Important Compound Classes
Title
Degraders of Wild-type and Mutant Forms of LRRK2.
Patent Publication Number
WO 2020/081682 A1
Publication Date
April 23, 2020
Priority Application
US 62/746,283 and US 62/884,410
Priority Date
October 16, 2018, and August 8, 2019
Inventors
Gray, N. S.; Hatcher, J.
Assignee Company
Dana-Farber Cancer Institute, INC.; 450 Brookline Avenue, Boston, Massachusetts 02215, USA.
Disease Area
Parkinson’s Disease (PD)
Biological Target
Leurine-rich repeat kinase 2 (LRRK2).
Summary
Parkinson’s disease (PD) is a chronic and the second most progressive neurodegenerative disorder after Alzheimer’s disease, affecting ∼1% of the population at age 65 years to ∼5% at age 85 years old, with onset of the disorder at mean age 55 years. PD is characterized by the loss of dopaminergic neurons of the substantia nigra pars compacta (SNpc) and the presence of intraneuronal proteinacious cytoplasmic inclusions (Lewy bodies) in surviving neurons. Symptoms associated with PD involve a large number of motoric and nonmotoric symptoms such as tremor, postural instability, rigidity, bradykinesia, cognitive dysfunction, sleep disruption, and autonomic dysfunction, which may greatly reduce the quality of life.
The typical hallmarks of PD in post-mortem brain tissue are loss of dopaminergic neurons of the substantia nigra, and also epidemiological studies have implicated environmental and inflammatory pathways. However, the exact etiology of this devastating disease is still largely unknown, and several mechanisms are implicated in the selective degeneration of the dopaminergic neurons in PD. For instance, PD pathogenesis may involve mitochondrial dysfunction, increasing oxidative stress and the dysfunction in the ubiquitin proteasome system, which potentially lead to the aggregation of oligomeric proteins.
There are more than 22 genes associated with PD, and mutations in most PD-associated genes are linked to the early onset or pathologically atypical forms of the disease. In addition, mutations in the most recently identified PD-associated gene, the leucine-rich repeat kinase 2 (LRRK2) having a missense mutation at G2019S, have been found in both familial and sporadic PD cases. The G2019S mutation increases kinase activity that leads to the activation of the neuronal death signal pathway. The genetic link of LRRK2 to PD is very strong; however, understanding exactly how mutations contribute to PD risk is complicated. LRRK2 has been detected in many essential areas such as the thymus, kidney, lung, lymph nodes, midbrain dopamine (DA) neurons, striatum, hippocampus, and cerebral cortex. However, the highest levels of LRRK2 are found in immune cells, including circulating B lymphocytes, dendritic cells, and macrophages.
The LRRK2 is located on chromosome 12 and consists of 51 exons encoding a 2527 amino acid protein with a complex domain structure. The encoded protein has several regions involved in protein–protein interactions and an unusual two domains with catalytic activity; a GTPase domain of the Ras of complex (ROC) protein family and a kinase domain of the tyrosine kinase like (TKL) family. The complex interplay between the catalytic GTPase and kinase activities ultimately lead to an increase in LRRK2 kinase activity, which has provided a rationale for the development of LRRK2 kinase as potential PD therapeutics.
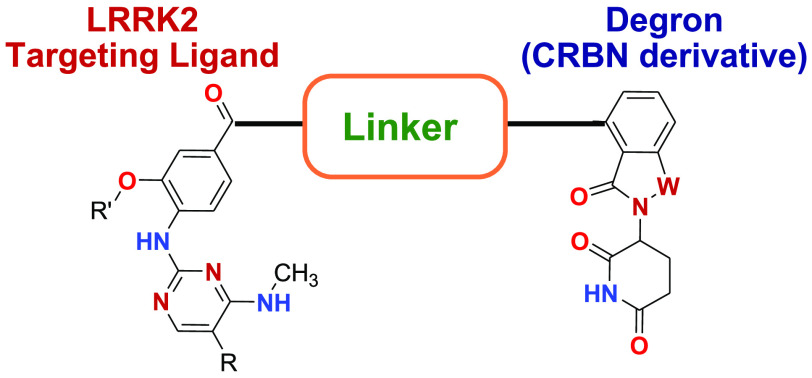
This Patent Highlight has representative bifunctional compounds (degrader or PROTAC), which consist of targeting ligand (an aminopyrimidine or indazole) that binds LRRK2, the degron which represents a ligand that binds an E3 ubiquitin ligase, and the linker which has a moiety that connects covalently the degron and the targeting ligand. The PROTAC compounds degrade LRRK2 that is involved in the genesis or progression of disease via the cell’s ubiquitin/proteasome system, whose function is to routinely identify and remove damaged proteins. The degron functional moiety recruits the E3 ubiquitin ligase to tag LRRK2 for ubiquitination and degradation through the proteasome, which is a large endogenous complex that degrades the ubiquitinated protein into small peptide fragments. After destruction of a LRRK2 molecule, the degrader is released and continues to be active in a catalytic fashion. The degraders may achieve an efficient degradation with lower affinity warheads and cause complete elimination of the protein by the proteasome, and the pharmacodynamic effects are dictated by protein resynthesis rates which are similar to that observed for covalent inhibitors.
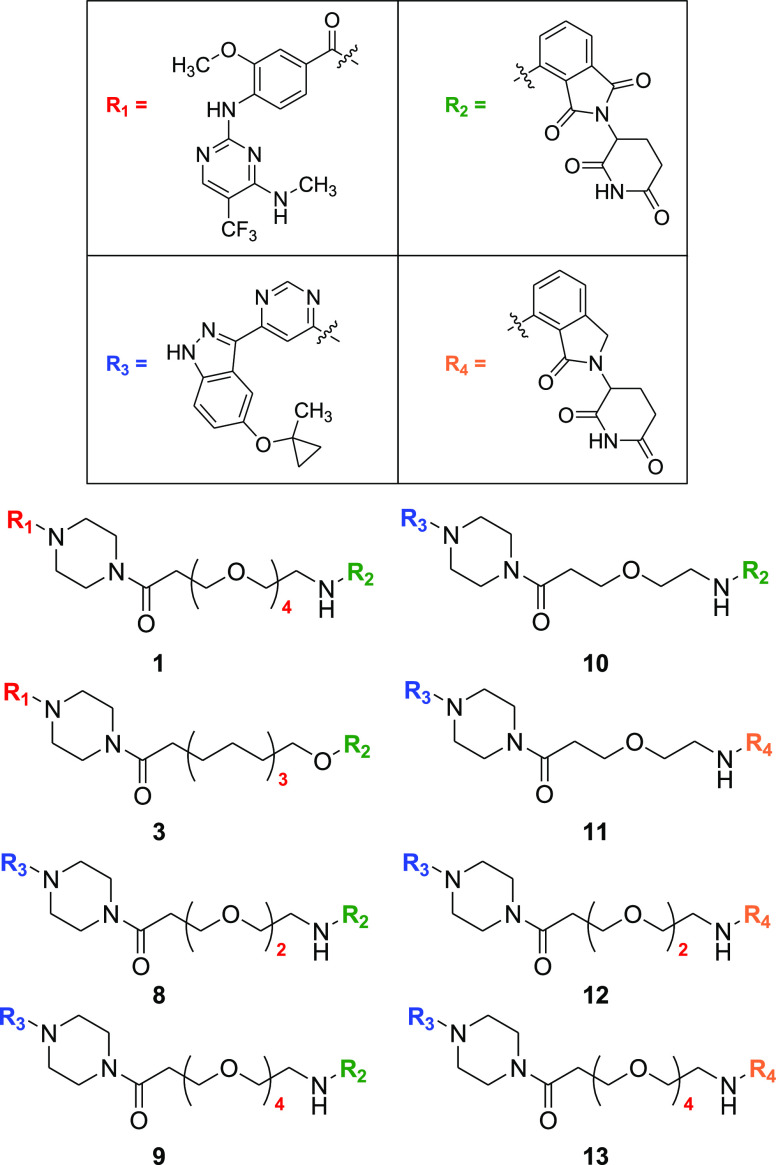
Key Structures
Biological Assay
Cell lines used were mouse embryonic fibroblast (MEF) WT, LRRK2 homozygous knock-ins in MEFs [R1441C; VPS25N(D620N); G2019S]. In addition, biological assays used were the Bradford protein assay, Western blot analysis, and Invitrogen’s Adapta assay
Biological Data
Compounds 1 and 3 inhibited the phosphorylation of S935 and Rab10 but did
not degrade LRRK2. On the other hand, compounds 8–13 inhibited
the phosphorylation of S935 and also decreased in the level of LRRK2.
The table below shows IC50 for inhibition of LRRK2 WT and
LRRK2 G2019S.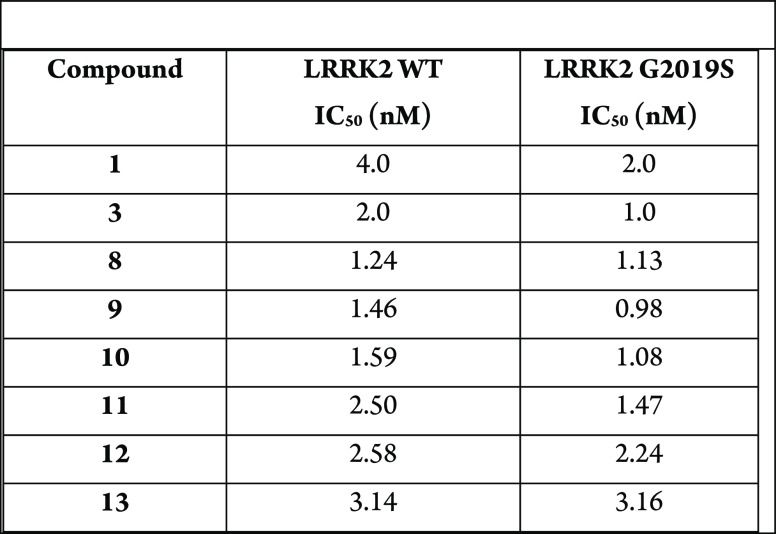
Recent Review Articles
-
1.
Madureira M.; Connor-Robson N.; Wade-Martins R.. Front. Neurosci. 2020, 14, 498.
-
2.
Tayla M.; Alessi D. R.. Curr. Opin. Cell Biol. 2020, 63, 102.
-
3.
Tolosa E.; Vila M.; Klein C.; Rascol O.. Nat. Rev. Neurol. 2020, 16, 97.
-
4.
Lamonaca G.; Volta M.. Cells 2020, 9, 1115.
-
5.
Kuusimaki T.; Korpela J.; Pekkonen E.; Martikinen M. H.; Antonini A.; Kaasinen V.. J. Neurol. 2020, 267, 883.
-
6.
Parekh P.; Sharma N.; Gadepalli A.; Shahane A.; Sharma M.; Khairnar A.. ACS Chem. Neurosci. 2019, 10, 3914.
The author declares no competing financial interest.