapping (μMap)
Free radical chemistry has historically been a powerful tool for the rapid construction of complex organic molecules due to the unique reactivity of radical intermediates. One electron bond construction has attracted chemists since its discovery and has led to broad application throughout the field of organic synthesis.1 These methods have traditionally relied upon the use of stoichiometric oxidizing or reducing reagents to generate reactive radicals.2,3 UV photochemistry is an alternative approach that involves a substrate absorbing high energy UV light to generate radical intermediates which then often engage in an isomerization or combination with another species, thus eliminating the requirement for harsh stoichiometric reagents.4,5 In recent years, visible-light photocatalysis has emerged as a new catalytic platform to generate free radicals, thereby circumventing the use of high energy UV light and harsh reagents.
In a general sense, photocatalysis relies on the ability of a catalyst to absorb low energy visible-light to give stable, long-lived photoexcited states. These photocatalysts are then able to engage in either single-electron transfer (SET) or energy transfer (ET) processes with organic or organometallic substrates (Figure 1).6,12 Given that the catalyst photoexcited species (*PC) is both more oxidizing and more reducing than the ground state species, single electron transfer with the *PC may proceed by either oxidative or reductive quenching (Figure 1a). During an oxidative quenching cycle, *PC functions as a reductant, reducing an electron-acceptor (A) via a single electron transfer step to generate the singly reduced species A•– and the oxidized form of the photocatalyst. PC•+ would then subsequently accept an electron from an electron-donor (D) to furnish the radical cation of D (D•+) and ground-state PC, thereby closing the catalytic cycle. In contrast, *PC may also function as an oxidant in a reductive quenching pathway, first accepting an electron from D to provide the reduced form of the photocatalyst and then donating an electron to A to give PC. A second pathway for the decay of a photocatalyst excited state is via energy transfer (ET) (Figure 1b). After irradiation, *PC can engage in direct energy transfer with an organic substrate A. This generates an energetically activated substrate *A and returns the photocatalyst to its ground state. Notably, energy transfer can take place via two distinct mechanisms, called Dexter and Förster energy transfer.9,10
Figure 1.
Typical photocatalytic cycles and common ruthenium, iridium, and organic photocatalysts.
Photoredox catalysis enables the use of extremely mild conditions at room temperature and typically employs commercially available light bulbs or blue LED light sources to access the excited state of the photocatalyst. Compared to UV light, which can directly photolyze bonds in organic molecules, low-energy visible light typically does not activate bonds in simple organic molecules, allowing for its broad use in diverse settings. Additionally, in contrast to methods requiring stoichiometric oxidants and reductants, photoredox methods enable the in situ generation of both strong oxidants and reductants within a single flask, often in a redox neutral manner.12 Due to these benign conditions and exquisite control of site selective radical generation, the past decade has witnessed a rapid rise in the application of photoredox catalysis in organic synthesis and the development of new reaction methodologies.
The first application of photoredox catalysis in organic synthesis was first reported over 40 years ago.11,12 Subsequently, pivotal reports from MacMillan, Yoon, and Stephenson in 2008–2009 began to unearth the potential for this activation mode in organic synthesis and led to the rapid rise in the development of these types of methods.13−15 Since these seminal publications, a wide range of visible light-photocatalyzed transformations have been developed, taking advantage of its unique reactivity to achieve novel bond-forming paradigms.12,16−18 Over this time multiple classes of photocatalysts have been developed including both organometallic complexes12 and organic dyes (Figure 1c).19 Modification of both the metal and ligand framework enables control of the redox parameters of these catalysts, allowing for fine-tuning of oxidation and reduction potentials. Recently, it has been found that adjustment of the ligand sphere can facilitate the aqueous solubility of these catalysts, allowing for use in biological settings.88
The combination of photoredox catalysis with alternative modes of catalytic activation has further demonstrated the versatility of this platform for the development of new, highly enabling synthetic methodologies that are not achievable with either catalytic platform alone.20 For example, photoredox catalysis has been successfully combined with organocatalysis to enable the development of multiple new transformations.13,21 Notably, the merger of visible light photocatalysis and transition metal catalysis, coined metallaphotoredox catalysis, has received broad attention from the synthetic community. This area has enabled the development of practical new bond-forming protocols which utilize native functionality (i.e., C–H bonds, alcohols, carboxylic acids, etc.) thereby creating a completely new paradigm for cross-coupling.22 Such dual catalytic platforms provide powerful tools for synthetic chemists to achieve transformations that are not currently possible using a single catalyst system.
The synthetic advancements in the field of visible-light photocatalysis and its inherently mild conditions have enabled a myriad of applications toward drug discovery.16,23−26 Aspects of this catalytic platform such as biocompatibility, harnessing of low-energy visible light, and low temperatures allow for broad functional group tolerance and minimize side reactivity. Specifically, these mild conditions are critical to enable compatibility of this platform in a biological setting to avoid protein denaturation or DNA fragmentation. All of these features have attracted the attention of the pharmaceutical industry, which recognized the potential application of photoredox catalysis to drug discovery and development (Figure 2). This innovation article will highlight the recent applications of visible-light photocatalysis in drug discovery, reported within the past decade. While this innovation article is not comprehensive, we have strived to highlight contributions that have had the most significant impact in this discipline.
Figure 2.
Technologies enabled through the application of visible-light photocatalysis towards drug discovery.
Peptide Functionalization and Protein Bioconjugation
Due to the increasing interest in peptides as drug candidates and the importance of antibody–drug conjugates in current drug discovery,27 the development of effective bioconjugation strategies is in high demand.28 A suite of robust transformations for direct peptide functionalization or protein bioconjugation that proceed under mild conditions with site specificity would be highly valuable. Early examples of protein modification via visible-light photocatalysis focused on peptide cross-linking, which set the stage for new bioconjugation protocols.29−31 Recently, with the rapid development of photoredox catalysis and its advantages (vide supra), numerous transformations have been reported to accomplish peptide modification and protein bioconjugation at select residues, namely cysteine, tyrosine, methionine, tryptophan, and histidine.32
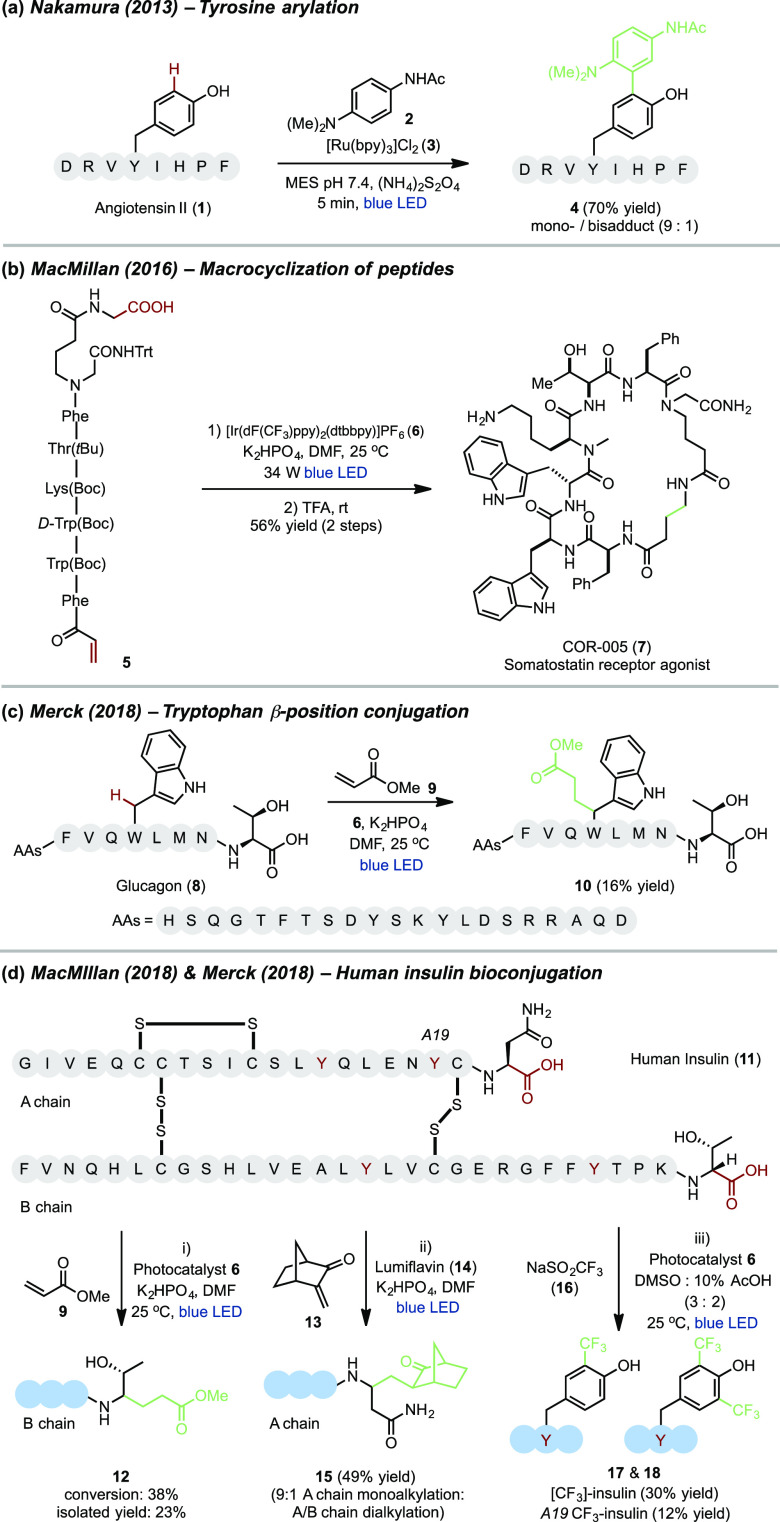
In 2013, Nakamura and co-workers developed a tyrosine-specific protein modification protocol through photoredox mediated radical generation followed by trapping with tyrosyl radical trapping reagents (TRTs).33 Angiotensin II (1) was treated with N’-acyl-N,N-dimethyl-1,4-phenylenediamine (2) in the presence of [Ru(bpy)3]Cl2 (3) and ammonium persulfate under visible-light irradiation to give arylated angiotensin II (4) as the monoadduct and bisadduct (9:1 ratio) in 70% yield (Figure 3a). In addition, tyrosine-specific modification was also applied to bovine serum albumin (BSA) with a fluorescent TRT substrate.
Figure 3.
Representative examples of complex peptide and protein bioconjugation via photoredox catalysis.
Recently, MacMillan and co-workers reported a method for the decarboxylative macrocyclization of peptides containing N-terminal Michael acceptors via photocatalytic intramolecular radical 1,4-addition. COR-005 (7), a somatostatin analogue, was successfully synthesized in 56% yield by this photoredox-mediated cyclization of linear peptide 5 using [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 (6) as the photocatalyst followed by acidic deprotection (Figure 3b).34
In 2018, Merck developed a chemoselective peptide modification for tryptophan residues at the benzylic position with acrylates via photocatalytic Michael addition.35 Glucagon (8), an important tryptophan-containing peptide hormone with 15 unique amino acids as shown in Figure 3c can be alkylated with methyl acrylate (9) under photoredox conditions to give about 45% conversion and 16% isolated yield of conjugated product 10. In order to investigate the chemoselectivity of this method in the absence of tryptophan residues, human insulin (11) as a substrate was subjected to conjugation with methyl acrylate (9) under the optimized reaction conditions (Figure 3di). Interestingly, exclusive B-chain C-terminal-selective decarboxylative conjugation products 12 were obtained in 38% conversion and 23% isolated yield.
Additionally, MacMillan and co-workers established a site-selective and chemoselective bioconjugation of native proteins via photocatalytic decarboxylative alkylation with enones.36 Specifically, they demonstrated the use of photoredox catalysis as a platform to selectively functionalize the C-terminus of proteins by exploiting the discrepancy in oxidation potentials between internal (E1/2red = ∼1.25 V vs SCE) and C-terminal carboxylates (E1/2red = ∼0.95 V vs SCE). Photocatalytic bioconjugation of native insulin (11) with 3-methylene-2-norbornanone (13) was investigated and monoalkylated adduct 15 was obtained in 49% isolated yield with modification exclusively at the A-chain C terminus (Figure 3dii). Notably, disulfide bonds were tolerated and no side-chain or heteroatom conjugation was observed under this alkylation condition. They propose the selectivity for A-chain modification could be explained by either (1) a change in the oxidation potential where the C terminus of the A-chain is favored over the B-chain and (2) nonspecific binding of the photocatalyst to the protein adjacent to the A-chain C terminus caused by lipophilic amino acids.
In the same year, Merck developed a tyrosine-specific and protecting group free trifluoromethylation of peptides using NaSO2CF3 as a trifluoromethyl radical source via visible light photoredox catalysis.37 This biocompatible method has been applied to the modification of complex tyrosine-containing peptides with up to 51 amino acids. Human insulin (11) with four tyrosine residues was subjected to visible-light irradiation in the presence of NaSO2CF3 and Ir catalyst 6, labeling all four residues to give a mixture of trifluoromethylated products 17 and 18 in 30% overall yield. In addition, trifluoromethylation of insulin exclusively at tyrosine A19 was obtained in 12% yield (Figure 3diii).
The reports highlighted in this section have shown important advances in the field of protein bioconjugation. However, many of these protocols have been demonstrated either on polypeptides or on small proteins, yet we anticipate that these strategies will be successfully applied on more complex systems in the near future.
Csp3–Csp2 Cross-coupling via Merger of Photoredox and Nickel Catalysis
The advent of metallaphotoredox catalysis has led to an explosion in novel transformations that has enabled a paradigm shift in retrosynthetic analysis. Within the broader field of visible light-mediated photocatalysis, the following seminal contributions have had the most significant impact in medicinal chemistry at Genentech based on an internal review of reaction utilization. In 2014, the Doyle and MacMillan laboratories developed a direct decarboxylative Csp3–Csp2 coupling of carboxylic acids with aryl halides via a dual photoredox/nickel catalytic platform (Figure 4a).38 This approach showed broad scope with regard to both aryl halides and cyclic or acyclic carboxylic acids including amino acids, providing high yields of desired alkyl-aryl products, thus illustrating a completely new bond disconnection. Concurrently, Molander and co-workers reported a mechanistically similar pathway for the Csp3–Csp2 cross-coupling of potassium trifluoroborates (RBF3K) with aryl bromides via the combination of photoredox and nickel catalysis (Figure 4b).39 A broad range of functional groups on both the benzylic trifluoroborates and aryl bromides are compatible with these mild reaction conditions, demonstrating its practicality toward Csp3–Csp2 cross-coupling.
Figure 4.
Seminal contributions toward photoredox-enabled Csp3–Csp2 cross-coupling.
In 2016, MacMillan and co-workers established a novel strategy for the cross-electrophile Csp3–Csp2 coupling of alkyl bromides with aryl or heteroaryl bromides in the presence of tris(trimethylsilyl)silane (TTMSS) (Figure 4c).40 Photocatalytically generated silyl radicals abstract halogen atoms from alkyl bromides to form the corresponding alkyl radicals which can then serve as competent nucleophilic cross-coupling partners. A wide range of alkyl and (hetero)aryl halides containing various functional groups are compatible under the optimized cross-coupling conditions. Recently, this method has been extended to unactivated alkyl chlorides, which greatly expands the scope of this transformation.41 Taken together, these protocols provide the medicinal chemist a toolbox of nucleophile replacements (alkyl carboxylic acid, alkyl BF3K, alkyl bromide/chloride) that can be chosen when employing a cross-coupling reaction.42 In fact, a recent report compared the robustness of these three methods to traditional Csp3–Csp2 cross-coupling conditions in the context of druglike manifolds and found them to be of similar utility to traditional methods.43 Additionally, these transformations have been further elaborated to Csp3–Csp3 cross-couplings.44,45 This represents a significant shift over traditional methods requiring organometallic nucleophiles, as these functional groups as handles for cross-coupling were considered inert by previous generations of medicinal chemists.
Late-Stage Functionalization of Pharmaceutically Relevant Compounds
In comparison to de novo synthesis, methods for chemoselective functionalization of pharmaceutically relevant compounds could provide a more straightforward and cost-effective approach to diversify molecules for lead optimization in drug discovery.46,47 Direct functionalization of aryl C–H bonds into C–C and C–heteroatom bonds can provide efficient access to arenes with diverse structural and physiochemical properties. Additionally, an established concept in medicinal chemistry is the development and application of bioisosteres, structural motifs that mimic common functional groups that can improve druglike properties.48,49 Recently, photoredox catalysis has emerged as a highly efficient and mild strategy for late stage diversification and access to synthetically challenging bioisosteres.50−53
Trifluoromethyl and difluoromethyl arenes are broadly utilized in drug design as their incorporation can be used to tune the physiochemical properties of a given biomolecule or block potential metabolic hot spots. Photoredox catalysis provides a powerful tool to enable the synthesis of trifluoromethyl and difluoromethyl compounds. In 2011, the MacMillan lab reported a mild and operationally simple strategy for the direct trifluoromethylation of unactivated arenes and heteroarenes by means of photoredox catalysis using commercial photocatalysts under visible light activation.54 A variety of biologically active molecules were successfully trifluoromethylated to obtain their CF3 substituted analogues under the optimized trifluoromethylation conditions. For instance, an Alzheimer’s drug precursor was selectively trifluoromethylated to deliver 20 in 94% yield (Figure 5a).
Figure 5.

Representative examples of late-stage functionalization of pharmaceutically relevant compounds via photoredox catalysis.
LY2784544 (21),55 a selective inhibitor of JAK2, has undergone clinical trials for the treatment of several myeloproliferative disorders. In 2014, Stephenson and co-workers developed a photocatalyzed direct coupling of N-methylmorpholine with imidazopyridazine 22 in good yield and selectivity (Figure 5b).56 This approach provided an efficient strategy for the direct introduction of the benzylic morpholine moiety to the functionalized imidazopyridazine.
In 2014, Merck reported a direct alkylation of a variety of biologically active heterocycles using organic peroxides as alkylating reagents via activation by visible-light photoredox catalysis.57 Fasudil (25), a potent Rho-kinase inhibitor and vasodilator, can be methylated using tert-butylperacetate as an alkylating reagent in 43% yield (Figure 5ci). Shortly thereafter, the MacMillan lab developed a dual catalytic alkylation of heteroarenes using alcohols as the alkylating reagent. This involved the successful merger of photoredox and hydrogen atom transfer catalysis.58 A broad range of functional groups on the heteroarenes and numerous unactivated alcohols are compatible with these mild reaction conditions, demonstrating their application in late-stage functionalization. Methylation of Fasudil can be achieved using methanol as the methylating reagent to form the desired fasudil derivative 26 in 82% yield (Figure 5cii).
The MacMillan lab reported a photocatalytic protocol which enables the alkylation of heteroarenes with ethers to form α-oxy heteroarenes, an important and broadly utilized functional group in medicinal chemistry.59 Various functional groups on both ether and heteroarene are tolerated under the optimized conditions providing consistently high yields of desired products. Isoquinoline (28) and tetrahydropyran (THP) were subjected to the optimized conditions using photocatalyst 6 in combination with Na2S2O8 as the oxidant under the irradiation of a 26 W household fluorescent light bulb (CFL) to give the desired α-arylation product 29 in 63% yield (Figure 5di). Shortly thereafter, Merck disclosed a method for the C–H hydroxymethylation of heteroarenes with methanol mediated by the same catalyst with benzoyl peroxide (BPO) as the terminal oxidant. This method showed excellent applicability to a variety of different heterocycles, such as the selective C–H hydroxymethylation of isoquinoline (28) to form isoquinolin-1-ylmethanol (30) in 88% yield (Figure 5dii).60 More recently, the Lei lab developed a new protocol for the C–H hydroxyalkylation of heteroarenes with alcohols, which is promoted by Selectfluor under visible-light irradiation.61 A broad range of functional groups on the heteroarenes and unactivated alcohols are compatible with these newly discovered hydroxyalkylation conditions.
Cyanoarenes are prevalent in drugs and bioactive molecules in addition to being a highly versatile intermediate. This is due to the nature of nitriles to serve as a masked functional group that can easily be converted to carboxylic acids, aldehydes, and amines. In 2017, the Nicewicz group reported a method for the construction of aromatic and heteroaromatic nitriles via direct C–H functionalization using acridinium photoredox catalyst 31 and trimethylsilyl cyanide (Me3SiCN) under an aerobic atmosphere (Figure 5e).62 The reaction conditions were compatible for the late-stage cyanation of more structurally complex bioactive molecules. For example, Naproxen methyl ester was selectively cyanated to afford the desired product 32 as a single regioisomer in 57% yield.
In 2015, Nicewicz and co-workers developed a novel site-selective C–H amination of unactivated arenes with secondary amines using acridinium-based photocatalyst 31 and a nitroxyl radical (TEMPO).63 A wide range of functional groups such as alcohols, esters, silyl ethers, halides, amides, alkenes, and protected amines are all compatible with the optimized amination conditions. The mildness of this protocol makes it an appealing tool to be applied to the late-stage amination of druglike molecules. For instance, naproxen methyl ester was selectively aminated with pyrazole to afford the desired product 33 in moderate yield (Figure 5f). In 2017 the Nicewicz lab developed another strategy for the direct C–H amination of unactivated arenes using primary amines as the coupling partner via organic photoredox catalysis.64 This approach showed broad scope with regard to both primary amine and arene substrates, providing consistently high yields of aminated arenes.
Astex Pharmaceuticals and Novartis developed an efficient strategy for the photoredox-mediated α-aminoalkylation of heteroarenes via Minisci-type addition of α-amino radicals to electron-deficient heteroarenes.65 A wide range of electron-withdrawing functional groups on the heteroarenes and Boc-protected cyclic amines were compatible with these reaction conditions. For example, in the case of Fasudil (25), the reaction occurs exclusively at the isoquinoline C2 in 43% yield (Figure 5g). Reaction condition screening was performed on the nanomolar scale in 1536-well microtiter plates (MTPs) using high-throughput experimentation (HTE) technology. The authors highlight that this technology would allow for the rapid expansion of a fragment-based library in a fragment to lead campaign.
In 2019, Jui and co-workers demonstrated a method for the defluoroalkylation and hydrodefluorination of unactivated trifluoromethylarenes using a phenoxazine photocatalyst under visible-light irradiation (Figure 5hi).66 This defluorinative radical process can be employed to access a diverse range of Ar–CF2R and Ar–CF2H products, especially difluoromethylarenes with electron donating groups. Triflupromazine can be functionalized to afford its derivative 35 in 66% yield. In 2020, Gouverneur and co-workers reported a photoredox-catalyzed protocol for the hydrodefluorination of electron-deficient trifluoromethylarenes.67 A wide range of functional groups and heterocycles commonly found in complex drug molecules were all tolerated under the optimized reductive defluorination conditions. This method provided an operationally simple protocol for the direct conversion of complex trifluoromethylated drugs into their difluoromethyl analogues (compound 36, Figure 5hii).
Isotopic labeling
Deuterium (2H, D) and tritium (3H, T) labeling of pharmaceutical compounds are pivotal diagnostic techniques in drug discovery research to trace and elucidate the biological fate of drugs. Recently, photoredox catalysis has been utilized as a highly effective strategy for the deuteration and tritiation of complex biomolecules and drugs. In 2017, MacMillan and co-workers reported a photoredox-mediated hydrogen atom transfer protocol which provides access to the selective deuteration and tritiation of α-amino sp3 C–H bonds in a single step, using deuterium oxide (D2O) or tritium oxide (T2O) as the source of hydrogen isotope (Figure 6a) in good yields.68 This deuteration or tritiation protocol was also applicable to various commercially available drugs containing a variety of alkyl amine scaffolds, such as the macrolide drug clarithromycin ([2H]-37, [3H]-38) showcasing the functional group tolerance of this method. Recently, Sanofi-Aventis reported a related protocol to accomplish the deuteration and tritiation of linear peptides under photoredox conditions.93
Figure 6.
Representative examples of isotopic labeling of pharmaceutical compounds via photoredox catalysis.
In addition, 18F radiolabeling is one of the most important labeling tools for noninvasive studies of biological systems. The direct radio-fluorination of pharmaceutical compounds or metabolites could be used as 18F radio-tracers for positron emission tomography (PET), with potential applications in biomedicine and oncology for elucidating the in vivo fate of pharmaceutical compounds or metabolites.69 Late-stage 18F radiolabeling of sp3C–H bonds has been demonstrated with a decatungstate photocatalyst and UV light.70 More recently in 2019, the Nicewicz lab and the Li lab developed a mild strategy for the [18F]-fluorination of aromatic C–H bonds by [18F]-TBAF using acridinium-based photoredox catalyst 39 under visible-light illumination (Figure 6b).71 This strategy has been applied to label various arenes containing a wide range of functional groups and heterocycles commonly found in bioactive molecules. For example, Fenoprofen methyl ester can be selectively [18F]-labeled through this method to give 40 in moderate yield followed by a deprotection to give [18F]-Fenoprofen which has been used as a tracer for PET. The hypolipidemic agents clofibrate (41) and a derivative of the biological neurotransmitter precursor DL-DOPA (42) can be selectively [18F]-labeled in moderate radiochemical yield (RCY).
DNA-Encoded Library Technology
DNA-encoded library technology (DELT) is a screening platform commonly used to discover small molecules that bind to a target of interest. DELT, originally envisioned by Brenner and Lerner in 1992,72 involves the synthesis of libraries of small molecules with conjugated DNA tags followed by screening against a target protein. Data analysis of hits requires DNA sequencing of binders to identify small molecule structures, off-DNA compound resynthesis and evaluation of the small molecule for activity.73 DELT has emerged as a powerful technology for generating and screening libraries of billions of small molecules with diverse chemical structure which has been adopted and validated by the pharmaceutical industry.
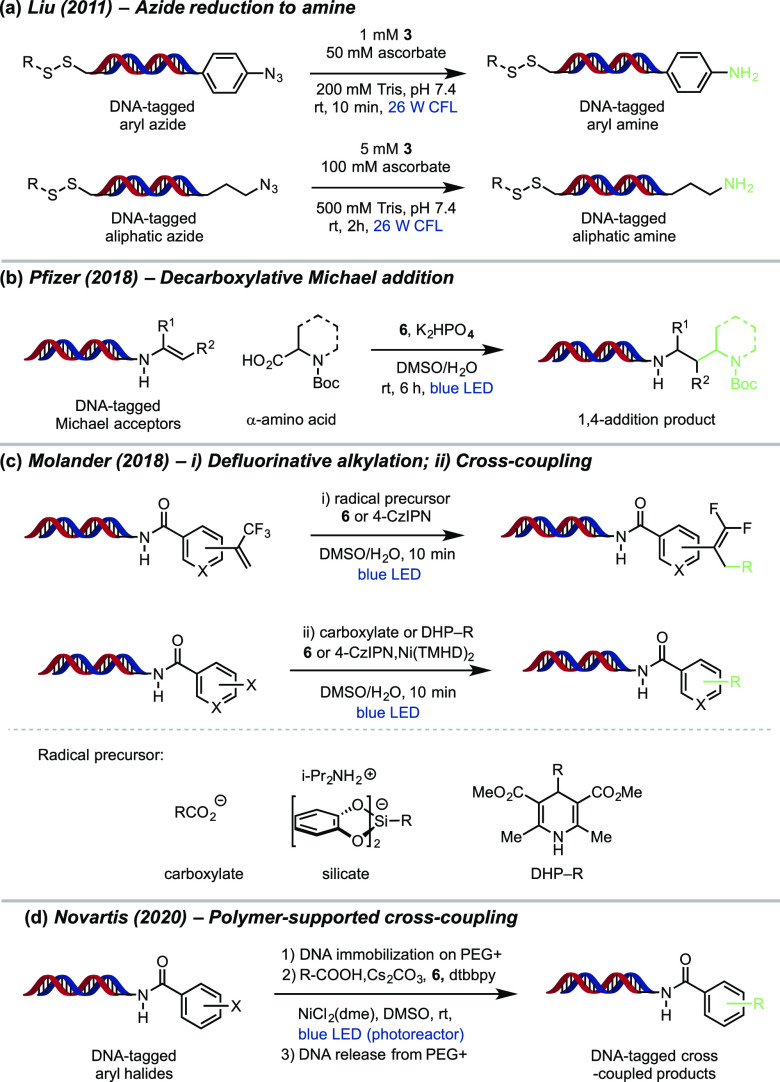
In 2010, the Liu lab developed the first biomolecule-compatible method for the reduction of DNA-tagged azides to amines via photoredox catalysis (Figure 7a).74 This reaction shows high chemoselectivity and excellent compatibility with alcohols, phenols, acids, alkenes, alkynes, aldehydes, alkyl halides, alkyl mesylates and disulfides, nucleic acid, and oligosaccharide substrates. This seminal publication demonstrated that the mild conditions of photoredox catalysis are compatible with DNA tags and could potentially lead to applications in DELT.
Figure 7.
Representative examples of DNA-encoded library (DEL) via photoredox catalysis.
In 2018, Pfizer developed a new procedure for the photoredox-mediated conjugate addition of α-amino acid-derived radicals to various DNA-tagged Michael acceptors. A broad array of structurally diverse radical precursors, including all of the 20 canonical amino acids, are tolerated in the optimized reaction conditions. Importantly, this transformation proceeds under aqueous conditions which allows this reaction platform to be well suited for the construction of DNA-encoded libraries (Figure 7b).75
Concurrently, the Molander lab and GlaxoSmithKline developed a DELT-compatible method for the photoredox-catalyzed alkylation of DNA-tagged substrates through two protocols. They demonstrated a photoredox-catalyzed radical/polar crossover defluorinative alkylation of DNA-tagged trifluoromethyl alkenes to form gem-difluoroalkenes (Figure 7ci).76,78 Three classes of water compatible, common radical precursors were selected for this transformation: alkyl bis(catecholato)silicates, DHPs, and amino acids. Organic photocatalyst 4-CZIPN was found to be the best catalyst for both the silicate and DHP radical precursors, while iridium catalyst 6 was optimal for carboxylic acids. They found that a wide range of functional groups were all well tolerated under the optimized conditions.
Recently, metallaphotoredox catalysis has emerged as a remarkably mild method for Csp3−Csp2 cross-coupling within complex molecular settings.17,22,77 Molander and co-workers additionally found that a Ni/photoredox-catalyzed Csp3−Csp2 cross-coupling could be applied to DELT libraries (Figure 7cii) with DHPs and amino acids as radical precursors.76,78 A variety of aryl bromides and iodides coupled efficiently with 2° alkyl, 3° alkyl, benzyl, and α-alkoxy DHP partners. For the coupling using amino acids as radical precursors, aryl iodide substrates are better coupling partners than bromides. Subsequently, Pfizer disclosed an extension of this approach focused on the decarboxylative arylation for DELT library synthesis with a broadly expanded scope.94
In 2020, Novartis developed a practical catch-and-release methodology under metallaphotoredox conditions on immobilized DNA conjugates utilizing a cationic, amphiphilic PEG-based polymer.79 This method includes three critical steps: (1) adsorption of DNA-labeled substrates on cationic support (PEG+) via nonbonded ionic interactions, (2) Ni/photoredox dual catalyzed decarboxylative cross-coupling, and (3) then the release of the DNA species from the resin. This method tolerates a wide variety of carboxylic acid substrates and DNA-conjugated aryl halides (Figure 7d). In particular, aliphatic acids bearing α-heteroatoms, such as oxygen or nitrogen, showed excellent conversion. While primary and secondary aliphatic acids could be coupled successfully, tertiary carboxylic acids gave poor conversion under the reaction conditions. This method was also demonstrated to be applicable to a wide range of DNA-conjugated haloaryl substrates bearing electron-donating or electron-withdrawing moieties. Additionally, they found that 48% of amplifiable DNA can be recovered after release from resin exposed to the photoredox decarboxylative coupling conditions.
Microenvironment Mapping Technology
Elucidation of the cell surface landscape can provide an insightful tool to understand many disease pathologies. Over the past decade, several techniques have been developed to assess protein–protein interactions and the local environment at the cell membrane, such as selective proteomic proximity labeling assay using tyramide (SPPLAT) and proximity labeling using ascorbic acid peroxidase (APEX).80,81 These novel approaches rely on the use of a tethered peroxidase to generate a long-lived phenoxy radical that can react with neighboring proteins. A challenge associated with both of these techniques is the promiscuous nature of these radicals with partner proteins due to its long half-life. As such, a precise map of these cellular microenvironments or landscapes would facilitate the development of useful therapeutic strategies.82−84 Seminal contributions from Brunner et al. demonstrated the ability to generate reactive carbenes from diazirines via direct excitation by UV light for photolabeling.87 Subsequent reports from the Salomon group showed that azides in combination with a photosensitizer and visible light can be used to label proteins in a biological setting.85,86 More recently in 2020, the MacMillan lab in collaboration with Merck developed a new technology called microenvironment mapping (μMap) which employs photocatalytic carbene generation to more precisely label the local environment of a specific receptor at the cell surface.88
The authors demonstrated that diazirine-based probes could be used to generate reactive carbene intermediates with concurrent loss of N2 via a photocatalytic Dexter energy transfer process (Figure 8a). They were able to conjugate a photocatalyst to an antibody targeting a receptor of interest, thereby generating these carbenes in close proximity to the target when irradiated with visible light. Due to the high reactivity and short half-life, these carbene intermediates either cross-link with a nearby protein or are quenched by the aqueous environment. Importantly this diazirine probe does not absorb visible light, thereby a fundamental requirement of this platform is to use the antibody photocatalyst conjugate to localize carbene generation.
Figure 8.
Photoaffinity labeling via photocatalytic energy transfer to enable microenvironment mapping.
To establish proof of concept, they demonstrated that biotinylation of bovine serum albumin (BSA) can be observed when BSA and diazirine Ar-PEG3-biotin were irradiated with visible light in the presence of a soluble photocatalyst 43 (Figure 8b). Photocatalytic labeling of BSA was further confirmed through intact protein mass spectrometry. With the desired reactivity profile in hand, they applied this antibody-targeted photocatalytic diazirine activation (μMap) to the surface of live cells. The constituent proteins of the programmed-death ligand 1 (PD-L1) microenvironment in live lymphocytes were successfully identified, as well as selectively labeling within an immunosynaptic junction (Figure 8c). A comparison between peroxidase and μMapping methods was undertaken, through both flow cytometry and confocal microscopy. μMapping was demonstrated to be less promiscuous for cross-linking protein partners and therefore provides enhanced granularity for the cell surface local environment for the cell lines tested.
Conclusion
In summary, the recent development of visible-light mediated photocatalysis within the context of drug discovery has been demonstrated through its applications to five fields: protein bioconjugation, late-stage functionalization, isotopic labeling, DNA-encoded library technology (DELT) and microenvironment mapping (μMap). As outlined in this innovation article, new methods that utilize visible light photocatalysis are breaking new ground in chemical reactivity that was not seen with standard two electron reaction manifolds. This is inherently due to the mild nature of visible-light photocatalysis and its compatibility to biologically relevant solvents. Additionally, this type of reactivity was not seen previously with reactions that generate stoichiometric quantities of reactive radical intermediates, an inherent advantage of these catalytic processes whereby only a catalytic amount of radical is present in the reaction mixture. Notably, these transformations do not require the use of harsh stoichiometric oxidants/reductants or high energy UV light thereby avoiding deleterious protein denaturation and functional group intolerance such as disulfide cleavage. While these attributes make this technology attractive, there are inherent challenges that need to be addressed to fully capitalize on its potential. Notably, scale up of these transformations can be hampered by a limitation of light penetration into the reaction vessel. These technical difficulties are being addressed by engineering solutions such as flow chemistry and immersion-well reactors.89−91 For the medicinal chemist, standardized equipment and photoreactors are continually being developed to address robustness and reproducibility.92 This is key in the current landscape of outsourcing synthetic chemistry, whereby translatability from internal team members to external partners is a necessity for success. Although the application of visible-light photocatalysis in drug discovery is still in its infancy, it has had an outsized impact in a short period of time. This catalytic platform has shown promising potential in drug discovery indicating a paradigm shift toward one electron pathways is occurring.
Acknowledgments
The authors are very grateful for significant input from Dr. Thomas F. Brewer for careful review of this manuscript while in preparation. P.L. thanks the Genentech Postdoctoral program for support.
Glossary
Abbreviations
- DELT
DNA-encoded library technology
- μMap
microenvironment mapping
- SET
single-electron transfer
- ET
energy transfer
- PC
photocatalyst
- LED
light-emitting diode
- TRTs
tyrosyl radical trapping reagents
- BSA
bovine serum albumin
- dtbbpy
4,4′-di-tert-butyl-2,2′-dipyridyl
- TTMSS
tris(trimethylsilyl)silane
- THP
tetrahydropyran
- CFL
compact fluorescent light
- BPO
benzoyl peroxide
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxy
- PMP
1,2,2,6,6-pentamethylpiperidine
- 4-HTP
4-hydroxythiophenol
- TMP
2,2,6,6-tetramethylpiperidine
- 4-DPA-IPN
2,4,5,6-tetrakis(diphenylamino)isophthalonitrile
- DCE
1,2-dichloroethane
- NMP
N-methyl-2-pyrrolidone
- DPBS
Dulbecco’s phosphate-buffered saline
- MTPs
microtiter plates
- HTE
high-throughput experimentation
- 4-CzIPN
2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile
- SPPLAT
selective proteomic proximity labeling assay using tyramide
- APEX
ascorbic acid peroxidase
The authors declare no competing financial interest.
References
- Yan M.; Lo J. C.; Edwards J. T.; Baran P. S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714. 10.1021/jacs.6b08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran D. P.; Rakiewicz D. M. Tandem radical approach to linear condensed cyclopentanoids. Total synthesis of (±)-hirsutene. J. Am. Chem. Soc. 1985, 107, 1448–1449. 10.1021/ja00291a077. [DOI] [Google Scholar]
- Curran D. P.; Rakiewicz D. M. Radical-initiated polyolefinic cyclizations in linear triquinane synthesis. Model studies and total synthesis of (±)-hirsutene. Tetrahedron 1985, 41, 3943–3958. 10.1016/S0040-4020(01)97175-3. [DOI] [Google Scholar]
- Karkas M. D.; Stephenson C. R. J.; Porco J. A. Jr. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. 2016, 116, 9683–9747. 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008, 108, 1052–1103. 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]
- Tucker J. W.; Stephenson C. R. Shining light on photoredox catalysis: theory and synthetic applications. J. Org. Chem. 2012, 77, 1617–1622. 10.1021/jo202538x. [DOI] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter D. L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. 10.1063/1.1699044. [DOI] [Google Scholar]
- Lin S. H.; Xiao W. Z.; Dietz W. Generalized Foerster-Dexter theory of photoinduced intramolecular energy transfer. Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top. 1993, 47, 3698–3706. 10.1103/PhysRevE.47.3698. [DOI] [PubMed] [Google Scholar]
- Van Bergen T. J.; Hedstrand D. M.; Kruizinga W. H.; Kellogg R. M. Chemistry of dihydropyridines. 9. Hydride transfer from 1,4-dihydropyridines to sp3-hybridized carbon in sulfonium salts and activated halides. Studies with NAD(P)H models. J. Org. Chem. 1979, 44, 4953–4962. 10.1021/jo00394a044. [DOI] [Google Scholar]
- Nicewicz D. A.; MacMillan D. W. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischay M. A.; Anzovino M. E.; Du J.; Yoon T. P. Efficient visible light photocatalysis of [2 + 2] enone cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]
- Narayanam J. M.; Tucker J. W.; Stephenson C. R. Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 2009, 131, 8756–8757. 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]
- Douglas J. J.; Sevrin M. J.; Stephenson C. R. J. Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents. Org. Process Res. Dev. 2016, 20, 1134–1147. 10.1021/acs.oprd.6b00125. [DOI] [Google Scholar]
- Shaw M. H.; Twilton J.; MacMillan D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. C.; McAtee R. C.; McClain E. J.; Stephenson C. R. J. Advancements in Visible-Light-Enabled Radical Csp2-H Alkylation of (Hetero)arenes. Synthesis 2019, 51, 1063–1072. 10.1055/s-0037-1611658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Geri J. B.; Oakley J. V.; Reyes-Robles T.; Wang T.; McCarver S. J.; White C. H.; Rodriguez-Rivera F. P.; Parker D. L. Jr.; Hett E. C.; Fadeyi O. O.; Oslund R. C.; MacMillan D. W. C. Microenvironment mapping via Dexter energy transfer on immune cells. Science 2020, 367, 1091–1097. 10.1126/science.aay4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubi K. L.; Blum T. R.; Yoon T. P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvi M.; Melchiorre P.; Melchiorre P. Enhancing the potential of enantioselective organocatalysis with light. Nature 2018, 554, 41–49. 10.1038/nature25175. [DOI] [PubMed] [Google Scholar]
- Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan D. W. C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- Bogdos M. K.; Murphy J. A.; Pinard E. Applications of organocatalysed visible-light photoredox reactions for medicinal chemistry. Beilstein J. Org. Chem. 2018, 14, 2035–2064. 10.3762/bjoc.14.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos K. R.; Coleman P. J.; Alvarez J. C.; Dreher S. D.; Garbaccio R. M.; Terrett N. K.; Tillyer R. D.; Truppo M. D.; Parmee E. R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805 10.1126/science.aat0805. [DOI] [PubMed] [Google Scholar]
- Crisenza G. E. M.; Melchiorre P. Chemistry glows green with photoredox catalysis. Nat. Commun. 2020, 11, 803. 10.1038/s41467-019-13887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D.; Campeau L.-C. Harder, better, faster. Nat. Chem. 2020, 12, 661–664. 10.1038/s41557-020-0510-8. [DOI] [PubMed] [Google Scholar]
- Beck A.; Goetsch L.; Dumontet C.; Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discovery 2017, 16, 315–337. 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos N.; Francis M. B. Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 2011, 7, 876–884. 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]
- Kim K.; Fancy D. A.; Carney D.; Kodadek T. Photoinduced Protein Cross-Linking Mediated by Palladium Porphyrins. J. Am. Chem. Soc. 1999, 121, 11896–11897. 10.1021/ja9916355. [DOI] [Google Scholar]
- Fancy D. A.; Kodadek T. Chemistry for the analysis of protein-protein interactions: rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 1317. 10.1073/pnas.97.3.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duroux-Richard I.; Vassault P.; Subra G.; Guichou J.-F.; Richard E.; Mouillac B.; Barberis C.; Marie J.; Bonnafous J.-C. Crosslinking Photosensitized by a Ruthenium Chelate as a Tool for Labeling and Topographical Studies of G-Protein-Coupled Receptors. Chem. Biol. 2005, 12, 15–24. 10.1016/j.chembiol.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Bottecchia C.; Noel T. Photocatalytic modification of amino acids, peptides, and proteins. Chem. - Eur. J. 2019, 25, 26–42. 10.1002/chem.201803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S.; Nakamura H. Ligand-Directed Selective Protein Modification Based on Local Single-Electron Transfer Catalysis. Angew. Chem., Int. Ed. 2013, 52, 8681–8684. 10.1002/anie.201303831. [DOI] [PubMed] [Google Scholar]
- McCarver S. J.; Qiao J. X.; Carpenter J.; Borzilleri R. M.; Poss M. A.; Eastgate M. D.; Miller M.; MacMillan D. W. C. Decarboxylative peptide macrocyclization through photoredox catalysis. Angew. Chem., Int. Ed. 2017, 56, 728–732. 10.1002/anie.201608207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Zhang L.-K.; Buevich A. V.; Li G.; Tang H.; Vachal P.; Colletti S. L.; Shi Z.-C. Chemoselective Peptide Modification via Photocatalytic Tryptophan β-Position Conjugation. J. Am. Chem. Soc. 2018, 140, 6797–6800. 10.1021/jacs.8b03973. [DOI] [PubMed] [Google Scholar]
- Bloom S.; Liu C.; Kölmel D. K.; Qiao J. X.; Zhang Y.; Poss M. A.; Ewing W. R.; MacMillan D. W. C. Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials. Nat. Chem. 2018, 10, 205–211. 10.1038/nchem.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiishi N.; Caldwell J. P.; Lin M.; Zhong W.; Zhu X.; Streckfuss E.; Kim H.-Y.; Parish C. A.; Krska S. W. Protecting group free radical C-H trifluoromethylation of peptides. Chem. Sci. 2018, 9, 4168–4175. 10.1039/C8SC00368H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellis J. C.; Primer D. N.; Molander G. A. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 2014, 345, 433–436. 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Le C. C.; MacMillan D. W. C. Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc. 2016, 138, 8084–8087. 10.1021/jacs.6b04818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H. A.; Liu W.; Le C. C.; MacMillan D. W. C. Cross-Electrophile Coupling of Unactivated Alkyl Chlorides. J. Am. Chem. Soc. 2020, 142, 11691–11697. 10.1021/jacs.0c04812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. A.; Phelan J. P.; Badir S. O.; Molander G. A. Alkyl Carbon-Carbon Bond Formation by Nickel/Photoredox Cross-Coupling. Angew. Chem., Int. Ed. 2019, 58, 6152–6163. 10.1002/anie.201809431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski A. W.; Gesmundo N. J.; Aguirre A. L.; Sarris K. A.; Young J. M.; Bogdan A. R.; Martin M. C.; Gedeon S.; Wang Y. Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)-C(sp3) Cross-Coupling Methods by Library Synthesis. ACS Med. Chem. Lett. 2020, 11, 597–604. 10.1021/acsmedchemlett.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C. P.; Smith R. T.; Allmendinger S.; MacMillan D. W. C. Metallaphotoredox-catalysed sp3-sp3 cross-coupling of carboxylic acids with alkyl halides. Nature 2016, 536, 322–325. 10.1038/nature19056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. T.; Zhang X.; Rincon J. A.; Agejas J.; Mateos C.; Barberis M.; Garcia-Cerrada S.; de Frutos O.; MacMillan D. W. C. Metallaphotoredox-Catalyzed Cross-Electrophile Csp3-Csp3 Coupling of Aliphatic Bromides. J. Am. Chem. Soc. 2018, 140, 17433–17438. 10.1021/jacs.8b12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak T.; Dykstra K. D.; Tyagarajan S.; Vachal P.; Krska S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. 10.1039/C5CS00628G. [DOI] [PubMed] [Google Scholar]
- Moir M.; Danon J. J.; Reekie T. A.; Kassiou M. An overview of late-stage functionalization in today’s drug discovery. Expert Opin. Drug Discovery 2019, 14, 1137–1149. 10.1080/17460441.2019.1653850. [DOI] [PubMed] [Google Scholar]
- Meanwell N. A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- Patani G. A.; LaVoie E. J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. 10.1021/cr950066q. [DOI] [PubMed] [Google Scholar]
- Nugent J.; Arroniz C.; Shire B. R.; Sterling A. J.; Pickford H. D.; Wong M. L. J.; Mansfield S. J.; Caputo D. F. J.; Owen B.; Mousseau J. J.; Duarte F.; Anderson E. A. A General Route to Bicyclo[1.1.1]pentanes through Photoredox Catalysis. ACS Catal. 2019, 9, 9568–9574. 10.1021/acscatal.9b03190. [DOI] [Google Scholar]
- Kolahdouzan K.; Khalaf R.; Grandner J. M.; Chen Y.; Terrett J. A.; Huestis M. P. Dual Photoredox/Nickel-Catalyzed Conversion of Aryl Halides to Aryl Aminooxetanes: Computational Evidence for a Substrate-Dependent Switch in Mechanism. ACS Catal. 2020, 10, 405–411. 10.1021/acscatal.9b03596. [DOI] [Google Scholar]
- VanHeyst M. D.; Qi J.; Roecker A. J.; Hughes J. M. E.; Cheng L.; Zhao Z.; Yin J. Continuous Flow-Enabled Synthesis of Bench-Stable Bicyclo[1.1.1]pentane Trifluoroborate Salts and Their Utilization in Metallaphotoredox Cross-Couplings. Org. Lett. 2020, 22, 1648–1654. 10.1021/acs.orglett.0c00242. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Smith R. T.; Le C.; MacMillan D. W. C.; McCarver S. J.; Shireman B. T.; Carruthers N. I. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 2020, 580, 220–226. 10.1038/s41586-020-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagib D. A.; MacMillan D. W. C. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 2011, 480, 224–228. 10.1038/nature10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovics R.; Passamonti F.; Buser A. S.; Teo S.-S.; Tiedt R.; Passweg J. R.; Tichelli A.; Cazzola M.; Skoda R. C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Douglas J. J.; Cole K. P.; Stephenson C. R. J. Photoredox Catalysis in a Complex Pharmaceutical Setting: Toward the Preparation of JAK2 Inhibitor LY2784544. J. Org. Chem. 2014, 79, 11631–11643. 10.1021/jo502288q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRocco D. A.; Dykstra K.; Krska S.; Vachal P.; Conway D. V.; Tudge M. Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew. Chem., Int. Ed. 2014, 53, 4802–4806. 10.1002/anie.201402023. [DOI] [PubMed] [Google Scholar]
- Jin J.; MacMillan D. W. C. Alcohols as alkylating agents in heteroarene C-H functionalization. Nature 2015, 525, 87–90. 10.1038/nature14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.; MacMillan D. W. C. Direct α-Arylation of Ethers through the Combination of Photoredox-Mediated C-H Functionalization and the Minisci Reaction. Angew. Chem., Int. Ed. 2015, 54, 1565–1569. 10.1002/anie.201410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff C. A.; Cohen R. D.; Dykstra K. D.; Streckfuss E.; DiRocco D. A.; Krska S. W. Photoredox-Catalyzed Hydroxymethylation of Heteroaromatic Bases. J. Org. Chem. 2016, 81, 6980–6987. 10.1021/acs.joc.6b00811. [DOI] [PubMed] [Google Scholar]
- Niu L.; Liu J.; Liang X.-A.; Wang S.; Lei A. Visible light-induced direct α C-H functionalization of alcohols. Nat. Commun. 2019, 10, 467. 10.1038/s41467-019-08413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus J. B.; Nicewicz D. A. Direct C-H Cyanation of Arenes via Organic Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 2880–2883. 10.1021/jacs.6b12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Margrey K. A.; Tay N. E.; Nicewicz D. A. Site-selective arene C-H amination via photoredox catalysis. Science 2015, 349, 1326–1330. 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]
- Margrey K. A.; Levens A.; Nicewicz D. A. Direct Aryl C-H Amination with Primary Amines using Organic Photoredox Catalysis. Angew. Chem., Int. Ed. 2017, 56, 15644–15648. 10.1002/anie.201709523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger R.; Heightman T. D.; Ley S. V.; Lima F.; Johnson C. N. Enabling synthesis in fragment-based drug discovery by reactivity mapping: photoredox-mediated cross-dehydrogenative heteroarylation of cyclic amines. Chem. Sci. 2019, 10, 2264–2271. 10.1039/C8SC04789H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D. B.; Seath C. P.; Wang H.; Jui N. T. Selective C-F Functionalization of Unactivated Trifluoromethylarenes. J. Am. Chem. Soc. 2019, 141, 13203–13211. 10.1021/jacs.9b06004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J. B. I.; Straathof N. J. W.; Knauber T.; Meyer C. F.; Medebielle M.; Buglioni L.; Genicot C.; Trabanco A. A.; Noel T.; am Ende C. W.; Gouverneur V. Organophotoredox Hydrodefluorination of Trifluoromethylarenes with Translational Applicability to Drug Discovery. J. Am. Chem. Soc. 2020, 142, 9181–9187. 10.1021/jacs.0c03881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. Y.; Nagao K.; Hoover A. J.; Hesk D.; Rivera N. R.; Colletti S. L.; Davies I. W.; MacMillan D. W. C. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 2017, 358, 1182–1187. 10.1126/science.aap9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F.; Fernandez-Rodriguez P.; Mishra A.; Weck R.; Bauer A.; Sandvoss M.; Ruf S.; Mendez M.; Mora-Rado H.; Rackelmann N.; Poverlein C.; Derdau V. Photoredox-Mediated Hydrogen Isotope Exchange Reactions of Amino-Acids, Peptides, and Peptide-Derived Drugs. Chem. - Eur. J. 2020, 26, 1–6. 10.1002/chem.202003464. [DOI] [PubMed] [Google Scholar]
- Preshlock S.; Tredwell M.; Gouverneur V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. 2016, 116, 719–766. 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- Yuan Z.; Nodwell M. B.; Yang H.; Malik N.; Merkens H.; Benard F.; Martin R. E.; Schaffer P.; Britton R. Site-Selective, Late-Stage C-H 18F-Fluorination on Unprotected Peptides for Positron Emission Tomography Imaging. Angew. Chem., Int. Ed. 2018, 57, 12733–12736. 10.1002/anie.201806966. [DOI] [PubMed] [Google Scholar]
- Chen W.; Huang Z.; Tay N. E. S.; Giglio B.; Wang M.; Wang H.; Wu Z.; Nicewicz D. A.; Li Z. Direct arene C-H fluorination with 18F− via organic photoredox catalysis. Science 2019, 364, 1170–1174. 10.1126/science.aav7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S.; Lerner R. A. Encoded combinatorial chemistry. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 5381–5383. 10.1073/pnas.89.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri D.; Lerner R. A. DNA-Encoded Chemical Libraries: A Selection System Based on Endowing Organic Compounds with Amplifiable Information. Annu. Rev. Biochem. 2018, 87, 479–502. 10.1146/annurev-biochem-062917-012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Kamlet A. S.; Steinman J. B.; Liu D. R. A biomolecule-compatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system. Nat. Chem. 2011, 3, 146–153. 10.1038/nchem.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölmel D. K.; Loach R. P.; Knauber T.; Flanagan M. E. Employing Photoredox Catalysis for DNA-Encoded Chemistry: Decarboxylative Alkylation of α-Amino Acids. ChemMedChem 2018, 13, 2159–2165. 10.1002/cmdc.201800492. [DOI] [PubMed] [Google Scholar]
- Phelan J. P.; Lang S. B.; Sim J.; Berritt S.; Peat A. J.; Billings K.; Fan L.; Molander G. A. Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis. J. Am. Chem. Soc. 2019, 141, 3723–3732. 10.1021/jacs.9b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badir S. O.; Sim J.; Zhang X.; Dong W.; Molander G. A.; Billings K.; Csakai A. Multifunctional Building Blocks Compatible with Photoredox-Mediated Alkylation for DNA-Encoded Library Synthesis. Org. Lett. 2020, 22, 1046–1051. 10.1021/acs.orglett.9b04568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellis J. C.; Kelly C. B.; Primer D. N.; Jouffroy M.; Patel N. R.; Molander G. A. Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3-sp2 Cross-Coupling. Acc. Chem. Res. 2016, 49, 1429–1439. 10.1021/acs.accounts.6b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölmel D. K.; Meng J.; Tsai M. H.; Que J.; Loach R. P.; Knauber T.; Wan J.; Flanagan M. E. On-DNA Decarboxylative Arylation: Merging Photoredox with Nickel Catalysis in Water. ACS Comb. Sci. 2019, 21, 588–597. 10.1021/acscombsci.9b00076. [DOI] [PubMed] [Google Scholar]
- Ruff Y.; Martinez R.; Pelle X.; Nimsgern P.; Fille P.; Ratnikov M.; Berst F. An Amphiphilic Polymer-Supported Strategy Enables Chemical Transformations under Anhydrous Conditions for DNA-Encoded Library Synthesis. ACS Comb. Sci. 2020, 22, 120–128. 10.1021/acscombsci.9b00164. [DOI] [PubMed] [Google Scholar]
- Rees J. S.; Li X.-W.; Perrett S.; Lilley K. S.; Jackson A. P. Selective Proteomic Proximity Labeling Assay Using Tyramide (SPPLAT): A Quantitative Method for the Proteomic Analysis of Localized Membrane-Bound Protein Clusters. Curr. Protoc. Protein Sci. 2017, 88, 19.27.11–19.27.18. 10.1002/cpps.27. [DOI] [PubMed] [Google Scholar]
- Chen C.-L.; Perrimon N.. Proximity-dependent labeling methods for proteomic profiling in living cells. Wiley Interdiscip. Rev.: Dev. Biol. 2017, 6, e272. 10.1002/wdev.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton B. Z.; Chory E. J.; Crabtree G. R. Chemically induced proximity in biology and medicine. Science 2018, 359, 1117. 10.1126/science.aao5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg E.; Borner G. H. H. Spatial proteomics: a powerful discovery tool for cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 285–302. 10.1038/s41580-018-0094-y. [DOI] [PubMed] [Google Scholar]
- Minter R. R.; Sandercock A. M.; Rust S. J. Phenotypic screening-the fast track to novel antibody discovery. Drug Discovery Today: Technol. 2017, 23, 83–90. 10.1016/j.ddtec.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Brunner J.; Senn H.; Richards F. M. 3-Trifluoromethyl-3-phenyldiazirine. A new carbene generating group for photolabeling reagents. J. Biol. Chem. 1980, 255, 3313–3318. [PubMed] [Google Scholar]
- Raviv Y.; Salomon Y.; Gitler C.; Bercovici T. Selective labeling of proteins in biological systems by photosensitization of 5-iodonaphthalene-1-azide. Proc. Natl. Acad. Sci. U. S. A. 1987, 84, 6103–6107. 10.1073/pnas.84.17.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv Y.; Bercovici T.; Gitler C.; Salomon Y. Selective photoinduced uncoupling of the response of adenylate cyclase to gonadotropins by 5-iodo-1-naphthylazide. Biochemistry 1984, 23, 503–508. 10.1021/bi00298a016. [DOI] [PubMed] [Google Scholar]
- Tucker J. W.; Zhang Y.; Jamison T. F.; Stephenson C. R. J. Visible-light photoredox catalysis in flow. Angew. Chem., Int. Ed. 2012, 51, 4144–4147. 10.1002/anie.201200961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambie D.; Bottecchia C.; Straathof N. J. W.; Hessel V.; Noel T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]
- Grimm I.; Hauer S. T.; Schulte T.; Wycich G.; Collins K. D.; Lovis K.; Candish L. Upscaling Photoredox Cross-Coupling Reactions in Batch Using Immersion-Well Reactors. Org. Process Res. Dev. 2020, 24, 1185–1193. 10.1021/acs.oprd.0c00070. [DOI] [Google Scholar]
- Le C. C.; Wismer M. K.; Shi Z.-C.; Zhang R.; Conway D. V.; Li G.; Vachal P.; Davies I. W.; MacMillan D. W. C. A general small-scale reactor to enable standardization and acceleration of photocatalytic reactions. ACS Cent. Sci. 2017, 3, 647–653. 10.1021/acscentsci.7b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]