Human herpesvirus 6A (HHV-6A) and human herpesvirus 6B (HHV-6B), collectively termed HHV-6A/B, are neurotropic viruses that permanently infect most humans from an early age. Although most people infected with these viruses appear to suffer no ill effects, the viruses are a well-established cause of encephalitis in immunocompromised patients. In this review, we summarize the evidence that the viruses may also be one trigger for febrile seizures (including febrile status epilepticus) in immunocompetent infants and children, mesial temporal lobe epilepsy, multiple sclerosis (MS), and, possibly, Alzheimer’s disease.
KEYWORDS: Alzheimer's disease, febrile seizures, febrile status epilepticus, mesial temporal lobe epilepsy, multiple sclerosis, human herpesvirus 6A, human herpesvirus 6B
SUMMARY
Human herpesvirus 6A (HHV-6A) and human herpesvirus 6B (HHV-6B), collectively termed HHV-6A/B, are neurotropic viruses that permanently infect most humans from an early age. Although most people infected with these viruses appear to suffer no ill effects, the viruses are a well-established cause of encephalitis in immunocompromised patients. In this review, we summarize the evidence that the viruses may also be one trigger for febrile seizures (including febrile status epilepticus) in immunocompetent infants and children, mesial temporal lobe epilepsy, multiple sclerosis (MS), and, possibly, Alzheimer’s disease. We propose criteria for linking ubiquitous infectious agents capable of producing lifelong infection to any neurologic disease, and then we examine to what extent these criteria have been met for these viruses and these diseases.
INTRODUCTION
Human herpesvirus 6A (HHV-6A) (1) and human herpesvirus 6B (HHV-6B) (2), collectively termed HHV-6A/B, were discovered about 30 years ago. Since then, an increasing body of evidence has linked these viruses to several important neurological diseases. The linkage is hard to prove conclusively because the viruses are ubiquitous: most humans are permanently infected from an early age (3), yet only a few experience disease potentially related to the viruses. In addition, for diseases of the central nervous system, the affected tissue is difficult to access for study in living patients.
In our opinion, it now is established that these viruses, particularly HHV-6B, cause encephalitis in immunocompromised patients (4–7). They can cause encephalitis in immunocompetent children (8–14) and adults (15, 16). Because several recent reviews and reports (4–7, 17) have summarized the extensive literature on the role of HHV-6A/B in causing encephalitis, we will discuss encephalitis only briefly in this review.
Instead, we first will summarize the relevant biology of HHV-6A/B and then discuss studies of the possible role of these viruses in several neurological diseases: febrile seizures (FS) in infants and young children, mesial temporal lobe epilepsy (MTLE), multiple sclerosis (MS). and Alzheimer’s disease (AD). Since not all early studies distinguished between HHV-6A and HHV-6B, we will simply refer throughout to HHV-6A/B rather than HHV-6 and indicate specifically when research has distinguished between the two related viruses.
Finally, we will propose explicit criteria for linking ubiquitous infectious agents like these viruses to a disease and then consider which of these criteria have been met in linking HHV-6A/B to each disease.
BIOLOGY OF HHV-6A/B
Virion Structure and Genome
HHV-6A/B are members of the Roseolovirus genus of the betaherpesvirus subfamily. They share with the other human herpesviruses a similar virion structure and genomic architecture. While most proteins of the two viruses have very similar sequences, a few proteins have substantially different sequences, and each virus has some unique gene products. Presumably, these genetic differences account for the differences between the viruses in their cellular tropism and disease associations. Some of the viral genes can regulate the transcription of human cellular genes. For example, HHV-6A induces cell surface expression of CD4 and downregulates cell surface expression of CD3 (18).
Primary Infection
Primary infection usually occurs between 6 months, when maternal antibodies wane, and 2 years of age (8, 19). As occurs with other herpesviruses, these viruses can establish latency within T cells and various central nervous system (CNS) cells and persist for the rest of a person’s life, retaining the capacity to reactivate and begin replicating again. The viral gene U94 may play an important role in establishing latency (20).
Whereas most herpesviruses achieve latency through the formation of circular episomes associated with nuclear proteins, it has been hypothesized that HHV-6A/B maintain their latent genomes by integration into the subtelomeric region of a somatic cell, a process facilitated by telomeric repeats at the end of its genome (21). Pathogenicity can occur during lytic reactivations, as well as during altered states associated with latency (22). Over 95% of adults are persistently infected with HHV-6B, and a smaller but substantial fraction are persistently infected with HHV-6A.
Initial infection occurs most often from the contact of infected saliva or nasal mucus with the respiratory tract, including the tonsils (epithelial cells and adjacent lymphoid cells) (18) and olfactory ensheathing cells of the nasal cavity (23). The viruses can reach the CNS by both hematogenous spread (infected lymphocytes penetrating the blood-brain barrier [BBB]) and retrograde movement up the olfactory nerve and perhaps other peripheral nerves (23). Initial primary infection can also occur through inheritance, as described just below, congenitally, and via organ transplantation. We are aware of no evidence of documented primary infection from blood transfusions or breastfeeding (20).
iciHHV-6
The capacity of HHV-6A/B to insert their entire genomes into the telomeres of host cell chromosomes was exploited on several occasions, early in human (and hominin) history, in germ cells. As a result, about 1% of the human race is born with the entire virus genome inside every cell, which is called inherited chromosomally integrated HHV-6 (iciHHV-6) (21, 24, 25). The inherited viral genome can be transcriptionally active, capable of producing viral proteins and even infectious virions. Investigators have begun to explore the health consequences of iciHHV-6 (26).
The ability of HHV-6A/B to integrate their genomes into the telomeres of both somatic cells and germ cells is illustrated in Fig. 1.
FIG 1.
Consequences of HHV-6A/B DNA integration into a chromosome of a somatic cell and into the chromosome of a germ cell. (A) Acquired virus integrating its DNA (in red) into the telomere of a chromosome of a somatic cell. It has been hypothesized that this is a mechanism by which HHV-6A/B achieves latency. Since the viral genome is not integrated into the DNA of sperm or ova, no vertical transmission of the viral genome occurs. (Note that this figure shows only 1 of the 23 chromosomes and assumes that viral infection and integration occur in the G1 phase and then the virus is replicated in the S phase and passed to both of the daughter cells during mitosis. When the integration event occurs during S or G2 phases [not shown], the daughter cells create a mosaic, since one contains the viral genome and the other does not.) (B) Description of an ancient event: on several occasions in human history, the viral genome integrated into the DNA of a haploid germ cell chromosome. This led to a fertilized ovum containing the viral genome and, hence, to a human with the viral genome integrated into a chromosome in every cell, i.e., with inherited chromosomally integrated HHV-6 (iciHHV-6). In Mendelian fashion, the integrated viral DNA is present in 50% of gametes (whether sperm or ova). About 1% of humans are born with iciHHV-6. The inherited presence of the viral genome in every cell, including germ cells, contrasts with the integration of acquired virus into only a small fraction of target somatic cells.
Cell and Tissue Tropism
Both viruses are unusual in their ability to infect a broad spectrum of cell types. This probably is because both viruses (particularly HHV-6A) can use CD46 as a cellular receptor (27). CD46 is the complement receptor and is expressed by all nucleated human cells. The cellular receptor used primarily by HHV-6B is CD134 (28). Organs infected by the viruses include the brain, salivary glands, tonsils, liver, kidneys, lymph nodes, endothelial cells, and multiple types of leucocytes (20). HHV-6A/B can also infect cell lines derived from endothelial cells, respiratory epithelial cells, and fibroblasts (29).
The viruses infect cells of the CNS, as summarized in Table 1. Compared to HHV-6B, HHV-6A more easily infects cultured cells of neuronal origin and is more likely to establish a productive lytic infection that results in cytopathic effects (30).
TABLE 1.
Central nervous system cells infectible by HHV-6A/B
| Cell category | Cell type |
|---|---|
| Glial cells | Fetal astrocytes (109, 230–232) |
| Adult astrocytes (109, 230, 231) | |
| Olfactory ensheathing cells (23, 230) | |
| Microglial cells (30, 229, 230) | |
| Primary adult oligodendrocytes (229, 230, 232) | |
| Primary oligodendrocyte precursor cells (190, 230, 307) | |
| Oligodendrocyte cell lines (30, 212, 230, 308) | |
| Neurons | Adult neurons (230, 232) |
| Cerebellar Purkinje cells (309) |
Figure 2 demonstrates HHV-6 infection of glial cells, as shown both by electron microscopy (31) (Fig. 2A) and by costaining to reveal both cellular and viral markers (32) (Fig. 2B).
FIG 2.
(A) Infection of human-derived astrocytes demonstrates many viral particles (asterisks), suggesting complete replication cycle in astrocytes. (Reproduced from reference 31 with permission of the publisher.) (B) Cultured primary astrocytes were isolated from fresh brain material obtained during MTLE brain resection and costained for the nonvariant-specific HHV-6 gp116 surface glycoprotein (green) and for glial fibrillary acidic protein (GFAP) as a marker for astrocytes (blue). (Reproduced from reference 32.)
With regard to the diseases considered here, HHV-6A/B can also infect various cells of the immune system (18, 20), particularly CD4+ T cells, NK cells, monocyte/macrophages (an important site of latency), myeloid cells, and endothelial cells (33). Infection of these cells leads to the production of various chemokines (34) as well as proinflammatory cytokines, including interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), alpha interferon (IFN-α), IFN-γ, and IL-6 (35–39). It also decreases expression of IL-10 (40, 41), a proinflammatory cytokine profile. With inflammation comes associated oxidative stress (38). As examples of viral molecular piracy, the HHV-6A/B U83 gene can also encode a human chemokine and the U12 and U51 genes can encode chemokine receptors (42). This almost surely is important in the ability of the viruses to produce neuroinflammation.
Much of what has been inferred about the pathogenic mechanisms employed by these viruses is based on data obtained in experiments that involve cultures of single cell types, e.g., lymphocytes or astrocytes. The biological reality in the infected host is much more complicated.
Role in Human Diseases
Clinically, HHV-6B is the major etiologic agent for roseola infantum (sixth disease or exanthem subitem) (43), a disease that generally occurs before 2 years of age. In very young children, HHV-6B also causes fevers and rashes that do not fully match the phenotype characteristic of roseola infantum.
In hematopoietic stem cell and cord blood transplant recipients, HHV-6B causes CNS disease, including encephalitis/encephalopathy and delirium (4–6). The virus has also been associated with drug-induced hypersensitivity syndrome (DIHS), which is also known as drug rash with eosinophilia and systemic symptoms (DRESS) (44–48). The clinical spectrum of HHV-6A is less well defined; several lines of evidence suggest a role in Hashimoto’s thyroiditis (49) and primary infertility (50). Both HHV-6A/B, particularly iciHHV-6A/B, have recently been associated with preeclampsia, the most common cause of maternal and infant mortality in pregnancy (51).
Animal Models
The effect of infection with HHV-6A/B on the brain can be studied using several animal models, including humanized SCID mice, CD46-transgenic mice, and pig-tailed macaques (34, 52, 53) and, more recently, the common marmoset (Callithrix jacchus), which also expresses CD46 (54).
The biology of HHV-6A/B is described in more detail elsewhere (18, 20, 42, 55, 56).
EVIDENCE REQUIRED TO LINK HHV-6A/B WITH A DISEASE
Linking a ubiquitous infectious agent capable of producing lifelong infection, like HHV-6A/B, to any disease is very difficult, particularly when the agent establishes lifelong infections in the host and appears to cause no long-term pathology in most infected individuals (57).
Over 130 years ago, Jakob Henle and Robert Koch proposed a set of criteria (now known as Koch’s postulates) for establishing that a bacterium caused a disease (58). In brief, the three postulates were fulfilled if (i) the agent in question was associated in every case with the disease of interest, (ii) the agent was associated with only the disease of interest, and (iii) material from infected individuals could be used to propagate the agent, which could then be transmitted to, and then cause the same disease in, another individual.
Since Koch’s time, understanding of infectious diseases has expanded greatly, including the discovery of viruses and the potential pathological consequences of the immune response to an infectious agent. With these discoveries, the limitations of Koch’s postulates have become evident. Rivers (59), Hill (60), Evans (61), Fredricks and Relman (62), and others have proposed alternative criteria that overcome most of the limitations of Koch’s postulates.
Some of these proposed alternative criteria focus on general principles of evidence: the strength and specificity of the association, whether the association is reproducible among different investigators, whether there is a dose-response relationship between the infectious agent and severity of the disease, whether the temporal relationships make sense, and whether the outcome is changed by elimination or reduction of exposure to the virus.
However, with regard to the possible role of HHV-6A/B as a cause of disease, these criteria do not fully address the following: (i) the ability of a ubiquitous agent, capable of lifelong latent infection, to cause disease in only some of those infected, either by direct cytopathology or by eliciting a pathological immune response, and (ii) the ability of an inherited, latent virus genome (e.g., iciHHV-6) to be activated, with the production of proteins or infectious virions that have pathological consequences.
Borrowing heavily from the previous discussions of causation, we suggest a set of criteria that seem helpful in evaluating associations between HHV-6A/B and the neurological diseases of interest here, which are summarized in Table 2.
TABLE 2.
Evidence required to suggest a causal role for HHV-6A/B in any disease
| HHV-6A/B nucleic acid is present in diseased tissue, in most cases, in higher abundance than in nondiseased tissue (by qPCR or other means). |
| The amount of HHV-6A/B nucleic acid in diseased tissue or blood, and/or antibody levels, correlates with the severity of the disease. |
| HHV-6A/B nucleic acid is demonstrated in cells relevant to disease pathology. |
| HHV-6A/B mRNA (by RT-PCR, or other means) and antigens by immunohistochemistry are present in diseased tissue. |
| Exposure to and then presence of the viruses and their gene products in affected tissue precede the development of the disease (temporal relationship). |
| Infectious agents other than HHV-6A/B are not generally detected in diseased tissue in a substantial number of cases. |
| There are cellular and/or humoral immune responses to HHV-6A/B in diseased tissue and/or in blood, and these responses correlate with the severity of the disease. |
| HHV-6A/B affect cellular function in diseased tissue in a manner able to cause or augment the disease pathology (in vitro or in vivo studies). |
| Specific antiviral therapy both reduces viral load in diseased tissue or blood and is followed by clinical improvement. |
In applying these criteria, we note the following provisos:
-
1.
We do not specify a threshold for how many of the criteria must be met to “certify” a causal connection; obviously, the more criteria that are met, the greater the likelihood that the connection is causal.
-
2.
We recognize that criteria involving viral nucleic acid or proteins in diseased tissue do not distinguish whether HHV-6A/B is a sole causal agent, a causal cofactor, or simply an innocent bystander that homes to diseased tissue but does not contribute to pathology.
-
3.
We realize that with each of the criteria, the evidence is strongest when different investigators, in different institutions, using different methods have come to the same conclusion.
As we discuss each disease that has been linked to HHV-6A/B, we summarize the evidence according to these criteria.
HHV-6A/B IN FEBRILE SEIZURES AND FEBRILE STATUS EPILEPTICUS
Background
Epidemiological and laboratory evidence indicates that HHV-6B may be a causal factor in several conditions that are themselves linked: febrile seizures (FS), febrile status epilepticus (FSE), mesial temporal sclerosis (MTS) and hippocampal sclerosis (HS), and mesial temporal lobe epilepsy (MTLE).
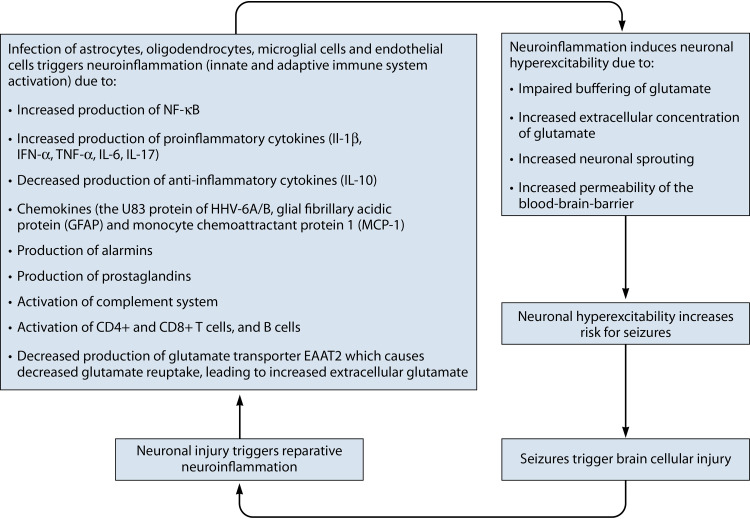
Infection triggers neuroinflammation.
Infection of the brain by neurotropic viruses can activate the innate and adaptive immune systems of the brain (neuroinflammation) (63), as seen in arbovirus infection (64), dengue infection (65), and influenza virus infection (66). Infection with herpesviruses—agents capable of achieving lifelong latency and of periodically reactivating from latency—can elicit recurring inflammatory responses (67).
In animal models of seizures induced by HHV-6A infection, seizures can appear within 3 days of infection, prior to extensive replication of the virus. This implicates the innate immune system in at least the initiation of the first seizures (68). Thereafter, CD4+ and CD8+ T cells, and B cells activated as part of the adaptive immune response, perpetuate an inflammatory milieu that is associated with seizures (68).
There are several mechanisms by which HHV-6A/B can induce neuroinflammation. Infection of astrocytes (32) or oligodendrocytes (69) results in the production of proinflammatory cytokines, including IL-1β, IFN-α, and TNF-α (63). The HHV-6A/B U83 protein is a monocyte-attracting chemokine. The production of two chemokines/cytokines, glial fibrillary acidic protein (GFAP) and monocyte chemoattractant protein 1 (MCP-1), is upregulated in the amygdala of people with MTLE and HHV-6A/B infection, and there is a linear relationship between the amount of HHV-6A/B DNA and the levels of these two proteins in both rats and humans (70, 71).
Infants with acute encephalopathy and HHV-6A/B DNA in the blood or cerebrospinal fluid (CSF), most of whom experience febrile seizures, are reported to have elevated levels of various proinflammatory cytokines, molecules involved in tissue injury and repair (such as metalloproteinases), markers of oxidative stress, and tau protein in the blood or CSF (72–75).
In addition, HHV-6A/B infection of endothelial cells induces the production of proinflammatory cytokines (33) and can thereby weaken the blood-brain barrier (76). Interactions between HHV-6A virion glycoproteins and CD46 can induce T cells to produce IL-17 (76) and suppress the production of IL-10. Finally, through molecular mimicry, some viral proteins may stimulate the production of T and B cells that react with structurally similar epitopes on neuronal or glial cells (68).
Seizures trigger neuroinflammation.
Seizures themselves can activate the brain’s immune system in a process called sterile or neurogenic neuroinflammation (68). Thus, infection of the brain can induce another vicious cycle: infection leads to neuroinflammation, which generates neuronal hyperexcitability—the tendency of the neuronal membrane to depolarize, measured by the firing rate—and more seizures.
Neuroinflammation triggers viral reactivation.
Proinflammatory cytokines can stimulate reactivation of HHV-6B in vitro (75). If true in vivo, it would suggest the possibility of yet another “vicious cycle” in which infection triggers an inflammatory response, which further increases the infectious burden.
Neuroinflammation induces neuronal hyperexcitability.
Neuroinflammation induced by HHV-6A/B leads to the production of several mediators of neuronal hyperexcitability, including chemokines, alarmins, prostaglandins, and complement factors (77).
A meta-analysis of 6 studies involving 243 children with FS found that CSF levels of IL-1β and serum levels of IL-6 were significantly associated with FS or FSE (78). Proinflammatory cytokines alter voltage- and receptor-gated ion channels and also alter the functioning of endothelial cells and glial cells in ways that enhance neuronal hyperexcitability, by (i) increasing permeability of the BBB, (ii) impairing the buffering of glutamate, the major excitatory neurotransmitter, (iii) increasing neurogenesis, and (iv) neuronal sprouting induced by the increased expression of NF-ĸB and activator protein 1 (AP1) (79).
Infection directly induces neuronal hyperexcitability.
HHV-6A/B may have epileptogenic potential via their direct effect on neurotransmitters as well. High levels of extracellular glutamate can cause neuronal hyperexcitability. Infection of astrocytes by either HHV-6A or -6B can raise extracellular levels of glutamate by decreasing production of the glutamate transporter EAAT2 (32, 80).
In addition, infection of astrocytes by these viruses also affects glutamate reuptake: acute infection leads to increased reuptake, whereas persistent infection leads to impaired reuptake and consequent increased levels of extracellular glutamate (80). HHV-6A/B encephalitis also has been reported to elicit autoantibodies against glutamic acid decarboxylase (GAD) in both CSF and serum (81).
HHV-6A/B can injure the CNS.
Seizures are triggered by a wide variety of brain injuries. There is little doubt that HHV-6B is not only neurotropic but also capable of causing injury to the CNS.
First, during primary infection of immunocompetent children, HHV-6A/B DNA frequently is detected in the CSF (9, 82, 83). Second, in some children with FSE, hippocampal injury can be visualized by magnetic resonance imaging (MRI; described in more detail below). Third, some children with exanthem subitem experience ataxia and developmental disabilities, typically after experiencing recurrent seizures (84). Other children develop chorea with developmental abnormalities (85). Fourth, in a small fraction of children with focal encephalitis, abundant HHV-6A/B DNA has been identified in brain biopsy specimens (86) or at autopsy (87). Fifth, primary HHV-6A/B infection of apparently immunocompetent adults also can lead to status epilepticus (88) and, rarely, fulminant demyelinating encephalomyelitis (89).
Thus, as summarized in Fig. 3, laboratory evidence supports the hypothesis that HHV-6A/B infection of the brain may be a trigger in FS, FSE, MTS, HS, and MTLE. This linkage is also supported by clinical and epidemiological studies, summarized below. The questions are whether HHV-6A/B are directly responsible for FS, FSE, MTS, HS, and MTLE and, if so, how frequently.
FIG 3.
Vicious cycle by which infection with HHV-6A/B may be one factor leading to neuroinflammation, neuronal hyperexcitability, seizures, brain injury, and further neuroinflammation.
Febrile Seizures and Febrile Status Epilepticus in Children
Relationship between FS, FSE, and MTLE.
FS, FSE, and MTLE cooccur in the same individuals often enough to suggest the possibility that they may share etiologic agents.
FS are the most common type of seizures in children younger than 5 years, occurring in 2% to 5% of these children, most often in the second year of life. A small fraction (5% to 8%) of FS last long enough to meet the criteria for FSE. Indeed, during the second year of life, FSE accounts for 70% of all cases of status epilepticus (90).
Children with a first episode of FSE, in turn, have a greater likelihood of future FSE (91) and also may have a greater likelihood of subsequently developing acute hippocampal injury (92–94) and temporal lobe epilepsy (TLE) 8 to 11 years later (95), although the linkage to TLE is not firmly established and is the subject of ongoing investigation (90, 96).
In children with FSE, hippocampal or temporal lobe injury is often seen on MRI. After multivariate adjustment for other risk factors, individuals with hippocampal injury evident by MRI (either T2 hyperintensity or impaired growth of hippocampal volume without T2 hyperintensity), in turn, have an increased risk of future FSE, as well as an increased risk for subsequent HS (91).
Thus, the cooccurrence of FS, FSE, hippocampal injury, and subsequent MTLE in the same individuals might theoretically be explained by ineradicable neurotropic agents like HHV-6B.
HHV-6B in FS.
The median age for acquiring HHV-6B infection is 9 months, with many new infections occurring in the second year of life (8). This is also a common time for FS. Primary infection with HHV-6B, defined by the presence of viral nucleic acid and newly appearing antiviral antibodies being detected in the blood, has been reported in 12% of young children seen in emergency rooms for fevers (8, 19, 97) and in 8% to 40% of cases of initial FS (8, 14, 82, 98–102). Most often, it is HHV-6B, rather than HHV-6A, that is associated with seizures, although there are occasional reports of cases linked to HHV-6A in geographic regions where HHV-6A may be more prevalent (103).
Children with febrile seizures and HHV-6B infection are more likely to be younger (13 months of age versus 39 months), to be female, and to have a longer interval between fever and the onset of seizures than children with febrile seizures not associated with HHV-6B infection (104).
HHV-6B in FSE.
Primary HHV-6B infection has been detected in about 20% of children with FSE, and reactivated HHV-6B infection has been detected in just under 10% of children with FSE (14, 82, 90, 105, 106). A rigorous prospective assessment of the role of HHV-6B in FSE is under way in the Consequences of Prolonged Febrile Seizures in Childhood (FEBSTAT) study and two affiliated studies (90, 96). In children ages 1 month to 5 years presenting with FSE, blood was promptly tested both for viral DNA and RNA by quantitative PCR and for viral antibodies (to distinguish primary infection from reactivation). Of 169 children with these virologic studies, HHV-6B viremia was detected in 32% at the time of FSE, about 70% with primary infection and 30% with reactivated infection. CSF was obtained in 75 children; HHV-6A/B DNA or RNA was not detected, even in the viremic children. A previous study found HHV-6B DNA in the CSF of some children with FSE (8). Children with FSE also sometimes had pleocytosis, elevated CSF protein, or reduced glucose (107).
While the FEBSTAT study included no control group, the investigators noted that a previous large prospective study had found no evidence of primary infection with HHV-6B in any of 582 infants and young children with acute nonfebrile illnesses nor in 352 healthy controls (8). The investigators concluded that primary infection with HHV-6B is correlated with both FS and FSE, although evidence linking reactivated infection to FS or FSE was less convincing.
Children with FSE are much more likely to have increased T2 signal in the hippocampus on MRI than those with just FS (11.5% versus 0%) (94). Given the predilection of HHV-6A/B for the hippocampus, and its ability to cause hippocampal inflammation and subsequent sclerosis (32, 71, 94, 108–111), this finding is consistent with a role for HHV-6A/B in some cases of FSE.
HHV-6B and FS and FSE: contrary evidence.
Despite considerable evidence linking HHV-6B to FS and FSE, there is some contrary evidence. First, not all investigators have found specific associations between primary HHV-6B infection and FS or FSE (112). In a study that detected HHV-6A/B in children with FS, other viruses that might have triggered FS were also detected (113).
Second, while HHV-6B infection is detectable in about 20% of children with FS, the converse is not true: FS are very infrequently seen in children with primary HHV-6B infection (3). This is likely a result of the relatively infrequent occurrence of FS.
Third, although primary HHV-6B infection is often seen in young children with FS, it is not clear if the virus, as contrasted to the fever induced by the virus, triggers the neuronal hyperexcitability. Surely, febrile seizures occur in the absence of primary or reactivated HHV-6B infection. Having said that, primary infection with HHV-6A/B has also been reported in children with afebrile seizures (114), suggesting that viral infection of the central nervous system can be sufficient to stimulate seizures in the absence of fever.
Fourth, some argue that injury from infection of the brain by HHV-6B may not directly cause FS or FSE. Instead, the seizures may simply reveal an underlying seizure diathesis, inherited or acquired (115). Indeed, one such predisposing factor, hippocampal malrotation, is found more often in FSE (94).
Nevertheless, a meta-analysis of 147 studies found a 21% detection rate of HHV-6A/B in FS patients, typically involving the detection of viral DNA in blood, reflecting either primary infection or reactivation (116). The evidence linking HHV-6A/B infection with FS is strongest in infants aged 12 to 15 months (8, 117) and stronger for FSE than for FS (103).
Evidence of HHV-6B in brain tissue in FS and FSE.
Biopsies are not done in children with FS and FSE, and no autopsies have been reported of the rare child who has died from FSE. Therefore, support from studies of brain tissue for the hypothesis that HHV-6B is a cause of FS and FSE is, by its nature, indirect, coming from evidence that the virus infects brain tissue in other, related illnesses.
Post-Hematopoietic Cell Transplantation Encephalitis and Epilepsy
Encephalitis due to HHV-6B is a recognized complication in immunocompromised individuals, particularly following hematopoietic cell transplantation (HCT), as described in several recent reviews (4–6, 17). The risk may be greater following umbilical cord blood transplantation (118, 119). Three children with posttransplant encephalitis who were followed for 2 to 8 years developed generalized seizures well after the acute encephalitis had resolved clinically and after the resolution of inflammatory markers; the seizures were frequent and resistant to antiepileptic drugs (120).
Eight patients who developed HHV-6A/B encephalitis following allogeneic HCT were followed longitudinally, and three developed severe bilateral hippocampal atrophy on follow-up MRI, accompanied by neurological deficits, although none of the three had seizures after the initial encephalitis (121).
One study found that insomnia, agitation, and hallucinations were seen more often in individuals with PCR-positive post-HCT encephalitis than in individuals who were PCR negative and who presumably had other types of encephalitis (122).
Thus, the course of HHV-6B infection in immunosuppressed children and adults, presumably reactivated infection in most instances, has some parallels with what has been observed in very young immunocompetent children with FS and FSE.
Conclusions: HHV-6A/B, Febrile Seizures, Febrile Status Epilepticus
Most published evidence supports the hypothesis that primary and (possibly) reactivated infections with HHV-6B are frequent causes of both FS and FSE in infants and young children, although some studies have concluded otherwise (Table 3). The evidence is stronger for FSE than for FS. While primary infection with HHV-6B is often seen in children with FS or FSE, most children experiencing primary infection with HHV-6B do not develop FS or FSE.
TABLE 3.
Criteria for causality and strength of evidence linking HHV-6A/B to febrile seizures and status epilepticus
| Disease causation criterion | Evidencea |
|---|---|
| HHV-6A/B nucleic acid is present in diseased tissue, in most cases, in higher abundance than in nondiseased tissue (by qPCR or other means). | Viral DNA is present in CSF (9, 82, 83) and in brain (87). |
| The amount of HHV-6A/B nucleic acid in diseased tissue or blood, and/or antibody levels, correlates with the severity of the disease. | Higher levels of viral nucleic acid are present in blood at the time of seizures (8, 9, 14, 19, 82, 83, 97–103). |
| HHV-6A/B nucleic acid is demonstrated in cells relevant to disease pathology. | Brain biopsies/autopsies are typically not performed in febrile seizures, even following status epilepticus. However, HHV-6A/B can infect adult neurons (230, 232), adult astrocytes (109, 230, 231), and microglial cells (30, 229, 230). |
| HHV-6A/B mRNA (by RT-PCR or other means) and antigens by immunohistochemistry are present in diseased tissue. | Brain biopsies/autopsies are typically not performed in febrile seizures, even following status epilepticus. |
| Exposure to and then presence of the viruses and their gene products in affected tissue precede the development of the disease (temporal relationship). | 95% of humans are infected with HHV-6B in early childhood. The number of humans infected with HHV-6A, and the typical age of primary infection, is less clear. |
| Infectious agents other than HHV-6A/B are not detected in diseased tissue in a substantial number of cases. | Negative evidence: Other agents capable of inducing seizures sometimes are found along with HHV-6B (113). |
| There are cellular or humoral immune responses to HHV-6A/B in diseased tissue and/or in blood, and these responses correlate with the severity of the disease. | In animal models, HHV-6A infection generates activated CD4+ and CD8+ T cells and B cells and proinflammatory cytokines (68). Primary HHV-6B infection and reactivated HHV-6B infection (each defined by blood nucleic acid and antibody studies) have been detected in 20% and 10% of children with FSE (14, 82, 90, 103, 105, 106). HHV-6B DNA has been found in the CSF of some children with FSE (8). |
| HHV-6A/B affect cellular function in diseased tissue in a manner known to cause or augment the disease pathology (in vitro or in vivo studies). | Positive evidence: Infection of astrocytes or oligodendrocytes results in the production of proinflammatory cytokines, and chemokines (32, 33, 63, 69–71, 76–79) … as well as mediators of neuronal hyperexcitability (32, 79, 80) … … and autoantibodies against glutamic acid decarboxylase (81). |
| Negative evidence: HHV-6B nucleic acid or antibodies have not been found in FS (112). | |
| Specific antiviral therapy both reduces viral load in diseased tissue or blood and is followed by clinical improvement. | No evidence yet. |
All evidence cited is positive evidence in support of the assertion unless specifically identified as negative evidence.
The association of primary HHV-6B infection with FS and FSE might not be unique to this virus: it is possible that people with FS and FSE are genetically prone to these conditions whenever dealing with an acute primary infection of any type and that HHV-6B simply happens to be a common primary infection in young children.
HHV-6A/B IN MESIAL TEMPORAL LOBE EPILEPSY
Possible Connections between FS, FSE, and MTLE
Mesial temporal lobe epilepsy (MTLE) is the most common form of epilepsy, and in most cases the presumed anatomic substrate is mesial temporal lobe sclerosis (most prominently involving the hippocampus) (108, 110). This form of epilepsy is often resistant to pharmacological treatment. While 50% to 80% of patients with all forms of epilepsy can become largely seizure-free with treatment, only 11% with MTLE and hippocampal sclerosis (HS) respond to treatment (108).
HHV-6A/B infects astrocytes in the temporal lobe and hippocampus (71, 109), and encephalitis caused by HHV-6A/B is frequently followed by HS (42, 123, 124). Patients with MTLE are more likely to have a history of FS during infancy (32), and infants with complex FS or status epilepticus may be more likely to develop HS (92, 125).
For some patients, surgical resection of diseased tissue offers the only hope of relief. Resected tissue has been studied to determine whether HHV-6A/B infection is associated with the sclerosis and resulting seizures.
Viral DNA, RNA, and Antigens in Resected Diseased Tissue, CSF, and Blood
Because HHV-6A/B is latent in lymphocytes in nearly all human beings, PCR detection of HHV-6A/B DNA (in blood or CSF) in diseased tissue does not in itself incriminate the virus as a pathological agent (126). The virus becomes a more plausible pathological agent if large amounts of DNA are found in the diseased tissue or if viral mRNA (detected using reverse transcription PCR [RT-PCR]) can be demonstrated. Even then, however, the presence of actively replicating virus does not necessarily mean the virus is causing pathology.
Over 60% of specimens resected to treat MTLE have evidence of active infection with HHV-6A/B (predominantly, if not exclusively, HHV-6B) (32, 71, 109, 127–131), although this linkage has not always been found (132). In contrast, HHV-6A/B DNA is found only rarely in the same anatomic areas in two comparison populations, those with neocortical and other forms of temporal lobe epilepsy and those with other forms of intractable nonmesial temporal lobe epilepsy (32, 109).
Viral DNA (32), viral mRNA detected by RT-PCR (71), and viral antigen (32) have been found more frequently in the hippocampus of people with MTLE, particularly when HS is present. One study assessed the presence of all nine human herpesviruses and found that HHV-6A/B was most frequently detected (71). A meta-analysis involving 10 studies and a total of 645 MTLE cases (most of which had HS) found HHV-6A/B DNA in 19.5% of cases versus 10.3% of hippocampal tissue from non-MTLE controls; 36% of MTLE patients with detectable HHV-6A/B DNA had a history of febrile seizures, whereas only 18% of MTLE patients without detectable HHV-6A/B DNA had such a history (133).
Conclusions: HHV-6A/B in Mesial Temporal Lobe Epilepsy
The evidence linking HHV-6A/B with MTLE is summarized in Table 4. HHV-6A/B is tropic for cells in the hippocampus (32, 71, 94, 108–111), and FSE as well as encephalitis caused by HHV-6A/B is frequently followed by HS, a pathological precursor for MTLE. HHV-6B (and, less often, HHV-6A) DNA and mRNA are often found in resected mesial temporal lobe tissue from people with MTLE at higher frequencies than are seen in comparison populations. While the DNA of other viruses may also be found in diseased tissue, HHV-6B DNA has been found most frequently.
TABLE 4.
Criteria for causality and strength of evidence linking HHV-6A/B to mesial temporal lobe epilepsy
| Disease causation criterion | Evidencea |
|---|---|
| HHV-6A/B nucleic acid is present in diseased tissue, in most cases, in higher abundance than in nondiseased tissue (by qPCR or other means). | Positive evidence: HHV-6A/B infects cells in the hippocampus (32, 71, 94, 108–111). Over 60% of hippocampus and temporal lobe specimens resected to treat MTLE have evidence of active infection with HHV-6A/B (32, 71, 109, 127–131). HHV-6A/B DNA is found only rarely in the same anatomic areas in other forms of temporal lobe epilepsy (32, 109). |
| Negative evidence: One study did not find HHV-6A/B in resected tissue (132). | |
| The amount of HHV-6A/B nucleic acid in diseased tissue or blood, and/or antibody levels, correlates with the severity of the disease. | No evidence yet. |
| HHV-6A/B nucleic acid is demonstrated in cells relevant to disease pathology. | HHV-6A/B can infect adult neurons (230, 232), adult astrocytes (109, 230, 231), and microglial cells (30, 229, 230). |
| HHV-6A/B mRNA (by RT-PCR or other means) and antigens by immunohistochemistry are present in diseased tissue. | Viral mRNA detected by RT-PCR (71) and viral antigen (32) are found more frequently in the hippocampus of people with MTLE. |
| Exposure to and then presence of the viruses and their gene products in affected tissue precede the development of the disease (temporal relationship). | 95% of humans are infected with HHV-6B in very early childhood. The number of humans infected with HHV-6A, and the typical age of primary infection, is less clear. |
| Infectious agents other than HHV-6A/B are not detected in diseased tissue in a substantial number of cases. | HHV-6A/B is present in resected tissue more often than other human herpesviruses (71). |
| There are cellular and/or humoral immune responses to HHV-6A/B in diseased tissue and/or in blood, and these responses correlate with the severity of the disease. | No evidence yet. |
| HHV-6A/B affect cellular function in diseased tissue in a manner known to cause or augment the disease pathology (in vitro or in vivo studies). | HHV-6A/B infect astrocytes in the temporal lobe and hippocampus and cause hippocampal and mesial temporal lobe sclerosis (42, 71, 109, 123, 124), anatomic correlates of mesial temporal lobe epilepsy. |
| Specific antiviral therapy both reduces viral load in diseased tissue or blood and is followed by clinical improvement. | No evidence yet. |
All evidence cited is positive evidence in support of the assertion unless specifically identified as negative evidence.
HHV-6A/B IN MULTIPLE SCLEROSIS
Clinical investigators have long speculated that infectious agents may be one trigger for multiple sclerosis (MS) (134, 135). However, many such putative infectious triggers of MS have failed the test of time.
Nevertheless, in recent years, there has been a resurgence of interest in this hypothesis, as evidence has accumulated that Epstein-Barr virus (136) and endogenous retroviruses (137, 138) may each trigger pathology in some cases of MS. In addition, since the mid-1990s, evidence has accumulated that suggests a causal role for HHV-6A/B in some individuals with MS.
Below, we summarize the several forms of data related to the HHV-6A/B association with MS.
Viral DNA, RNA, and Antigens in Plaques and CSF
HHV-6A/B DNA in plaques.
Most (69, 139–146) but not all (147, 148) studies have found higher levels of HHV-6A/B DNA in MS plaques than in (i) areas of the same brains distant from the plaques, (ii) the periventricular areas of brains from subjects with other neurological diseases, or (iii) brain-healthy subjects who have died from other causes, such as trauma. HHV-6A/B DNA levels are reportedly greater in acute lesions than in chronic lesions, including in MS patients who had not received immunomodulatory therapy (145). Although many of these studies did not distinguish a viral DNA signal coming from CNS cells, as contrasted to a signal from infiltrating lymphocytes, many studies have found that CNS cells involved in the pathogenesis of MS are infected by HHV-6A/B (Table 1).
Studies (typically employing PCR) to quantitate the amount of viral nucleic acid can come to quite different results, depending on the sensitivity and specificity of the PCR techniques and the adequacy of the techniques used to distinguish active from latent infection. This caveat must be considered every time (and there will be several) that we report conflicting results in detecting or quantifying HHV-6A/B nucleic acid.
In situ PCR localizes HHV-6A/B DNA to oligodendrocytes, microglia, lymphocytes, and macrophages in acute-phase lesions of patients with MS (145), although immunocytochemical staining has detected only one HHV-6A/B glycoprotein in those cells, primarily in oligodendrocytes (139).
HHV-6A/B mRNA and antigens in plaques.
Increased levels of HHV-6A/B mRNAs and the proteins they produce (both early and late antigens) have been found in the lesions of patients with MS in comparison to controls (69). This is particularly true in oligodendrocytes and in MS lesions as opposed to normal-appearing white matter (69, 149). Another early study found HHV-6A/B antigens in 47% of brains from MS patients versus none in brains from non-MS controls, apparently otherwise healthy people who died from trauma (143). HHV-6A/B expression may be greater in active than in chronic lesions of MS (144). Finally, a case report of a fulminant, fatal demyelinating disease clinically diagnosed as progressive MS found dense concentrations of HHV-6A/B proteins by immunohistochemical staining in plaques and elsewhere in the brain, a finding not found in other inflammatory demyelinating diseases (such as subacute sclerosing panencephalitis [SSPE]) (150).
HHV-6A/B DNA in CSF.
Several studies have reported significantly more HHV-6A/B DNA in the CSF of patients with MS than in healthy controls (151–154), although others have not (148, 155–158). One group reported detecting cell-free HHV-6A/B DNA in about 20% of CSF from patients with MS (159), but other groups have not (160, 161). One study found that MS patients with HHV-6A DNA in the CSF have more contrast-enhancing lesions than those without viral DNA (162).
Viral load in the blood.
Several groups have found cell-free viral DNA in serum more frequently in patients with MS than in healthy controls, predominantly HHV-6A (152, 163–169); others have not been able to confirm this finding (155–157, 170). For example, one study found cell-free DNA in 60.2% of 78 patients with either relapsing-remitting MS (RRMS) or secondary progressive MS (SPMS) compared to 14.6% of 123 healthy control subjects (169). MS patients with cell-free HHV-6A/B DNA in serum appear more likely to have a particular polymorphism in the MHC2TA gene, which encodes a transcription factor important in the expression of major histocompatibility complex (MHC) class II genes (171).
HHV-6A/B DNA may be present more often in circulating white blood cells in patients with MS than in healthy controls (166, 172); the same has not been found with other lymphotropic herpesviruses (172). Moreover, those with higher viral loads for HHV-6A were more likely to have subsequent exacerbations of disease (173). One group could not confirm these findings, however (142).
Correlation of the level of viral DNA in serum and blood with activity of disease.
Increases in virus DNA levels in the blood have been observed in individuals with RRMS during times of disease exacerbation (167, 174, 175). The correlation of viral load with exacerbations may be stronger for HHV-6A than HHV-6B (164). The same correlation of viral load with disease exacerbations is not clearly present with SPMS (176), although exacerbations may be harder to identify clinically in SPMS than in RRMS. Finally, MS patients who respond well clinically to beta interferon treatment are likely to have lower levels of HHV-6A/B DNA in serum than poor responders (177).
Prevalence of viral mRNA in circulating white blood cells.
Compared to healthy control subjects, the circulating white blood cells of patients with RRMS were more likely to have transcription of HHV-6A/B genes involved in active infection (immediate early genes) (178). Moreover, those with active infection had higher viral DNA loads during acute attacks than during remissions (178), whereas the same is not true for expression of genes involved in latent infection (165).
Prevalence of viral proteins in circulating white blood cells.
Proteins indicative of active infection with HHV-6A/B were present in circulating leukocytes from 54% of 41 patients with MS compared with none of 61 normal controls (149). If confirmed, this could be the basis of a useful new diagnostic tool.
Viral DNA in urine.
One study isolated cell-free HHV-6A/B DNA indicative of active infection from the urine of 26% of patients with RRMS and from none of matched healthy control subjects (163).
Meta-analyses.
A meta-analysis of 39 studies that used either molecular or serological techniques to study the possible association of HHV-6A/B infection with MS found a statistically significant association, with an odds ratio (OR) of 2.23 and a 95% confidence interval (95% CI) of 1.5 to 3.3. When the analysis was restricted to the studies deemed of highest quality, according to prespecified and specific criteria, the association became more significant, with an OR of 6.7 and a 95% CI of 4.8 to 8.6 (P < 0.00001) (179).
Immune Response to the Virus
Peripheral antibodies to HHV-6A/B as correlates of disease.
Most studies have found that levels of serum IgM antibodies to HHV-6A/B are higher in people with MS than in healthy control subjects (169, 180–186), although levels of IgG antibodies are not always higher (187, 188). A study involving over 300 patients followed for 2 years after disease-modifying therapy found that MS patients who had a decrease in anti-HHV-6A/B IgG titers after 2 years were much more likely to be free of relapses and progression than those in whom titers increased (69.0% versus 40.7%, P = 0.00002) (189). The finding was particularly striking in patients treated with natalizumab.
One study found higher titers of antibodies to an HHV-6A/B latency-associated protein (U94/REP) in patients with MS than in healthy control subjects (168). A subsequent study used a lentiviral vector to prompt human oligodendrocyte precursor cells (OPCs) to express the U94 protein and observed significant impairment of the ability of OPCs to migrate to areas of demyelination (190).
A recent study examined serum from a Swedish study of 8,742 persons with MS and from 7,215 matched controls. The study demonstrated increased serological responses against a virus-specific peptide for the HHV-6A variant (IE1A) (191). The IgG response to this peptide was positively associated with all subtypes of MS (OR of 1.55, P = 9 × 10−22). No such association was seen with HHV-6B-specific peptides. IgG to the same IE1A peptide was then measured in a pre-MS cohort (persons with presymptomatically drawn blood samples): the presence of the antibody conferred an increased risk of developing MS in the future (OR of 2.2, P = 2 × 10−5). The antibody profile also correlated with HLA haplotypes previously linked to MS and with serological responses to Epstein-Barr virus (EBV). This compelling, extraordinarily well-powered study supports a role for HHV-6A in the etiology of MS (191).
Peripheral antibodies as predictors of relapse.
Several longitudinal studies have found that rising peripheral antibody levels predict relapse in patients with MS (189, 192, 193), although some studies have not (194). For example, one study of 145 persons with RRMS followed for 3 years found that anti-HHV-6 IgG titers predicted the hazard of relapse, after adjusting for concomitant infection with EBV: the higher the level of anti-HHV-6 IgG, the greater the hazard of relapse (P = 0.003) (193). However, a later study by the same group did not come to the same conclusion (194). Both IgG and IgM antibodies seem to predict relapse, as do antibodies directed at the latency-promoting protein U94/REP (154, 168, 195).
Lymphoproliferative responses of circulating lymphocytes to HHV-6A/B antigens.
Some studies have reported that HHV-6-specific CD4 T cell responses in peripheral blood are seen more often in people with MS (both progressive and relapsing-remitting subtypes) (196, 197). For example, circulating lymphocytes proliferated in response to HHV-6A (strain U1102)-infected cell lysates in 67% of patients with MS versus 33% of healthy control subjects (198). Other investigators have not found this (152, 187, 199).
Lymphoproliferative responses of CSF lymphocytes to HHV-6A/B antigens.
Intrathecal CD4+ and CD8+ T cells in patients with relapsing-remitting and progressive MS exhibit striking reactivity against HHV-6A/B antigens (and EBV antigens), compared to disease controls with other inflammatory neurological diseases. The reactivity was abolished by long-term treatment with daclizumab, a monoclonal antibody that modulates production of IL-2 (196).
Intrathecal antibodies in CSF.
Intrathecal antibodies against HHV-6A/B have been identified more often in RRMS and chronic progressive MS (CPMS) than in other neurological diseases (200).
In virtually all diseases of the CNS associated with a microbial agent, the intrathecal antibodies or oligoclonal bands (OCBs) are specific for the inciting pathogen. For example, in neurosyphilis, OCBs are specific for Treponema pallidum; in human T cell leukemia virus 1 (HTLV-1)-associated myelopathy (HAM/TSP), the OCBs are specific for HTLV-1; in subacute sclerosing panencephalitis (SSPE), the OCBs are specific for measles virus, etc. (162).
Moreover, between 26% and 38% of patients with MS have OCBs in CSF specific for either HHV-6A/B or EBV (162, 201, 202), results that remain durable on repeated testing (201), a finding not present in other inflammatory neurological disorders (162).
NK cell dysfunction against herpesviruses in MS.
Specific, HLA-linked, inhibitory receptors on natural killer (NK) cells (specifically the KIR2DL2/C1 genotype) reduce the effectiveness of the NK cell attack on herpesviruses. This genotype reportedly is found more often in people with MS (203, 204) and Alzheimer’s disease (204), another putative mechanism by which herpesviruses may be linked to these diseases.
Proinflammatory potential of CNS infection with HHV-6.
Infection of T cells by HHV-6A/B, in vitro, leads to the production of various proinflammatory mediators, including IFN-γ, granzyme B, chemokine receptor CCR9, and IL-17A (76). Patients with RRMS who are HHV-6 seropositive have significantly higher levels of TNF-α, IFN-γ, IL-1β, IL-6, and CCL-5 and significantly lower levels of IL-12, IL-10, and CCL-2, a cytokine profile indicating enhanced inflammation, than those who are seronegative (205).
Molecular mimicry.
The HHV-6A/B U24 membrane protein and human myelin basic protein (MBP) share a stretch of identical amino acids, and one study has found that patients with MS, in comparison to healthy controls, have a higher frequency of circulating T cells that are reactive against both HHV-6A/B U24 and MBP (206, 207). Thus, molecular mimicry is a possible mechanism by which HHV-6A/B infection might trigger MS.
The complement system.
Serum and CSF levels of soluble CD46 are significantly elevated in patients with MS (208). CD46 is one of a family of glycoproteins involved in regulating the complement cascade. It also is the cellular receptor for HHV-6A (27). Expression of CD46 is increased in patients with MS, compared to healthy control subjects (166), and immunoaffinity purification has demonstrated HHV-6A/B DNA bound to soluble CD46 (209). It is plausible that HHV-6A/B infection, by engaging CD46, increases activation of the complement system, contributing to the pathology of MS. It has also been suggested that during replication, the virus can incorporate host antigens into its virion (including CD46), thereby triggering an autoimmune reaction (208).
Effect of Virus on Remyelination
Supernatant from HHV-6-infected SupT1 cells appears to inhibit the proliferation, and shorten the life span, of an oligodendroglial cell line (210). Expression of HHV-6A U94 protein, a marker of latency in oligodendrocyte progenitor cells, reportedly disrupted their migration and led to impairment of remyelination (190). Furthermore, viral infection of oligodendrocytes or oligodendrocyte progenitor cells can induce the cells to express MHC class I antigens and other genes that facilitate prolonged inflammation (211).
Effect of Virus on Cell Lines
Exposure to HHV-6A, but not HHV-6B, reportedly induces apoptosis in a time-dependent manner in human neuronal (SK-N-SH), astrocytic (CRT), and oligodendrocyte (TC620) cell lines (212). Experiments in human adult postmortem astrocytes suggest that infection by HHV-6A, combined with exposure to a combination of proinflammatory cytokines (TNF-α, IL-1β, or IFN-γ), induces production of a variety of other proinflammatory and anti-inflammatory cytokines (e.g., IL-11), chemotactic proteins, and cell growth factors (e.g., VEGF-C) (40).
Thus, as summarized in Fig. 4, there is evidence that HHV-6A/B (particularly HHV-6A) can serve as a stimulus to three essential components of the pathology of MS: neuroinflammation, demyelination, and impaired remyelination.
FIG 4.
Speculative schema for how HHV-6A/B might be one stimulus of three cardinal features of multiple sclerosis, i.e., neuroinflammation, demyelination, and impaired remyelination, alone or in combination with other agents, including Epstein-Barr virus (EBV) and endogenous retroviruses and the proteins they produce. MBP, human myelin basic protein. The term “oligodendrocyte cells” includes oligodendrocyte precursor cells.
Animal Models
In addition to humanized SCID mice, CD46-transgenic mice, and pig-tailed macaques (34, 52, 53), a nonhuman primate model, the common marmoset (Callithrix jacchus), which also expresses CD46 and can be infected with HHV-6A/B, has recently been described (54). The common marmoset has long been used as a model for MS: induction of experimental autoimmune encephalomyelitis (EAE) can be achieved by inoculation with whole-brain homogenates or peptides of human myelin oligodendrocyte (MOG) protein. Intranasal inoculation of marmosets with HHV-6A/B (the typical route of infection for humans) results in an asymptomatic infection. However, induction of EAE in these virus-infected animals results in accelerated disease (213). In animals that have been infected with HHV-6A/B prior to EAE induction, the time to first symptoms of neurologic disease, lesion development by MRI, and HHV-6A/B viral antigen expression in brain lesions are all increased (213). This animal-virus model is highly reminiscent of what is observed in MS patients and therefore supports a role for HHV-6A/B in the pathogenesis of MS.
HHV-6A/B, EBV, and Endogenous Retroviruses
Several possible viral triggers of MS besides HHV-6A/B have been identified, including EBV (134, 214) and human endogenous retroviruses (HERVs) (137, 138). Both of these viruses can be transactivated by HHV-6 and raise the possibility that coinfection with HHV-6A may sometimes participate in the pathogenesis of MS (215, 216).
Epstein-Barr virus.
Epidemiological studies identify infection with Epstein-Barr virus (EBV) as a risk factor for MS (214). EBV infects over 90% of adults globally, with higher rates in developing nations. Specifically, in developing nations, there is a 90% seropositivity by the age of 4, whereas in developed nations, seropositivity typically develops during the teenage years (214).
More impressive, in longitudinal studies of large populations, MS is extraordinarily rare in the small fraction of people who remain seronegative to EBV (214). Instead, MS virtually always develops within 4 to 6 years after a person has seroconverted to EBV. While the vast majority of people do not develop MS following primary infection with EBV, when MS does develop, it is in a narrow window of time following primary infection (214). In addition, the risk of developing MS is nearly 3-fold higher in EBV-seropositive individuals who develop infectious mononucleosis immediately following primary infection with EBV than in EBV-seropositive individuals who do not develop infectious mononucleosis (214). EBV may even affect the risk of MS across generations: mothers with the highest levels of IgG to EBV viral capsid antigen, compared to those with the lowest levels, bear offspring with an increased relative risk (RR) of developing MS (RR = 2.44; 95% CI, 1.20 to 5.00) (217).
Various biological mechanisms have been proposed as to how EBV infection might trigger MS, including a direct cytopathic effect and a destructive immune response to EBV infection, as described in detail in a recent review (134). The recent recognition of the importance of B cells in the pathology of MS (218–221), as secretors of cytokines and chemokines, presenters of autoantigens, and producers of pathogenic antibodies, has increased the plausibility of EBV as an etiologic agent, given that EBV is tropic for B cells.
Human endogenous retroviruses.
Multiple reports find higher levels of HERV DNA, RNA, and reverse transcriptase in MS lesions than in nonlesions from the same brains, brains from subjects with other neurological diseases, or brains from healthy control subjects. Studies also find higher levels of these HERV biomarkers in serum and circulating white blood cells from patients with MS than in healthy control subjects (137, 138). Perhaps the factor most clearly linked to MS is upregulation of the transcription of the HERV-W env gene, leading to the production of the proinflammatory HERV-W Env protein (138).
Infection with HHV-6A, and stimulation of its receptor (CD46), upregulates the expression of HERV-W Env protein, which is dependent on CD46 Cyt-1 signaling and is abolished by inhibitors of protein kinase C (216). Both HHV-6A and -6B have also been found to transactivate the production of other HERV proteins (216). Thus, in addition to several direct effects by which HHV-6A/B infection might promote MS, it may also act indirectly by transactivating HERVs. Studies are needed that assay simultaneously for all three viruses in tissue from individuals with MS.
Conclusions Regarding HHV-6A/B in MS: Fingerprints but Lingering Doubt
While there are areas of disagreement and inconsistency, considerable evidence from numerous sources and using a variety of approaches supports the hypothesis that HHV-6A (and possibly -6B) may trigger some cases of MS. Evidence also supports the possibility that other viruses, particularly EBV, varicella-zoster virus (VZV), and endogenous retroviruses, may also trigger the pathology in some cases and sometimes work in concert with HHV-6A/B in the pathogenesis of MS.
HHV-6A/B DNA, mRNA, and antigens have been found closely associated with plaques of MS and in the spinal fluid of people with MS. Viral DNA load in serum, as well as anti-HHV-6A/B antibody levels, correlates with the level of activity of the disease and predicts relapse. Intrathecal antibodies against HHV-6A/B have been found more often in RRMS and CPMS than in other neurological diseases, and OCBs are often specific for HHV-6A/B.
Mechanistically, HHV-6A proteins and MBP share a stretch of identical amino acids. HHV-6A/B infection impairs remyelination. In mouse and primate models, HHV-6A/B generate pathology similar to that seen in MS.
In summary, as shown in Table 5, while the fingerprints of HHV-6A are all over the crime scene, evidence remains insufficient to convict beyond a reasonable doubt.
TABLE 5.
Criteria for causality and strength of evidence linking HHV-6A/B to multiple sclerosis
| Disease causation criterion | Evidencea |
|---|---|
| HHV-6A/B nucleic acid is present in diseased tissue, in most cases, in higher abundance than in nondiseased tissue (by qPCR or other means). | Viral DNA in plaques Positive evidence: 69, 139–146 Negative evidence: 147, 148 |
| Viral DNA in CSF Positive evidence: 151–154, 159, 162 Negative evidence: 148, 155–158 | |
| Viral DNA in cell-free CSF Positive evidence: 159 Negative evidence: 160, 161 | |
| Viral DNA in serum more often in MS than healthy controls Positive evidence: 152, 163–169, 171 Negative evidence: 155–157, 170 | |
| Viral DNA in circulating WBCs Positive evidence: 166, 172 Negative evidence: 142 | |
| Viral mRNA in circulating WBCs (178) | |
| Viral proteins indicating active infection in circulating WBCs (149) | |
| The amount of HHV-6A/B nucleic acid in diseased tissue or blood, and/or antibody levels, correlates with the severity of the disease. | Viral load in blood correlates with disease activity (164, 167, 174, 175, 177). |
| IgM and IgG antibodies to HHV-6A/B are more often and at higher levels in MS patients than in healthy controls and correlate with disease activity. Positive evidence: 154, 168, 169, 180–186, 189–193, 195 Negative evidence: 187, 188, 194 | |
| HHV-6A/B nucleic acid is demonstrated in cells relevant to disease pathology. | HHV-6A/B can infect adult neurons (230, 232), adult astrocytes (109, 230, 231), microglial cells (30, 229, 230), primary adult oligodendrocytes (229, 230, 232), primary oligodendrocyte precursor cells (190, 230, 307), oligodendrocyte cell lines (30, 212, 230, 308), CD4+ T cells (18, 20), monocytes/macrophages (18, 20), endothelial cells (18, 20, 33) … … and is found in MS lesions in neurons (139), oligodendrocytes (139, 145), microglia (145), and lymphocytes (145). |
| HHV-6A/B mRNA (by RT-PCR or other means) and antigens by immunohistochemistry are present in diseased tissue. | Viral mRNA and/or antigens/proteins in plaques (69, 143, 144, 149, 150) |
| Exposure to and then presence of the viruses and their gene products in affected tissue precede the development of the disease (temporal relationship). | 95% of humans are infected with HHV-6B in very early childhood. The number of humans infected with HHV-6A, and the typical age of primary infection, is less clear. Thus, people with MS have almost surely been infected with HHV-6A/B before the development of the disease. |
| Infectious agents other than HHV-6A/B are not detected in diseased tissue in a substantial number of cases. | EBV and endogenous retroviruses, as discussed separately |
| There are cellular and/or humoral immune responses to HHV-6A/B in diseased tissue and/or in blood, and these responses correlate with the severity of the disease. | Lymphocytes in CSF proliferate in response to HHV-6A/B antigens (196). |
| Intrathecal antibodies/oligoclonal bands against HHV-6A/B (162, 200–202). | |
| HHV-6A/B proteins share potential epitopes with host tissue (molecular mimicry) (206, 207). | |
| No studies correlate these immune responses to the severity of disease. | |
| HHV-6A/B affect cellular function in diseased tissue in a manner known to cause or augment the disease pathology (in vitro or in vivo studies). | HHV-6A, by engaging the complement receptor, may explain the increased activation of the complement system seen in MS (27, 166, 208, 209). |
| HHV-6A infects oligodendrocytes (and precursor cells), affecting their ability to remyelinate (190, 210). | |
| HHV-6A induces apoptosis in neuronal, astrocytic, and oligodendrocyte cell lines (212). | |
| Specific antiviral therapy both reduces viral load in diseased tissue or blood and is followed by clinical improvement. | No evidence yet. |
All evidence cited is positive evidence in support of the assertion, unless specifically identified as negative evidence.
HHV-6A/B IN ALZHEIMER’S DISEASE
Several recent reports have suggested that HHV-6A/B may contribute to the pathogenesis of Alzheimer’s disease (AD). To put those reports in perspective, we (i) summarize the current paradigm regarding the pathogenesis of AD, (ii) discuss how infection might contribute to pathogenesis, (iii) summarize claims that the production of amyloid beta (Aβ) might be a response to infection, (iv) discuss evidence linking other herpesviruses to AD, and (v) consider the evidence for HHV-6A/B.
The Pathogenesis of AD: the Current Paradigm
Aβ, Tau, and APOE.
Over several decades, a substantial body of evidence has demonstrated the important roles of amyloid beta, or Aβ (found in plaques), tau (found in neurofibrillary tangles), apolipoprotein E (APOE), and neuroinflammation in the pathogenesis of AD (222). Aβ, tau, and inflammation can each lead to the destruction of neurons and synapses (222).
With inherited forms of early-onset AD, it is clear that aberrations in the production and processing of amyloid precursor protein (APP) and Aβ are the primary drivers of pathology. But in the much more common sporadic late-onset AD, it is not entirely clear which of these key molecules is the primary driver of pathology and whether other factors may also be involved in pathogenesis.
In late-onset AD, there is strong evidence that Aβ is a primary driver of pathology (222). Several forms of Aβ, including Aβ 1-40 and Aβ 1-42, then form soluble oligomers that progress to form fibrils and then fibrillar plaques. The production of Aβ, in turn, can stimulate both the development of neurofibrillary tangles and neuroinflammation (223). That is, under experimental conditions, Aβ can precede the production of tau and neuroinflammation and be the primary driver of pathology.
Neuroinflammation in AD.
Neuroinflammation is also present in AD (224), and both neurons and synapses can be damaged by multiple components of inflammation: cytokines and chemokines, reactive oxygen species, and activation of cyclooxygenase-2 and of the classical and alternate complement pathways (225). Furthermore, biomarkers of inflammation are upregulated most strikingly in the areas of the brain that are most affected by AD neuropathology—the frontal and temporal neocortex and limbic system (225).
Almost as many “risk genes” for AD involve immune function as involve the production and clearance of Aβ (226, 227). In particular, microglia and astrocytes may play a central role in the pathogenesis of AD. Originally thought of as structural “scaffolding” to hold neurons in place, astrocytes and microglia now are known to influence neurogenesis, synaptic plasticity, and learning and memory (228). Microglia are of myeloid origin, immune cells that enter the CNS early in embryogenesis and become resident there. Both microglia (30, 229, 230) and astrocytes (109, 230–232) are infected by HHV-6A/B.
Microglia surround amyloid plaques, perhaps in an attempt to remove debris (228). In addition, aberrant microglial signaling can cause astrocytes to become neurotoxic (228). Microglia can also generate neurotoxic release of inflammatory cytokines (228).
Perhaps most relevant to the pathogenesis of AD, microglia, which are now recognized to be responsible for beneficial synaptic pruning during development, also appear capable of detrimental synaptic pruning during neurodegeneration. This synaptic pruning, in turn, is facilitated by the TREM2 receptor, which is prominent on microglia and important in stimulating phagocytosis and suppressing neuroinflammation (226), as well as by transforming growth factor β (TGF-β), which is highly expressed by microglia in response to injury (233). Detrimental synaptic pruning by microglia is initiated by the deposition of complement proteins (particularly C1qa) on synapses and postsynaptic dendritic spines, particularly in the hippocampus (227). Finally, activation of the complement cascade is inhibited by most alleles of APOE except APOE-ɛ4, suggesting that one mechanism by which the APOE-ɛ4 allele may increase the risk of AD is by encouraging complement activation and subsequent pathological synaptic pruning (227).
Evidence Linking Infection-Generated Neuroinflammation to AD
What causes the neuroinflammation seen in AD?
Neuroinflammation is associated with the neurodegeneration seen in AD and may contribute to causing neurodegeneration. But what triggers the inflammation? Two causes are well established: neuroinflammation can occur in response to the production and accumulation of Aβ (223), and it can also occur in response to neurodegeneration (225). Might it also be triggered by infection?
The infection hypothesis.
Theoretically, in at least some cases of AD, neuroinflammation might also be triggered by infection with any infectious agent or with combinations of those infectious agents.
Although infection of the CNS is the most obvious potential cause of neuroinflammation, peripheral infections can produce antigen-specific CD8+ memory T cells in the brain (234). In addition, inflammation in the gut generated by the microbiome can also activate the innate immune system of the brain through humoral signals that breach a porous blood-brain barrier and also through retrograde signals up the vagus nerve (235). Indeed, pharmaceutical alteration of the gut microbiota has reportedly slowed the progression of mild cognitive impairment to AD (236).
The infection hypothesis in no way contradicts the current paradigm regarding the pathogenesis of AD. Instead, the infection hypothesis reinforces the importance of Aβ, tau, APOE, and neuroinflammation.
Among the infectious agents putatively linked to AD, the most widely discussed claims are those for human herpesviruses. Several human herpesviruses, particularly herpes simplex virus 1 (HSV-1) (237), HHV-6A/B (63), varicella-zoster virus (VZV) (238, 239), and Epstein-Barr virus (EBV) (141), can establish latency in the brain. Several can also infect white blood cells. Thus, these viruses can become targets of the inflammatory response and can also infect cells that mediate the inflammatory response.
Neuroinflammation generates the accumulation of Aβ and tau.
Neuroinflammation elicits production of Aβ: reactive astrocytes generate amyloid precursor protein, β-secretase (BACE1), and γ-secretase, all necessary for the production of Aβ (222, 240). The accumulation of Aβ, in turn, elicits neuroinflammation (222, 240). Thus, neuroinflammation can trigger a vicious cycle in which neuroinflammation and the accumulation of Aβ trigger each other.
Several polymorphisms increase the risk of both infection and AD.
One observation favoring the infection hypothesis is that several gene variants which impair the immune response to infection appear to be more common in people with late-onset AD (241–244). Such polymorphisms theoretically could foster a smoldering, recurrent reactivation of latent infection. This is hard to prove in humans, but it is consistent with the infection hypothesis.
Claims linking multiple infectious agents to AD.
Several infectious agents have been linked to AD. In this review, we will discuss in more detail HSV-1, VZV, and HHV-6A/B. However, hepatitis C virus (245), Helicobacter pylori (246), Porphyromonas gingivalis (247–249), Chlamydia pneumoniae (250, 251), fungal organisms (252), and different spirochetes, including Treponema pallidum and Borrelia burgdorferi (253), have also been linked to AD.
Some infectious disease phenotypes are linked to single infectious agents, e.g., Legionnaires’ disease to Legionella bacteria and AIDS to human immunodeficiency virus (HIV). This has led some observers to conclude that when multiple agents are putatively linked to a disease, one should be dubious of the claims for any one of them.
However, most infection-related disease phenotypes are linked to multiple infectious agents (e.g., the common cold with a host of respiratory viruses, gastroenteritis with many infectious agents, hepatitis with multiple viruses). In our view, the fact that multiple infectious agents have been linked to AD does not invalidate the claim for any one of them. Instead, such claims are consistent with a model that attributes pathogenesis to neuroinflammation elicited by any of multiple microbes.
To be clear, however, at this time no infectious agent has been persuasively shown to cause AD when evaluated according to formal elements of etiologic proof, such as described and defined here.
Is Aβ a Natural Antimicrobial Peptide, and Does This Link Infection to AD?
Antimicrobial properties of Aβ.
Recognition that Aβ may be the primary driver of pathogenesis in AD begs a question: what triggers the production of Aβ? Several investigators have found that Aβ has the ability to function as a natural antimicrobial peptide (254–258), active against a variety of microbes, including viruses (such as HSV-1 [258, 259], HHV-6A/B [258], and influenza A virus [260]), bacteria (such as Salmonella enterica serovar Typhimurium in mice [261]), and fungi (such as Candida albicans in nematodes [261]).
Mechanistically, the oligomerization of Aβ 1-40 and Aβ 1-42 generates protofibrils that engulf unattached microbes and that inhibit pathogen adhesion to host cells, in vitro (258, 261). Aβ also binds to and disrupts lipid bilayers of bacterial membranes, causing calcium influx and bacteriolysis (256). The antiviral action of Aβ has been demonstrated in vivo even in transgenic worm and mouse models (259).
Moir et al. (257) and Soscia et al. (254) note that Aβ has been evolutionarily conserved: it is produced in animals as ancient as coelacanths (257, 258, 261). They speculate that Aβ may have been conserved because of its antimicrobial properties (257). That is a plausible hypothesis; given the limited access of the adaptive immune system to the brain, the innate immune system (including antimicrobial peptides) is particularly important in the brain’s defense against pathogens.
However, although Aβ has antimicrobial properties, whether it functions as a natural antimicrobial peptide in the human brain in response to invading microbes remains to be shown and would be difficult to prove.
Be that as it may, the production of Aβ ultimately leads to neurotoxicity and plaque formation and thereby contributes to the pathogenesis of AD. Thus, Aβ has both potentially protective and definitely destructive potential.
It might seem curious that a molecule with such destructive potential would be evolutionarily conserved. However, late-onset AD takes decades to develop and usually becomes apparent only after age 60, well past child-bearing age for women. Moreover, even in the developed nations, life expectancy was not much longer than 60 years until the past century. Thus, the destructive potential of Aβ would have had little negative effect on natural selection throughout most of human history.
Evidence Linking HSV-1/2 with AD
Because it has been postulated that HHV-6A/B may be among several infectious agents capable of triggering AD, we summarize here the more extensive evidence incriminating another herpesvirus, HSV-1.
Laboratory evidence in mice.
In cell culture, HSV-1 elicits the production of Aβ42, catalyzes its aggregation and nucleation, and thereby enhances the subsequent growth of amyloid fibrils. This was shown by transmission electron microscopy in cell culture (262).
HSV-1 infection of mice leads to the production of Aβ deposits and cognitive deficits, although the pathological changes are not pathognomonic of AD (67, 263). In the widely used mouse model of AD (the 5XFAD mouse), infection by HSV-1 was followed by the accumulation of Aβ 1-42 in the hippocampus and cortex within 48 h, compared with sham infection (262), confirming the prior reports in the same mouse model (254). When mice are repeatedly infected with HSV-1, the number of repeated infections correlates with the development of multiple markers or neurodegeneration (67).
Laboratory evidence in humans.
As outlined in recent reviews (237, 264), HSV-1 DNA in the brains of people with AD was first reported in 1991 (265) and confirmed not long after (266). HSV-1 DNA is located predominantly within Aβ plaques (267).
In human neural cell culture, HSV-1 infection elicits the formation of both Aβ and tau protein and induces hyperexcitability (263, 268). The formation of Aβ and tau can be reduced by treatment with acyclovir, an effective antiviral against HSV-1 (269). Why does HSV-1 infection elicit Aβ and tau? Besides the possibility that Aβ is a natural antimicrobial peptide, HSV-1 infection of various cell types, including neuronal and glial cells, can induce autophagy, which in turn can lead to the accumulation of both Aβ and tau protein (38, 270–282).
HSV-1 DNA may be found more often in the brains of people with AD who are APOE-ɛ4 carriers (266, 283), although others have not confirmed this observation (284). APOE-ɛ4 carriers may be at increased risk for HSV-1-related cold sores (266) and HSV-2-related genital ulcers (285). Thus, APOE-ɛ4 carriers may be at greater risk for failing to contain HSV-1/2 infections and definitely are at greater risk for developing AD. It is therefore plausible that an increased vulnerability from HSV-1 infections might be manifest as an increased risk of developing AD.
Interestingly, APOE-ɛ4 carriers with mesial temporal lobe epilepsy may have an increased frequency of seizures (286) and may be more vulnerable to COVID-19 as well (287), suggesting that increased neuroinflammation seen in APOE-ɛ4 carriers contributes to the pathogenesis of each of these very different diseases.
Finally, HSV-1 infection of animal and human cell lines modifies the expression of many host genes that have been identified as susceptibility genes for AD (242).
Laboratory evidence in human organoids.
Two groups created human brain models (“organoids”) from induced pluripotent stem (iPS) cells (257, 288). The organoids contain multiple cell types, including neurons and glial cells, and exhibit electrical activity. Low-multiplicity infections with HSV-1 led to the formation of plaque-like structures composed of β-amyloid, phosphorylation of tau protein, multiple markers of neuroinflammation, gliosis, and slowed electrical activity, all phenomena seen in human AD. No such changes were seen with sham-infected organoids. Finally, in the HSV-infected organoids, all of these changes could be prevented by treating the organoids with valacyclovir (288).
Epidemiological evidence linking HSV-1 to AD.
Epidemiological evidence linking HSV-1 to AD has been summarized in detail elsewhere (289). For example, in a prospective longitudinal study involving 512 subjects over age 65 years and free of dementia, elevated levels of IgM antibody against HSV-1/2 (reflecting primary or reactivated infection) at the start of the study was significantly associated with the subsequent development of dementia (hazard ratio [HR] = 2.55; 95% CI, 1.38 to 4.72) (284).
In another prospective epidemiological study of over 8,000 people, symptomatic infection with HSV-1 or HSV-2 that was sufficient to cause repeated medical visits was associated with a nearly 3-fold increased relative risk of dementia later in life (290).
Remarkably, the relative risk of subsequently developing dementia in patients treated with antivirals (in an uncontrolled fashion) was 91% lower than that in the untreated group. Moreover, there was a dose-response relationship: those treated for longer periods had a lower risk of subsequent dementia (290). A risk reduction of this magnitude, even granting that this was an uncontrolled observational study, conducted in just one community, and with just 1 decade of follow-up, is provocative and demands attempts at replication.
A randomized, controlled, double-blind trial of valacyclovir in people with mild AD is under way at New York University to see if cognitive decline can be slowed, with the estimated date of completion in the summer of 2022 (ClinicalTrials.gov identifier NCT03282916).
Conclusion: HSV-1/2 and AD.
While not fully proven, HSV-1 and HSV-2 remain viable candidates for having etiologic roles in at least some cases of AD.
Evidence Linking VZV with AD
Epidemiologic evidence linking VZV to AD.
Another herpesvirus, varicella-zoster virus (VZV), has also been linked to AD. A large epidemiologic study compared people seeking care for herpes zoster to matched control subjects (approximately 80,000 total subjects). The study found a slightly but significantly increased risk of dementia in those with a history of zoster, after adjusting for potential confounders (HR = 1.11; 95% CI, 1.04 to 1.17) (291). Another study involving 3,384 subjects also found a strong association of herpes zoster with the later development of dementia, particularly for zoster involving the face (HR = 2.83; 95% CI, 1.83 to 4.37) (292).
Echoing the study of people with HSV-1/2 infection, zoster patients treated with antivirals had a considerably reduced risk of developing dementia than people left untreated (HR = 0.55; 95% CI, 0.40 to 0.77) (291).
Although not as extensive as for HSV-1/2, current data suggest, but do not yet prove, an etiologic relationship between VZV and AD.
Evidence Specifically Linking HHV-6A/B with AD
Serologic studies.
Serologic studies attempting to link HHV-6A/B with AD have been uninformative and not entirely consistent (293, 294).
Effect of HHV-6A/B infection on glial cells.
Infection of primary glial cell cultures by HHV-6A produces proinflammatory cytokines and chemokines via stimulation of toll-like receptors (34). When transgenic mice bearing the human CD46 receptor (the receptor for HHV-6A) are inoculated with HHV-6A, they develop infection of glial cells that produce proinflammatory chemokines (34).
Most relevant, in cultures of human neuroglioma cells and in mice engineered to express human Aβ, infection with HHV-6A, HHV-6B, or HSV-1 dramatically accelerated production of Aβ (258). In addition, when microglial cells and T cells are infected with HHV-6A, the expression of Aβ 1-42, tau, and ApoE are all significantly increased (204, 295).
HHV-6A/B and autophagy.
Autophagy is important for maintenance of healthy homeostasis in postmitotic cells, like neurons (296). Impaired autophagy is seen in neurodegenerative diseases, so it is notable that HHV-6A infection of HSB2 cells induces autophagy whereas HHV-6B infection of peripheral blood mononuclear cells (PBMCs) inhibits autophagy (282). HHV-6A infection of astrocytoma cells and primary neurons reduces autophagy. This leads to the accumulation of misfolded and aggregated proteins, increases the production of Aβ, and activates endoplasmic reticulum stress, which in turn promotes both the hyperphosphorylation of tau protein and the unfolded protein response, all phenomena prominent in AD (282, 296–298).
Shared gene expression patterns in HHV-6A/B infection and AD.
The transcriptome of white blood cells (WBCs) infected with HHV-6A/B was compared to the transcriptome in WBCs from patients with AD. There were 95 differentially expressed genes shared by the two groups, most prominently genes involved in antigen presentation by MHC class II molecules (244).
Nucleic acid in blood and brain.
The first suggestion that HHV-6A/B might be one trigger for AD was a report using nested PCR. The investigators found HHV-6A/B DNA in a much higher proportion of brains from the frontal and temporal cortex of patients with AD than in age-matched normal brains (70% versus 40%, P = 0.003). DNA from both HHV-6A and HHV-6B was identified. In contrast, the prevalence of DNA for two other human herpesviruses, HSV-2 and cytomegalovirus (CMV), was not different between AD patients and matched controls (299).
More recently, also using nested PCR, HHV-6A/B DNA was found in peripheral lymphocytes of 23% of patients with AD versus 4% in matched healthy controls (P = 0.002). In contrast, there was only a marginally significant difference in the prevalence of EBV DNA between AD patients and controls (300).
In 2018, Readhead et al. generated considerable excitement when they reported on an examination of DNA and mRNA sequence data obtained from postmortem entorhinal cortex and hippocampal tissues of over 1,000 patients, from four brain repositories, including patients with AD, healthy aging, or progressive supranuclear palsy (PSP) (243). The team looked for 515 known human viruses (243) in genomic (whole-exome DNA) and transcriptome sequencing (RNA-seq) data sets.
In individuals with AD, but not in those with PSP or healthy aging, the team reported finding viral DNA and viral mRNA (indicative of active infection for DNA viruses). The strongest signals were from HHV-6A, HHV-7, and HSV-1. Viral RNA load correlated positively with clinical dementia scores and the density of Aβ plaques and negatively with the number of neurons (243).
As had others (241–244), these investigators found that DNA loci associated with impaired defense against viral infections were more common in people with AD. Finally, host DNA variants known to affect processing of amyloid precursor protein (APP), such as β-secretase (BACE-1), were found to be associated with antiviral signaling (particularly IFN-γ1 signaling). These variants were seen most often in AD patients with the highest viral loads of HHV-6A (243).
Unfortunately, no confirmatory assays (such as immunohistochemistry) were reported, and no data were presented on the exact nucleic acid sequences that “scored” a sample as containing HHV-6A and how those sequences compared to previously published virus sequences. Such evidence would have strengthened the study’s conclusions. Finally, there are questions about the specificity (false-positive rate) of the results. For example, 587 (98%) of 602 specimens were reportedly positive for sequences attributed to variola virus, the causative agent of smallpox, which was declared eradicated in 1980.
Allnutt et al. (301) attempted to replicate the Readhead study in two ways. First, they reexamined the RNA sequencing (RNA-seq) data from Readhead, using a tool developed for virus detection. They found little evidence of HHV-6A/B mRNA sequences in either AD-definite specimens (4 of 308, 1.3%) or in non-AD controls (1 of 244, 0.4%) (301).
Second, Allnutt et al. probed directly for HHV-6A and HHV-6B DNA by digital droplet PCR in several independent collections of brain samples from AD cases and non-AD controls (including samples from the same repository used by Readhead): 20 of 510 (3.9%) AD cases and 5 of 166 (3.0%) controls were positive (301). The digital droplet PCR technique allows absolute quantification of the viral target, is highly sensitive and specific when tested in saliva, serum, and peripheral blood mononuclear cells, and can distinguish HHV-6A from HHV-6B (302). Thus, Allnutt et al. did not confirm that increased HHV-6A DNA or mRNA was present in AD brains compared to healthy control subjects.
Jeong and Liu attempted to replicate the findings of Readhead et al. by reexamining some of the same data (303). They noted that HHV-6A DNA and mRNA were sparse in the Readhead data set and argued that the analytical methods employed by Readhead et al. were inappropriate for such sparse data sets. When they used bioinformatics methods that they deemed more appropriate for such data, no statistically significant associations between AD and virus sequences were detected. Indeed, the authors concluded that that the extremely low levels of viral RNA and DNA sequences in the Readhead and other AD repository data sets preclude making robust conclusions in support of the potential role of any viruses in AD.
Finally, Chorlton found numerous problems in the original analysis and demonstrated that the virus detection algorithm used by Readhead et al. “likely vastly overestimates viral read counts” and “probably identifies viral reads when none are present” (304). Readhead et al. challenged this conclusion (305).
Conclusions: HHV-6A/B and AD
As summarized in Table 6, for HHV-6A/B, the evidence of an etiologic role in AD is much more limited than for HSV-1/2 and several other microbes. Nested-PCR studies have suggested an association between HHV-6A/B and AD. However, because false positives can be easily generated by nested PCR (306), follow-up studies using nonnested, well-validated quantitative PCR assays are needed to confirm these findings. Moreover, the results from studies using nested PCR were not borne out in a recent study (301) that employed a well-validated assay (302) that is less likely to produce false-positive results.
TABLE 6.
Criteria for causality and strength of evidence linking HHV-6A/B to Alzheimer’s disease
| Disease causation criterion | Evidencea |
|---|---|
| HHV-6A/B nucleic acid is present in diseased tissue, in most cases, in higher abundance than in nondiseased tissue (by qPCR or other means). | Positive evidence: HHV-6A/B DNA is reportedly present at higher levels in AD brains than in healthy or disease controls (243). |
| Negative evidence: Examination of brains from AD patients failed to find HHV-6A/B DNA more often than in controls (301). Reexamination of the same data sets examined in reference 243 did not support the original conclusions about the presence of HHV-6A/B in AD brains (301, 303, 304). | |
| The amount of HHV-6A/B nucleic acid in diseased tissue or blood, and/or antibody levels, correlates with the severity of the disease. | Positive evidence: Viral load correlated positively with clinical dementia scores and density of Aβ plaques and correlated negatively with neuron number (243). |
| Negative evidence: Reexamination of the same data sets examined in reference 243 did not support the original conclusions about the presence of HHV-6A/B in AD brains (301, 303, 304). | |
| HHV-6A/B nucleic acid is demonstrated in cells relevant to disease pathology by in situ nucleic acid studies. | HHV-6A/B can infect adult neurons (230, 232), adult astrocytes (109, 230, 231), and microglial cells (30, 229, 230). |
| HHV-6A/B mRNA (by RT-PCR or other means) and antigens by immunohistochemistry are present in diseased tissue. | Positive evidence: HHV-6A/B mRNA is reportedly present at higher levels in AD brains than in healthy or disease controls; viral RNA load correlated positively with clinical dementia scores and the density of Aβ plaques and negatively with neuron number (243). |
| Negative evidence: Reexamination of the same RNA data set by three other groups failed to confirm an association with AD (301, 303, 304). Examination of brains from AD patients failed to find HHV-6A/B mRNA more often than in controls (301). | |
| Exposure to and then presence of the viruses and their gene products in affected tissue precede the development of the disease (temporal relationship). | 95% of humans are infected with HHV-6B in very early childhood. The number of humans infected with HHV-6A, and the typical age of primary infection, is less clear. Thus, people with AD have almost surely been infected with HHV-6A/B before the development of the disease. |
| Infectious agents other than HHV-6A/B are not detected in diseased tissue in a substantial number of cases. | HSV-1 DNA has been detected in the brains of people with AD (237, 264–266). |
| There are cellular and/or humoral immune responses to HHV-6A/B in diseased tissue and/or in blood, and these responses correlate with the severity of the disease. | No evidence yet. |
| HHV-6A/B affect cellular function in diseased tissue in a manner known to cause or augment the disease pathology (in vitro or in vivo studies). | HHV-6A infection of CNS cells leads to the accumulation of misfolded and aggregated proteins, increases the production of Aβ, and promotes both the hyperphosphorylation of tau protein and the unfolded protein response, all phenomena prominent in AD (282, 296–298). |
| Specific antiviral therapy both reduces viral load in diseased tissue or blood and is followed by clinical improvement. | No evidence yet. |
All evidence cited is positive evidence in support of the assertion unless specifically identified as negative evidence.
The antiviral activity of Aβ against HHV-6A/B in vitro in cell culture assays is interesting. However, as yet, there is no evidence that HHV-6A/B is a specific target of Aβ in vivo in humans. Because of this, the Aβ reactivity with HHV-6A/B does not of itself constitute evidence of a role for HHV-6A/B in AD pathogenesis.
It remains possible that generation of new DNA and RNA data sets that provide even deeper sequence coverage, and that include a larger number of cells and cell types, would demonstrate an association between HHV-6A and AD.
Even if improved methodologies do find strong and consistent associations of HHV-6A with AD, studies that demonstrate clinical benefits from immunization and/or antimicrobials directed specifically at these viruses would be needed to formally prove that the viruses play a causal role in AD pathogenesis.
Indeed, the infection hypothesis of AD causality argues that immunization and/or antimicrobials directed at HHV-6A would not be guaranteed to reduce AD incidence and pathogenicity. The hypothesis postulates that multiple pathogens (of which HHV-6A is just one) may be capable of triggering neuroinflammation and the production of Aβ and tau, which converge to cause neurodegeneration. Only if HHV-6A proved to be a dominant infection in AD (as suggested by the unreplicated study by Readhead et al. [243]) would immunization and/or antivirals specifically for HHV-6A be likely to demonstrate a benefit. It seems more likely that treatments directed at diminishing neuroinflammation, regardless of its cause, may prove useful in the prevention or treatment of AD.
CONCLUDING REMARKS
HHV-6A/B are ubiquitous agents capable of producing lifelong infection. They are neurotropic and capable of reactivation throughout life. Furthermore, HHV-6B is well established as the leading cause of encephalitis following hematopoietic stem cell transplantation (4–7, 17). The claim that such ubiquitous agents cause a particular disease is sometimes dismissed with the following question: if this agent, which infects most humans permanently, causes this disease, then why does everybody not have the disease?
Clearly, infectious diseases can result not just from the presence of the infectious agent but also can be shaped by the genetically influenced immune response to the agent, as well as by other host and stochastic factors. Thus, it is entirely plausible that a ubiquitous agent might cause a particular uncommon disease in just a subset of individuals it has infected.
In Table 2, we proposed a set of criteria for judging whether HHV-6A/B might be an etiologic agent of the diseases we have discussed, and for each disease we have provided a table summarizing the evidence according to these criteria.
For FS, FSE, and MTLE, several criteria are met. For FS and FSE, which occur in young children and are usually not fatal, some forms of proof about proximity of the agent to the disease-associated tissue will be difficult to obtain, since there is no justification for invasive sampling of brain tissue. Noninvasive, virus-specific diagnostic methods for studying virus activity in the CNS are needed.
For MS, many of the criteria are met, although the results from multiple laboratories have not been entirely consistent.
For AD, while there is growing evidence that multiple infectious agents may be capable of triggering the disease, current data provide only hints of the possible etiologic involvement of HHV-6A/B. Further pursuit of the possible role of various infectious agents in the pathogenesis of AD will require much deeper sequencing than has been employed thus far, as well as the application of more specific and sensitive probes for these viruses in brain tissues from AD patients.
For FS, FSE, MTLE, MS, and AD, no clinical trials have yet been conducted with agents that target these viruses. Thus, the last criterion has not been met: it has not been demonstrated that elimination or reduction of exposure to the virus changes the outcome of the disease.
Resolution of the outstanding questions for all of these complex diseases will require research governed by a series of principles, as outlined in Table 7, and filling in the gaps in knowledge that are summarized in Tables 3 to 6. The evidence that HHV-6A/B may be an etiologic agent of some of the important neurological diseases discussed here justifies and should encourage expanded research consistent with these principles.
TABLE 7.
Principles to guide future research
| 1. Develop consensus case definitions and diagnostic criteria so that studies can be designed with comparable endpoints and thereby more easily compared. |
| 2. Use the World Health Organization quantitative DNA standard for HHV-6B (http://www.nibsc.org/documents/ifu/15-266.pdf), quantitative PCR that distinguishes HHV-6A from -6B, and antigens and antibodies that appear to be reliable and reproducible, shared reagents, and HHV-6A/B specificity controls. |
| 3. In any publication, include detailed information about PCR assays such as how many cells were lysed, how many cell equivalents were included per reaction, detection limits based on shared standards, etc. |
| 4. Conduct collaborative evaluations of diagnostic assays on panels of coded specimens. |
| 5. Establish multicenter collaborations for disease association studies to ensure reasonable population sizes and geographic generalizability. |
| 6. Develop the capabilities needed to conduct well-controlled multicenter clinical trials that attempt to alter disease course or progression by blocking virus replication (antiviral therapies) or by preventing viral infection (vaccines). |
ACKNOWLEDGMENTS
We are indebted to Kristin Loomis and Dharam V. Ablashi, whose vision and leadership have greatly accelerated progress in studying human herpesvirus 6A, 6B, and 7, through the HHV-6A/B Foundation. Eva Eliassen and Jill Mazzetta were invaluable in identifying, retrieving, and cataloguing the large literature we reviewed, only a small fraction of which is cited; we could not have done it without them.
We have no conflicts of interest to declare.
Biographies

Anthony L. Komaroff is a clinical epidemiologist and general internist who is a Senior Physician at Brigham & Women's Hospital (BWH) and the Simcox-Clifford-Higby Distinguished Professor of Medicine at Harvard Medical School. He received an A.B. degree from Stanford University, an M.D. from the University of Washington, and internal medicine training at Beth Israel Hospital, Boston. He served as Director of the Division of General Medicine at BWH and is known for research on common infectious syndromes, such as pharyngitis, community-acquired pneumonia, and dysuria in adult women, and studies of the pathogenesis, clinical presentation, and epidemiology of myalgic encephalomyelitis/chronic fatigue syndrome. Since the discovery of human herpesviruses 6A/B in the 1980s, he has studied the possible role of these viruses in illness and contributed to the discovery of inherited chromosomally integrated HHV6. He has coauthored the published summaries of each of the biannual international research conferences on these viruses.

Philip E. Pellett is a virologist and has been on the faculty of the Wayne State University School of Medicine since 2007, where he chairs the Department of Biochemistry, Microbiology & Immunology. He served as Chief of the Herpesvirus Section of the U.S. Centers for Disease Control and Prevention from 1986 to 2003 and Director of Herpesvirus Translational and Basic Research at the Cleveland Clinic from 2003 to 2007. Dr. Pellett obtained his B.S. in Chemistry from Ohio University and his Ph.D. in Virology from the University of Chicago. His research is aimed at understanding the biology of human herpesviruses and improving clinical outcomes of herpesvirus infections, with a focus on human cytomegalovirus. Dr. Pellett began studying roseoloviruses in 1986, immediately following the discovery of human herpesvirus 6A, and has made numerous contributions to our understanding of their molecular biology, diagnosis, and spectrum of disease.

Steven Jacobson is currently Chief of the Viral Immunology Section at the National Institutes of Health, National Institutes of Neurologic Diseases and Stroke. Dr. Jacobson obtained his Ph.D. in virology at the Rensselaer Polytechnic Institute in 1980. He came to NIH for his postdoctoral fellowship in neuroimmunology under the direction of the late Dale McFarlin and Henry McFarland. In 1991, he was promoted to Senior Investigator and became tenured Chief of his own section in Viral Immunology in 1993. He was named acting Branch Chief of the Neuroimmunology Branch from 2009 to 2014. Research in his laboratory has focused on the virologic, immunological, and molecular biological characteristics of chronic, progressive neurological disease that are associated with human viruses and the relationship of these disorders to the disease of multiple sclerosis. He has a large clinical program in which he translates basic laboratory observations into clinical interventional strategies.
REFERENCES
- 1.Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, Gallo RC. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 2.Lopez C, Pellett P, Stewart J, Goldsmith C, Sanderlin K, Black J, Warfield D, Feorino P. 1988. Characteristics of human herpesvirus-6. J Infect Dis 157:1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- 3.Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, Rhoads MP, Nguy L, Borneman R, Morrow RA, Corey L. 2005. A population-based study of primary human herpesvirus 6 infection. N Engl J Med 352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 4.Hill JA, Venna N. 2014. Human herpesvirus 6 and the nervous system, p 327–355. In Tselis AC, Booss J (ed), Neurovirology, vol 123 Elsevier, Amsterdam, the Netherlands. [DOI] [PubMed] [Google Scholar]
- 5.Zerr DM, Gooley TA, Corey L, Anasetti C, Huang ML, Carpenter P, Yeung L, Wade JC. 2001. Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clin Infect Dis 33:763–771. doi: 10.1086/322642. [DOI] [PubMed] [Google Scholar]
- 6.Zerr DM, Komaroff AL. 2014. Cognitive dysfunction from HHV-6A and HHV-6B, p 99–143. In Flamand L, Lautenschlager I, Krueger GRF, Ablashi DV (ed), Human herpesviruses HHV-6A, HHV-6B and HHV-7: diagnosis and clinical management, 3rd ed Elsevier, Oxford, United Kingdom. [Google Scholar]
- 7.Eliassen E, Hemond CC, Santoro JD. 2020. HHV-6-associated neurological disease in children: epidemiologic, clinical, diagnostic, and treatment considerations. Pediatr Neurol 105:10–20. doi: 10.1016/j.pediatrneurol.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S, Insel RA, Epstein LG. 1994. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med 331:432–438. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- 9.Caserta MT, Hall CB, Schnabel K, McIntyre K, Long C, Costanzo M, Dewhurst S, Insel R, Epstein LG. 1994. Neuroinvasion and persistence of human herpesvirus 6 in children. J Infect Dis 170:1586–1589. doi: 10.1093/infdis/170.6.1586. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa T, Asano Y, Morishima T, Nagashima M, Sobue G, Watanabe K, Yamaguchi H. 1993. Epidemiology of acute childhood encephalitis. Aichi Prefecture, Japan, 1984–1990. Brain Dev 15:192–197. doi: 10.1016/0387-7604(93)90064-F. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim AI, Obeid MT, Jouma MJ, Roemer K, Mueller-Lantzsch N, Gärtner BC. 2005. Prevalence of herpes simplex virus (types 1 and 2), varicella-zoster virus, cytomegalovirus, and human herpesvirus 6 and 7 DNA in cerebrospinal fluid of Middle Eastern patients with encephalitis. J Clin Microbiol 43:4172–4174. doi: 10.1128/JCM.43.8.4172-4174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou J, Wu Y, Cai M, Wu X, Shang S. 2011. Subtype-specific, probe-based, real-time PCR for detection and typing of human herpesvirus-6 encephalitis from pediatric patients under the age of 2 years. Diagn Microbiol Infect Dis 70:223–229. doi: 10.1016/j.diagmicrobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, Saito Y, Takanashi J, Hirose S, Yamagata T, Yamanouchi H, Mizuguchi M. 2012. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev 34:337–343. doi: 10.1016/j.braindev.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Ward KN, Andrews NJ, Verity CM, Miller E, Ross EM. 2005. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child 90:619–623. doi: 10.1136/adc.2004.062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser CA, Gilliam S, Schnurr D, Forghani B, Honarmand S, Khetsuriani N, Fischer M, Cossen CK, Anderson LJ, California Encephalitis P, California Encephalitis Project, 1998–2000. 2003. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis 36:731–742. doi: 10.1086/367841. [DOI] [PubMed] [Google Scholar]
- 16.Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, Jacobson S. 2009. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol 65:257–267. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward KN, Hill JA, Hubacek P, de la Camara R, Crocchiolo R, Einsele H, Navarro D, Robin C, Cordonnier C, Ljungman P, European Conference on Infections in Leukaemia. 2019. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica 104:2155–2163. doi: 10.3324/haematol.2019.223073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun DK, Dominguez G, Pellett PE. 1997. Human herpesvirus 6. Clin Microbiol Rev 10:521–567. doi: 10.1128/CMR.10.3.521-567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruksananonda P, Hall CB, Insel RA, McIntyre K, Pellett PE, Long CE, Schnabel KC, Pincus PH, Stamey FR, Dambaugh TR, Stewart JA. 1992. Primary human herpesvirus 6 infection in young children. N Engl J Med 326:1445–1450. doi: 10.1056/NEJM199205283262201. [DOI] [PubMed] [Google Scholar]
- 20.Agut H, Bonnafous P, Gautheret-Dejean A. 2015. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev 28:313–335. doi: 10.1128/CMR.00122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbuckle JH, Medveczky MM, Luka J, Hadley SH, Luegmayr A, Ablashi D, Lund TC, Tolar J, de Meirleir K, Montoya JG, Komaroff AL, Ambros PF, Medveczky PG. 2010. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci U S A 107:5563–5568. doi: 10.1073/pnas.0913586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogestyn JM, Mock DJ, Mayer-Proschel M. 2018. Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology. Neural Regen Res 13:211–221. doi: 10.4103/1673-5374.226380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harberts E, Yao K, Wohler JE, Maric D, Ohayon J, Henkin R, Jacobson S. 2011. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci U S A 108:13734–13739. doi: 10.1073/pnas.1105143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, Flamand L, Gautheret-Dejean A, Hall CB, Kamble RT, Kuehl U, Lassner D, Lautenschlager I, Loomis KS, Luppi M, Lusso P, Medveczky PG, Montoya JG, Mori Y, Ogata M, Pritchett JC, Rogez S, Seto E, Ward KN, Yoshikawa T, Razonable RR. 2012. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 22:144–155. doi: 10.1002/rmv.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang E, Bell AJ, Wilkie GS, Suarez NM, Batini C, Veal CD, Armendariz-Castillo I, Neumann R, Cotton VE, Huang Y, Porteous DJ, Jarrett RF, Davison AJ, Royle NJ. 2017. Inherited chromosomally integrated human herpesvirus 6 genomes are ancient, intact, and potentially able to reactivate from telomeres. J Virol 91:e01137-17. doi: 10.1128/JVI.01137-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravel A, Dubuc I, Morissette G, Sedlak RH, Jerome KR, Flamand L. 2015. Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proc Natl Acad Sci U S A 112:8058–8063. doi: 10.1073/pnas.1502741112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 28.Tang H, Serada S, Kawabata A, Ota M, Hayashi E, Naka T, Yamanishi K, Mori Y. 2013. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc Natl Acad Sci U S A 110:9096–9099. doi: 10.1073/pnas.1305187110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robert C, Aubin JT, Visse B, Fillet AM, Huraux JM, Agut H. 1996. Difference in permissiveness of human fibroblast cells to variants A and B of human herpesvirus-6. Res Virol 147:219–225. doi: 10.1016/0923-2516(96)89652-X. [DOI] [PubMed] [Google Scholar]
- 30.De Bolle L, Van Loon J, De Clercq E, Naesens L. 2005. Quantitative analysis of human herpesvirus 6 cell tropism. J Med Virol 75:76–85. doi: 10.1002/jmv.20240. [DOI] [PubMed] [Google Scholar]
- 31.Ahlqvist J, Donati D, Martinelli E, Akhyani N, Hou J, Major EO, Jacobson S, Fogdell-Hahn A. 2006. Complete replication cycle and acquisition of tegument in nucleus of human herpesvirus 6A in astrocytes and in T-cells. J Med Virol 78:1542–1553. doi: 10.1002/jmv.20737. [DOI] [PubMed] [Google Scholar]
- 32.Fotheringham J, Donati D, Akhyani N, Fogdell-Hahn A, Vortmeyer A, Heiss JD, Williams E, Weinstein S, Bruce DA, Gaillard WD, Sato S, Theodore WH, Jacobson S. 2007. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med 4:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caruso A, Rotola A, Comar M, Favilli F, Galvan M, Tosetti M, Campello C, Caselli E, Alessandri G, Grassi M, Garrafa E, Cassai E, Di Luca D. 2002. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory cytokines. J Med Virol 67:528–533. doi: 10.1002/jmv.10133. [DOI] [PubMed] [Google Scholar]
- 34.Reynaud JM, Jegou JF, Welsch J, Horvat B. 2014. Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via TLR9. J Virol 88:5421–5436. doi: 10.1128/JVI.03763-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flamand L, Gosselin J, D'Addario M, Hiscott J, Ablashi DV, Gallo RC, Menezes J. 1991. Human herpesvirus 6 induces interleukin-1β and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J Virol 65:5105–5110. doi: 10.1128/JVI.65.9.5105-5110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arena A, Capozza AB, Di Luca D. 1996. Role of IFN gamma on TNF alpha, IL-1 and IL-6 release during HHV-6 infection. New Microbiol 19:183–191. [PubMed] [Google Scholar]
- 37.Mayne M, Cheadle C, Soldan SS, Cermelli C, Yamano Y, Akhyani N, Nagel JE, Taub DD, Becker KG, Jacobson S. 2001. Gene expression profile of herpesvirus-infected T cells obtained using immunomicroarrays: induction of proinflammatory mechanisms. J Virol 75:11641–11650. doi: 10.1128/JVI.75.23.11641-11650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tallóczy Z, Virgin HW IV, Levine B. 2006. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2:24–29. doi: 10.4161/auto.2176. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K, Segal E, Kondo T, Mukai T, Moriyama M, Takahashi M, Yamanishi K. 1992. Interferon and natural killer cell activity in patients with exanthem subitum. Pediatr Infect Dis J 11:369–373. doi: 10.1097/00006454-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Meeuwsen S, Persoon-Deen C, Bsibsi M, Bajramovic JJ, Ravid R, De Bolle L, van Noort JM. 2005. Modulation of the cytokine network in human adult astrocytes by human herpesvirus-6A. J Neuroimmunol 164:37–47. doi: 10.1016/j.jneuroim.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Flamand L, Stefanescu I, Menezes J. 1996. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J Clin Invest 97:1373–1381. doi: 10.1172/JCI118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Bolle L, Naesens L, de Clercq E. 2005. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev 18:217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet 1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki Y, Inagi R, Aono T, Yamanishi K, Shiohara T. 1998. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch Dermatol 134:1108–1112. doi: 10.1001/archderm.134.9.1108. [DOI] [PubMed] [Google Scholar]
- 45.Sekiguchi A, Kashiwagi T, Ishida-Yamamoto A, Takahashi H, Hashimoto Y, Kimura H, Tohyama M, Hashimoto K, Iizuka H. 2005. Drug-induced hypersensitivity syndrome due to mexiletine associated with human herpes virus 6 and cytomegalovirus reactivation. J Dermatol 32:278–281. doi: 10.1111/j.1346-8138.2005.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 46.Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, Urano Y, Matsumoto K, Iijima M, Shear NH. 2007. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol 157:934–940. doi: 10.1111/j.1365-2133.2007.08167.x. [DOI] [PubMed] [Google Scholar]
- 47.Descamps V. 2009. Drug reaction with eosinophila and systemic symptoms: a cause of human herpesvirus 6-related fulminant myocarditis and hepatitis in immunocompetent patients. Hum Pathol 40:1667–1667. doi: 10.1016/j.humpath.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Mardivirin L, Descamps V, Lacroix A, Delebassee S, Ranger-Rogez S. 2009. Early effects of drugs responsible for DRESS on HHV-6 replication in vitro. J Clin Virol 46:300–302. doi: 10.1016/j.jcv.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Caselli E, Zatelli MC, Rizzo R, Benedetti S, Martorelli D, Trasforini G, Cassai E, Degli Uberti EC, Di Luca D, Dolcetti R. 2012. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog 8:e1002951. doi: 10.1371/journal.ppat.1002951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marci R, Gentili V, Bortolotti D, Lo Monte G, Caselli E, Bolzani S, Rotola A, Di Luca D, Rizzo R. 2016. Presence of HHV-6A in endometrial epithelial cells from women with primary unexplained infertility. PLoS One 11:e0158304. doi: 10.1371/journal.pone.0158304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaccioli F, Lager S, de Goffau MC, Sovio U, Dopierala J, Gong S, Cook E, Sharkey A, Moffett A, Lee WK, Delles C, Venturini C, Breuer J, Parkhill J, Peacock SJ, Charnock-Jones DS, Smith GCS. 2020. Fetal inheritance of chromosomally integrated human herpesvirus 6 predisposes the mother to pre-eclampsia. Nat Microbiol 5:901–908. doi: 10.1038/s41564-020-0711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horvat B, Berges BK, Lusso P. 2014. Recent developments in animal models for human herpesvirus 6A and 6B. Curr Opin Virol 9:97–103. doi: 10.1016/j.coviro.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynaud JM, Horvat B. 2013. Animal models for human herpesvirus 6 infection. Front Microbiol 4:174. doi: 10.3389/fmicb.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leibovitch E, Wohler JE, Cummings Macri SM, Motanic K, Harberts E, Gaitan MI, Maggi P, Ellis M, Westmoreland S, Silva A, Reich DS, Jacobson S. 2013. Novel marmoset (Callithrix jacchus) model of human herpesvirus 6A and 6B infections: immunologic, virologic and radiologic characterization. PLoS Pathog 9:e1003138. doi: 10.1371/journal.ppat.1003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamanishi K, Mori Y, Pellett PE. 2013. Human herpesviruses 6 and 7, p 2059–2111. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2 Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 56.Zanella MC, Cordey S, Kaiser L. 2020. Beyond cytomegalovirus and Epstein-Barr Virus: a review of viruses composing the blood virome of solid organ transplant and hematopoietic stem cell transplant recipients. Clin Microbiol Rev 33:e00027-20. doi: 10.1128/CMR.00027-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitley RJ, Lakeman FD. 2005. Human herpesvirus 6 infection of the central nervous system: is it just a case of mistaken association? Clin Infect Dis 40:894–895. doi: 10.1086/427953. [DOI] [PubMed] [Google Scholar]
- 58.Koch R. 1890. Über bakteriologische Forschung, abstr [none]. Verhandlung des X internationalen medischinischen Congresses, Berlin, 1891. August Hirschwald, Berlin, Germany. [Google Scholar]
- 59.Rivers TM. 1937. Viruses and Koch's postulates. J Bacteriol 33:1–12. doi: 10.1128/JB.33.1.1-12.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill AB. 1965. The environment and disease: association or causation? Proc R Soc Med 58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans AS. 1976. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med 49:175–195. [PMC free article] [PubMed] [Google Scholar]
- 62.Fredricks DN, Relman DA. 1996. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev 9:18–33. doi: 10.1128/CMR.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartolini L, Theodore WH, Jacobson S, Gaillard WD. 2019. Infection with HHV-6 and its role in epilepsy. Epilepsy Res 153:34–39. doi: 10.1016/j.eplepsyres.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Solomon T, Vaughn DW. 2002. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol 267:171–194. doi: 10.1007/978-3-642-59403-8_9. [DOI] [PubMed] [Google Scholar]
- 65.Murthy JMK. 2010. Neurological complications of dengue infection. Neurol India 58:581–584. doi: 10.4103/0028-3886.68654. [DOI] [PubMed] [Google Scholar]
- 66.Ekstrand JJ, Herbener A, Rawlings J, Turney B, Ampofo K, Korgenski EK, Bonkowsky JL. 2010. Heightened neurologic complications in children with pandemic H1N1 influenza. Ann Neurol 68:762–766. doi: 10.1002/ana.22184. [DOI] [PubMed] [Google Scholar]
- 67.De Chiara G, Piacentini R, Fabiani M, Mastrodonato A, Marcocci ME, Limongi D, Napoletani G, Protto V, Coluccio P, Celestino I, Li Puma DD, Grassi C, Palamara AT. 2019. Recurrent herpes simplex virus-1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog 15:e1007617. doi: 10.1371/journal.ppat.1007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartolini L, Libbey JE, Ravizza T, Fujinami RS, Jacobson S, Gaillard WD. 2019. Viral triggers and inflammatory mechanisms in pediatric epilepsy. Mol Neurobiol 56:1897–1907. doi: 10.1007/s12035-018-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Opsahl ML, Kennedy PGE. 2005. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 128:516–527. doi: 10.1093/brain/awh390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Z, Xue T, Zhang Z, Wang X, Xu P, Zhang J, Lei X, Li Y, Xie Y, Wang L, Fang M, Chen Y. 2011. Role of signal transducer and activator of transcription-3 in up-regulation of GFAP after epilepsy. Neurochem Res 36:2208–2215. doi: 10.1007/s11064-011-0576-1. [DOI] [PubMed] [Google Scholar]
- 71.Kawamura Y, Nakayama A, Kato T, Miura H, Ishihara N, Ihira M, Takahashi Y, Matsuda K, Yoshikawa T. 2015. Pathogenic role of human herpesvirus 6B infection in mesial temporal lobe epilepsy. J Infect Dis 212:1014–1021. doi: 10.1093/infdis/jiv160. [DOI] [PubMed] [Google Scholar]
- 72.Ichiyama T, Ito Y, Kubota M, Yamazaki T, Nakamura K, Furukawa S. 2009. Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev 31:731–738. doi: 10.1016/j.braindev.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Kawamura Y, Yamazaki Y, Ohashi M, Ihira M, Yoshikawa T. 2014. Cytokine and chemokine responses in the blood and cerebrospinal fluid of patients with human herpesvirus 6B-associated acute encephalopathy with biphasic seizures and late reduced diffusion. J Med Virol 86:512–518. doi: 10.1002/jmv.23788. [DOI] [PubMed] [Google Scholar]
- 74.Kittaka S, Hasegawa S, Ito Y, Ohbuchi N, Suzuki E, Kawano S, Aoki Y, Nakatsuka K, Kudo K, Wakiguchi H, Kajimoto M, Matsushige T, Ichiyama T. 2014. Serum levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in human herpesvirus-6-infected infants with or without febrile seizures. J Infect Chemother 20:716–721. doi: 10.1016/j.jiac.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 75.Tamai M, Kobayashi N, Shimada K, Oka N, Takahashi M, Tanuma A, Tanemoto T, Namba H, Saito Y, Wada Y, Okamoto A, Ida H, Kondo K. 2017. Increased interleukin-1beta and basic fibroblast growth factor levels in the cerebrospinal fluid during human herpesvirus-6B (HHV-6B) encephalitis. Biochem Biophys Res Commun 486:706–711. doi: 10.1016/j.bbrc.2017.03.102. [DOI] [PubMed] [Google Scholar]
- 76.Yao K, Graham J, Akahata Y, Oh U, Jacobson S. 2010. Mechanism of neuroinflammation: enhanced cytotoxicity and IL-17 production via CD46 binding. J Neuroimmune Pharmacol 5:469–478. doi: 10.1007/s11481-010-9232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vezzani A, Fujinami RS, White HS, Preux PM, Blumcke I, Sander JW, Loscher W. 2016. Infections, inflammation and epilepsy. Acta Neuropathol 131:211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon A, Kwak BO, Kim K, Ha J, Kim SJ, Bae SH, Son JS, Kim SN, Lee R. 2018. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure 59:5–10. doi: 10.1016/j.seizure.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 79.Bartolini L, Piras E, Sullivan K, Gillen S, Bumbut A, Lin C-TM, Leibovitch EC, Graves JS, Waubant EL, Chamberlain JM, Gaillard WD, Jacobson S. 2018. Detection of HHV-6 and EBV and cytokine levels in saliva from children with seizures: results of a multi-center cross-sectional study. Front Neurol 9:834. doi: 10.3389/fneur.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fotheringham J, Williams EL, Akhyani N, Jacobson S. 2008. Human herpesvirus 6 (HHV-6) induces dysregulation of glutamate uptake and transporter expression in astrocytes. J Neuroimmune Pharmacol 3:105–116. doi: 10.1007/s11481-007-9084-0. [DOI] [PubMed] [Google Scholar]
- 81.Niehusmann P, Widman G, Eis-Hubinger AM, Greschus S, Robens BK, Grote A, Becker AJ. 2016. Non-paraneoplastic limbic encephalitis and central nervous HHV-6B reactivation: causality or coincidence? Neuropathology 36:376–380. doi: 10.1111/neup.12283. [DOI] [PubMed] [Google Scholar]
- 82.Kondo K, Nagafuji H, Hata A, Tomomori C, Yamanishi K. 1993. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J Infect Dis 167:1197–1200. doi: 10.1093/infdis/167.5.1197. [DOI] [PubMed] [Google Scholar]
- 83.Suga S, Yoshikawa T, Asano Y, Kozawa T, Nakashima T, Kobayashi I, Yazaki T, Yamamoto H, Kajita Y, Ozaki T, Nishimura Y, Yamanaka T, Yamada A, Imanishi J. 1993. Clinical and virological analyses of 21 infants with exanthem subitum (roseola infantum) and central nervous system complications. Ann Neurol 33:597–603. doi: 10.1002/ana.410330607. [DOI] [PubMed] [Google Scholar]
- 84.Mannonen L, Herrgard E, Valmari P, Rautiainen P, Uotila K, Aine MR, Karttunen-Lewandowski P, Sankala J, Wallden T, Koskiniemi M. 2007. Primary human herpesvirus-6 infection in the central nervous system can cause severe disease. Pediatr Neurol 37:186–191. doi: 10.1016/j.pediatrneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Pulickal AS, Ramachandran S, Rizek P, Narula P, Schubert R. 2013. Chorea and developmental regression associated with human herpes virus-6 encephalitis. Pediatr Neurol 48:249–251. doi: 10.1016/j.pediatrneurol.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 86.McCullers JA, Lakeman FD, Whitley RJ. 1995. Human herpesvirus 6 is associated with focal encephalitis. Clin Infect Dis 21:571–576. doi: 10.1093/clinids/21.3.571. [DOI] [PubMed] [Google Scholar]
- 87.Miyahara H, Miyakawa K, Nishida H, Yano S, Sonoda T, Suenobu S, Izumi T, Daa T, Ihara K. 2018. Unique cell tropism of HHV-6B in an infantile autopsy case of primary HHV-6B encephalitis. Neuropathology 38:400–406. doi: 10.1111/neup.12462. [DOI] [PubMed] [Google Scholar]
- 88.Shahani L. 2014. HHV-6 encephalitis presenting as status epilepticus in an immunocompetent patient. BMJ Case Rep 2014:bcr2014205880. doi: 10.1136/bcr-2014-205880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Novoa LJ, Nagra RM, Nakawatase T, Edwards-Lee T, Tourtellotte WW, Cornford ME. 1997. Fulminant demyelinating encephalomyelitis associated with productive HHV-6 infection in an immunocompetent adult. J Med Virol 52:301–308. doi:. [DOI] [PubMed] [Google Scholar]
- 90.Epstein LG, Shinnar S, Hesdorffer DC, Nordli DR, Hamidullah A, Benn EKT, Pellock JM, Frank LM, Lewis DV, Moshe SL, Shinnar RC, Sun S, FEBSTAT Study Team. 2012. Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study. Epilepsia 53:1481–1488. doi: 10.1111/j.1528-1167.2012.03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hesdorffer DC, Shinnar S, Lax DN, Pellock JM, Nordli DR Jr, Seinfeld S, Gallentine W, Frank LM, Lewis DV, Shinnar RC, Bello JA, Chan S, Epstein LG, Moshe SL, Liu B, Sun S, FEBSTAT Study Team. 2016. Risk factors for subsequent febrile seizures in the FEBSTAT study. Epilepsia 57:1042–1047. doi: 10.1111/epi.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. 1998. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol 43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 93.Provenzale JM, VanLandingham KE, Lewis DV, Mukundan S Jr, White LE. 2008. Extrahippocampal involvement in human herpesvirus 6 encephalitis depicted at MR imaging. Radiology 249:955–963. doi: 10.1148/radiol.2492071917. [DOI] [PubMed] [Google Scholar]
- 94.Shinnar S, Bello JA, Chan S, Hesdorffer DC, Lewis DV, Macfall J, Pellock JM, Nordli DR, Frank LM, Moshé SL, Gomes W, Shinnar RC, Sun S, FEBSTAT Study Team. 2012. MRI abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology 79:871–877. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shinnar S. 2003. Febrile seizures and mesial temporal sclerosis. Epilepsy Curr 3:115–118. doi: 10.1046/j.1535-7597.2003.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lewis DV, Shinnar S, Hesdorffer DC, Bagiella E, Bello JA, Chan S, Xu Y, MacFall J, Gomes WA, Moshe SL, Mathern GW, Pellock JM, Nordli DR Jr, Frank LM, Provenzale J, Shinnar RC, Epstein LG, Masur D, Litherland C, Sun S, FEBSTAT Study Team. 2014. Hippocampal sclerosis after febrile status epilepticus: the FEBSTAT study. Ann Neurol 75:178–185. doi: 10.1002/ana.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hattori F, Kawamura Y, Kozawa K, Miura H, Miyake M, Yoshikawa A, Ihira M, Yoshikawa T. 2019. Clinical characteristics of primary HHV-6B infection in children visiting the emergency room. Pediatr Infect Dis J 38:e248–e253. doi: 10.1097/INF.0000000000002379. [DOI] [PubMed] [Google Scholar]
- 98.Barone SR, Kaplan MH, Krilov LR. 1995. Human herpesvirus-6 infection in children with first febrile seizures. J Pediatr 127:95–97. doi: 10.1016/s0022-3476(95)70263-6. [DOI] [PubMed] [Google Scholar]
- 99.Zeller A, Schaub N, Steffen I, Battegay E, Hirsch HH, Bircher AJ. 2003. Drug hypersensitivity syndrome to carbamazepine and human herpes virus 6 infection: case report and literature review. Infection 31:254–256. doi: 10.1007/s15010-002-3099-5. [DOI] [PubMed] [Google Scholar]
- 100.Millichap JG, Millichap JJ. 2006. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol 35:165–172. doi: 10.1016/j.pediatrneurol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Laina I, Syriopoulou VP, Daikos GL, Roma ES, Papageorgiou F, Kakourou T, Theodoridou M. 2010. Febrile seizures and primary human herpesvirus 6 infection. Pediatr Neurol 42:28–31. doi: 10.1016/j.pediatrneurol.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 102.You SJ. 2020. Human herpesvirus-6 may be neurologically injurious in some immunocompetent children. J Child Neurol 35:132–136. doi: 10.1177/0883073819879284. [DOI] [PubMed] [Google Scholar]
- 103.Tembo J, Chandwe K, Kabwe M, Chilufya M, Ciccone O, Mpabalwani E, Ablashi D, Zumla A, Chen T, Bates M. 2018. Children infected by human herpesvirus 6B with febrile seizures are more likely to develop febrile status epilepticus: a case-control study in a referral hospital in Zambia. J Med Virol 90:1757–1764. doi: 10.1002/jmv.25269. [DOI] [PubMed] [Google Scholar]
- 104.Miyake M, Kawamura Y, Hattori F, Miura H, Ishihara N, Yoshikawa T. 2020. Clinical features of complex febrile seizure caused by primary human herpesvirus 6B infection. Pediatr Neurol 109:52–55. doi: 10.1016/j.pediatrneurol.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 105.Suga S, Suzuki K, Ihira M, Yoshikawa T, Kajita Y, Ozaki T, Iida K, Saito Y, Asano Y. 2000. Clinical characteristics of febrile convulsions during primary HHV-6 infection. Arch Dis Child 82:62–66. doi: 10.1136/adc.82.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Juntunen A, Herrgård E, Mannonen L, Korppi M, Linnavuori K, Vaheri A, Koskiniemi M. 2001. A major role of viruses in convulsive status epilepticus in children: a prospective study of 22 children. Eur J Pediatr 160:37–42. doi: 10.1007/pl00008414. [DOI] [PubMed] [Google Scholar]
- 107.Frank LM, Shinnar S, Hesdorffer DC, Shinnar RC, Pellock JM, Gallentine W, Nordli DR Jr, Epstein LG, Moshe SL, Lewis DV, Sun S, FEBSTAT Study Team. 2012. Cerebrospinal fluid findings in children with fever-associated status epilepticus: results of the consequences of prolonged febrile seizures (FEBSTAT) study. J Pediatr 161:1169–1171. doi: 10.1016/j.jpeds.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Engel J. 2001. Mesial temporal lobe epilepsy: what have we learned? Neuroscientist 7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- 109.Donati D, Akhyani N, Fogdell-Hahn A, Cermelli C, Cassiani-Ingoni R, Vortmeyer A, Heiss JD, Cogen P, Gaillard WD, Sato S, Theodore WH, Jacobson S. 2003. Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections. Neurology 61:1405–1411. doi: 10.1212/01.wnl.0000094357.10782.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, Pfäfflin M, Elger C, Widman G, Schramm J, Becker A, Braun KP, Leijten F, Baayen JC, Aronica E, Chassoux F, Hamer H, Stefan H, Rössler K, Thom M, Walker MC, Sisodiya SM, Duncan JS, McEvoy AW, Pieper T, Holthausen H, Kudernatsch M, Meencke HJ, Kahane P, Schulze-Bonhage A, Zentner J, Heiland DH, Urbach H, Steinhoff BJ, Bast T, Tassi L, Lo Russo G, Özkara C, Oz B, Krsek P, Vogelgesang S, Runge U, Lerche H, Weber Y, Honavar M, Pimentel J, Arzimanoglou A, Ulate-Campos A, Noachtar S, Hartl E, EEBB Consortium, et al. 2017. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med 377:1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- 111.Hubele F, Bilger K, Kremer S, Imperiale A, Lioure B, Namer IJ. 2012. Sequential FDG PET and MRI findings in a case of human herpes virus 6 limbic encephalitis. Clin Nucl Med 37:716–717. doi: 10.1097/RLU.0b013e31824c5e2f. [DOI] [PubMed] [Google Scholar]
- 112.Hukin J, Farrell K, MacWilliam LM, Colbourne M, Waida E, Tan R, Mroz L, Thomas E. 1998. Case-control study of primary human herpesvirus 6 infection in children with febrile seizures. Pediatrics 101:E3. doi: 10.1542/peds.101.2.e3. [DOI] [PubMed] [Google Scholar]
- 113.Francis JR, Richmond P, Robins C, Lindsay K, Levy A, Effler PV, Borland M, Blyth CC. 2016. An observational study of febrile seizures: the importance of viral infection and immunization. BMC Pediatr 16:202. doi: 10.1186/s12887-016-0740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zerr DM, Yeung LC, Obrigewitch RM, Huang ML, Frenkel LM, Corey L. 2002. Case report: primary human herpesvirus-6 associated with an afebrile seizure in a 3-week-old infant. J Med Virol 66:384–387. doi: 10.1002/jmv.2156. [DOI] [PubMed] [Google Scholar]
- 115.McClelland AC, Gomes WA, Shinnar S, Hesdorffer DC, Bagiella E, Lewis DV, Bello JA, Chan S, MacFall J, Chen M, Pellock JM, Nordli DR Jr, Frank LM, Moshe SL, Shinnar RC, Sun S, FEBSTAT Study Team. 2016. Quantitative evaluation of medial temporal lobe morphology in children with febrile status epilepticus: results of the FEBSTAT Study. AJNR Am J Neuroradiol 37:2356–2362. doi: 10.3174/ajnr.A4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohammadpour Touserkani F, Gainza-Lein M, Jafarpour S, Brinegar K, Kapur K, Loddenkemper T. 2017. HHV-6 and seizure: a systematic review and meta-analysis. J Med Virol 89:161–169. doi: 10.1002/jmv.24594. [DOI] [PubMed] [Google Scholar]
- 117.Kimberlin DW, Whitley RJ. 1998. Human herpesvirus-6: neurologic implications of a newly-described viral pathogen. J Neurovirol 4:474–485. doi: 10.3109/13550289809113492. [DOI] [PubMed] [Google Scholar]
- 118.Sakai R, Kanamori H, Motohashi K, Yamamoto W, Matsuura S, Fujita A, Ohshima R, Kuwabara H, Tanaka M, Fujita H, Maruta A, Ishigatsubo Y, Fujisawa S. 2011. Long-term outcome of human herpesvirus-6 encephalitis after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 17:1389–1394. doi: 10.1016/j.bbmt.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 119.Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, Armand P, Alyea EP III, Baden LR, Antin JH, Soiffer RJ, Marty FM. 2012. Cord-blood hematopoietic stem-cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant 18:1638–1648. doi: 10.1016/j.bbmt.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Howell KB, Tiedemann K, Haeusler G, Mackay MT, Kornber AJ, Freeman JL, Harvey AS. 2012. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia 53:e122–e126. doi: 10.1111/j.1528-1167.2012.03494.x. [DOI] [PubMed] [Google Scholar]
- 121.Fida M, Hamdi AM, Bryson A, Razonable RR, Abu Saleh O. 2019. Long-term outcomes of patients with human herpesvirus 6 encephalitis. Open Forum Infect Dis 6:ofz269. doi: 10.1093/ofid/ofz269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Provenzale JM, van Landingham K, White LE. 2010. Clinical and imaging findings suggesting human herpesvirus 6 encephalitis. Pediatr Neurol 42:32–39. doi: 10.1016/j.pediatrneurol.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 123.Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, Morgan MA, Hulette C, Kurtzberg J, Bushnell C, Epstein L, Lewis DV. 2001. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol 50:612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 124.Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, Baden LR, Bromfield EB. 2007. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology 69:156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- 125.Theodore WH, Bhatia S, Hatta J, Fazilat S, DeCarli C, Bookheimer SY, Gaillard WD. 1999. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology 52:132–136. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 126.Weiss EF, Masur D, Shinnar S, Hesdorffer DC, Hinton VJ, Bonner M, Rinaldi J, Van de Water V, Culbert J, Shinnar RC, Seinfeld S, Gallentine W, Nordli DR, Frank LM, Epstein L, Moshé SL, Sun S, FEBSTAT Study Team. 2016. Cognitive functioning one month and one year following febrile status epilepticus. Epilepsy Behav 64:283–288. doi: 10.1016/j.yebeh.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uesugi H, Shimizu H, Maehara T, Arai N, Nakayama H. 2000. Presence of human herpesvirus 6 and herpes simplex virus detected by polymerase chain reaction in surgical tissue from temporal lobe epileptic patients. Psychiatry Clin Neurosci 54:589–593. doi: 10.1046/j.1440-1819.2000.00758.x. [DOI] [PubMed] [Google Scholar]
- 128.Theodore WH, Epstein L, Gaillard WD, Shinnar S, Wainwright MS, Jacobson S. 2008. Human herpes virus 6B: a possible role in epilepsy? Epilepsia 49:1828–1837. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li JM, Lei D, Peng F, Zeng YJ, Li L, Xia ZL, Xia XQ, Zhou D. 2011. Detection of human herpes virus 6B in patients with mesial temporal lobe epilepsy in West China and the possible association with elevated NF-kB expression. Epilepsy Res 94:1–9. doi: 10.1016/j.eplepsyres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 130.Niehusmann P, Mittelstaedt T, Bien CG, Drexler JF, Grote A, Schoch S, Becker AJ. 2010. Presence of human herpes virus 6 DNA exclusively in temporal lobe epilepsy brain tissue of patients with history of encephalitis. Epilepsia 51:2478–2483. doi: 10.1111/j.1528-1167.2010.02741.x. [DOI] [PubMed] [Google Scholar]
- 131.Millichap JJ, Millichap JG. 2015. Role of HHV-6B infection in mesial temporal lobe epilepsy. Pediatr Neurol Briefs 29:40. doi: 10.15844/pedneurbriefs-29-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Esposito L, Drexler JF, Braganza O, Doberentz E, Grote A, Widman G, Drosten C, Eis-Hubinger AM, Schoch S, Elger CE, Becker AJ, Niehusmann P. 2015. Large-scale analysis of viral nucleic acid spectrum in temporal lobe epilepsy biopsies. Epilepsia 56:234–243. doi: 10.1111/epi.12890. [DOI] [PubMed] [Google Scholar]
- 133.Wipfler P, Dunn N, Beiki O, Trinka E, Fogdell-Hahn A. 2018. The viral hypothesis of mesial temporal lobe epilepsy—is human herpes virus-6 the missing link? A systematic review and meta-analysis. Seizure 54:33–40. doi: 10.1016/j.seizure.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 134.Bar-Or A, Pender MP, Khanna R, Steinman L, Hartung HP, Maniar T, Croze E, Aftab BT, Giovannoni G, Joshi MA. 2020. Epstein-Barr virus in multiple sclerosis: theory and emerging immunotherapies. Trends Mol Med 26:296–310. doi: 10.1016/j.molmed.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tselis A. 2011. Evidence for viral etiology of multiple sclerosis. Semin Neurol 31:307–316. doi: 10.1055/s-0031-1287656. [DOI] [PubMed] [Google Scholar]
- 136.Guan Y, Jakimovski D, Ramanathan M, Weinstock-Guttman B, Zivadinov R. 2019. The role of Epstein-Barr virus in multiple sclerosis: from molecular pathophysiology to in vivo imaging. Neural Regen Res 14:373–386. doi: 10.4103/1673-5374.245462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tao C, Simpson S Jr, Taylor BV, van der Mei I. 2017. Association between human herpesvirus & human endogenous retrovirus and MS onset & progression. J Neurol Sci 372:239–249. doi: 10.1016/j.jns.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 138.Kury P, Nath A, Creange A, Dolei A, Marche P, Gold J, Giovannoni G, Hartung HP, Perron H. 2018. Human endogenous retroviruses in neurological diseases. Trends Mol Med 24:379–394. doi: 10.1016/j.molmed.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, Schad PA, Steward PM, Nowinski RC, Brown JP, Burmer GC. 1995. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A 92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gordon L, McQuaid S, Cosby SL. 1996. Detection of herpes simplex virus (types 1 and 2) and human herpesvirus 6 DNA in human brain tissue by polymerase chain reaction. Clin Diagn Virol 6:33–40. doi: 10.1016/0928-0197(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 141.Sanders VJ, Felisan S, Waddell A, Tourtellotte WW. 1996. Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. J Neurovirol 2:249–258. doi: 10.3109/13550289609146888. [DOI] [PubMed] [Google Scholar]
- 142.Merelli E, Bedin R, Sola P, Barozzi P, Mancardi GL, Ficarra G, Franchini G. 1997. Human herpes virus 6 and human herpes virus 8 DNA sequences in brains of multiple sclerosis patients, normal adults and children. J Neurol 244:450–454. doi: 10.1007/s004150050121. [DOI] [PubMed] [Google Scholar]
- 143.Friedman JE, Lyons MJ, Cu G, Ablashl DV, Whitman JE, Edgar M, Koskiniemi M, Vaheri A, Zabriskie JB. 1999. The association of the human herpesvirus-6 and MS. Mult Scler 5:355–362. doi: 10.1191/135245899678846311. [DOI] [PubMed] [Google Scholar]
- 144.Cermelli C, Berti R, Soldan SS, Mayne M, D’ambrosia JM, Ludwin SK, Jacobson S. 2003. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis 187:1377–1387. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- 145.Goodman AD, Mock DJ, Powers JM, Baker JV, Blumberg BM. 2003. Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis 187:1365–1376. doi: 10.1086/368172. [DOI] [PubMed] [Google Scholar]
- 146.Blumberg BM, Mock DJ, Powers JM, Ito M, Assouline JG, Baker JV, Chen B, Goodman AD. 2000. The HHV6 paradox: ubiquitous commensal or insidious pathogen? A two-step in situ PCR approach. J Clin Virol 16:159–178. doi: 10.1016/s1386-6532(99)00084-0. [DOI] [PubMed] [Google Scholar]
- 147.Hon GM, Erasmus RT, Matsha T. 2014. Low prevalence of human herpesvirus-6 and varicella zoster virus in blood of multiple sclerosis patients, irrespective of inflammatory status or disease progression. J Clin Neurosci 21:1437–1440. doi: 10.1016/j.jocn.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 148.Gustafsson R, Reitsma R, Stralfors A, Lindholm A, Press R, Fogdell-Hahn A. 2014. Incidence of human herpesvirus 6 in clinical samples from Swedish patients with demyelinating diseases. J Microbiol Immunol Infect 47:418–421. doi: 10.1016/j.jmii.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 149.Knox KK, Brewer JH, Henry JM, Harrington DJ, Carrigan DR. 2000. Human herpesvirus 6 and multiple sclerosis: systemic active infections in patients with early disease. Clin Infect Dis 31:894–903. doi: 10.1086/318141. [DOI] [PubMed] [Google Scholar]
- 150.Carrigan DR, Harrington DP, Knox KK. 1996. Subacute leukoencephalitis caused by CNS infection with human herpesvirus-6 manifesting as acute multiple sclerosis. Neurology 47:145–148. doi: 10.1212/wnl.47.1.145. [DOI] [PubMed] [Google Scholar]
- 151.Liedtke W, Malessa R, Faustmann PM, Eis-Hübinger AM. 1995. Human herpesvirus 6 polymerase chain reaction findings in human immunodeficiency virus associated neurological disease and multiple sclerosis. J Neurovirol 1:253–258. doi: 10.3109/13550289509114021. [DOI] [PubMed] [Google Scholar]
- 152.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Killian JM, Zhang JZ. 2002. Detection of viral DNA and immune responses to the human herpesvirus 6 101-kilodalton virion protein in patients with multiple sclerosis and in controls. J Virol 76:6147–6154. doi: 10.1128/jvi.76.12.6147-6154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alvarez-Lafuente R, García-Montojo M, De Las Heras V, Domínguez-Mozo MI, Bartolome M, Benito-Martin MS, Arroyo R. 2008. Herpesviruses and human endogenous retroviral sequences in the cerebrospinal fluid of multiple sclerosis patients. Mult Scler 14:595–601. doi: 10.1177/1352458507086425. [DOI] [PubMed] [Google Scholar]
- 154.Alenda R, Alvarez-Lafuente R, Costa-Frossard L, Arroyo R, Mirete S, Alvarez-Cermeno JC, Villar LM. 2014. Identification of the major HHV-6 antigen recognized by cerebrospinal fluid IgG in multiple sclerosis. Eur J Neurol 21:1096–1101. doi: 10.1111/ene.12435. [DOI] [PubMed] [Google Scholar]
- 155.Beck R, Wiendl H, Jahn G, Melms A, Neipel F. 2003. Human herpesvirus 6 in serum and spinal fluid of patients with multiple sclerosis? Arch Neurol 60:639. doi: 10.1001/archneur.60.4.639-a. [DOI] [PubMed] [Google Scholar]
- 156.Kuusisto H, Hyöty H, Kares S, Kinnunen E, Elovaara I. 2008. Human herpes virus 6 and multiple sclerosis: a Finnish twin study. Mult Scler 14:54–58. doi: 10.1177/1352458507080063. [DOI] [PubMed] [Google Scholar]
- 157.Franciotta D, Bestetti A, Sala S, Perucca P, Jarius S, Price RW, Di Stefano AL, Cinque P. 2009. Broad screening for human herpesviridae DNA in multiple sclerosis cerebrospinal fluid and serum. Acta Neurol Belg 109:277–282. [PubMed] [Google Scholar]
- 158.Ahram M, El-Omar A, Baho Y, Lubad MA. 2009. Association between human herpesvirus 6 and occurrence of multiple sclerosis among Jordanian patients. Acta Neurol Scand 120:430–435. doi: 10.1111/j.1600-0404.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 159.Lusso P, de Filippis L, Foglieni C, Vescovi AL, Locatelli G, Comi G, Martino G, Malnati MS. 2006. Human herpesvirus 6 and multiple sclerosis: detection by quantitative real-time PCR and role of complement-mediated damage secondary to CD46 inactivation. J Clin Virol 37:S117. doi: 10.1016/S1386-6532(06)70092-0. [DOI] [Google Scholar]
- 160.Mancuso R, Hernis A, Cavarretta R, Caputo D, Calabrese E, Nemni R, Ferrante P, Delbue S, Clerici M. 2010. Detection of viral DNA sequences in the cerebrospinal fluid of patients with multiple sclerosis. J Med Virol 82:1051–1057. doi: 10.1002/jmv.21764. [DOI] [PubMed] [Google Scholar]
- 161.van Nierop GP, Hintzen RQ, Verjans GM. 2014. Prevalence of human Herpesviridae in cerebrospinal fluid of patients with multiple sclerosis and noninfectious neurological disease in the Netherlands. J Neurovirol 20:412–418. doi: 10.1007/s13365-014-0248-4. [DOI] [PubMed] [Google Scholar]
- 162.Virtanen JO, Wohler JE, Fenton K, Reich DS, Jacobson S. 2014. Oligoclonal bands in multiple sclerosis reactive against two herpesviruses and association with magnetic resonance imaging findings. Mult Scler 20:27–34. doi: 10.1177/1352458513490545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Akhyani N, Berti R, Brennan MB, Soldan SS, Eaton JM, McFarland HF, Jacobson S. 2000. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis 182:1321–1325. doi: 10.1086/315893. [DOI] [PubMed] [Google Scholar]
- 164.Alvarez-Lafuente R, Martin-Estefania C, Las Heras V, Castrillo C, Picazo JJ, Varela de Seijas E, Gonzalez RA. 2002. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch Neurol 59:929–933. doi: 10.1001/archneur.59.6.929. [DOI] [PubMed] [Google Scholar]
- 165.Alvarez-Lafuente R, De las Heras V, Bartolome M, Garcia-Montojo M, Arroyo R. 2006. Human herpesvirus 6 and multiple sclerosis: a one-year follow-up study. Brain Pathol 16:20–27. doi: 10.1111/j.1750-3639.2006.tb00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alvarez-Lafuente R, Garcia-Montojo M, De Las Heras V, Dominguez-Mozo MI, Bartolome M, Arroyo R. 2009. CD46 expression and HHV-6 infection in patients with multiple sclerosis. Acta Neurol Scand 120:246–250. doi: 10.1111/j.1600-0404.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 167.Behzad-Behbahani A, Mikaeili MH, Entezam M, Mojiri A, Pour GY, Arasteh MM, Rahsaz M, Banihashemi M, Khadang B, Moaddeb A, Nematollahi Z, Azarpira N. 2011. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. J Microbiol Immunol Infect 44:247–251. doi: 10.1016/j.jmii.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 168.Ben-Fredj N, Ben-Selma W, Rotola A, Nefzi F, Benedetti S, Frih-Ayed M, Di Luca D, Aouni M, Caselli E. 2013. Prevalence of human herpesvirus U94/REP antibodies and DNA in Tunisian multiple sclerosis patients. J Neurovirol 19:42–47. doi: 10.1007/s13365-012-0138-6. [DOI] [PubMed] [Google Scholar]
- 169.Ramroodi N, Sanadgol N, Ganjali Z, Niazi AA, Sarabandi V, Moghtaderi A. 2013. Monitoring of active human herpes virus 6 infection in Iranian patients with different subtypes of multiple sclerosis. J Pathog 2013:1–10. doi: 10.1155/2013/194932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nejati A, Shoja Z, Farahmand M, Shahmahmoodi S, Akrami K, Hamid KM, Tafakhori A, Doosti R, Sahraian MA, Marashi SM. 2017. Human herpes virus 6 status in relapsing-remitting multiple sclerosis patients. Intern Med J 47:339–341. doi: 10.1111/imj.13363. [DOI] [PubMed] [Google Scholar]
- 171.Martinez A, Alvarez-Lafuente R, Mas A, Bartolome M, Garcia-Montojo M, de Las Heras V, de la Concha EG, Arroyo R, Urcelay E. 2007. Environment-gene interaction in multiple sclerosis: human herpesvirus 6 and MHC2TA. Hum Immunol 68:685–689. doi: 10.1016/j.humimm.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 172.Alvarez-Lafuente R, Martin-Estefanía C, De las Heras V, Castrillo C, Cour I, Picazo JJ, Varela de Seijas E, Arroyo R. 2002. Prevalence of herpesvirus DNA in MS patients and healthy blood donors. Acta Neurol Scand 105:95–99. doi: 10.1034/j.1600-0404.2002.1o050.x. [DOI] [PubMed] [Google Scholar]
- 173.Alvarez-Lafuente R, Garcia-Montojo M, De las Heras V, Bartolome M, Arroyo R. 2006. Clinical parameters and HHV-6 active replication in relapsing-remitting multiple sclerosis patients. J Clin Virol 37:S24–S26. doi: 10.1016/S1386-6532(06)70007-5. [DOI] [PubMed] [Google Scholar]
- 174.Berti R, Brennan MB, Soldan SS, Ohayon JM, Casareto L, McFarland HF, Jacobson S. 2002. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J Neurovirol 8:250–256. doi: 10.1080/13550280290049615-1. [DOI] [PubMed] [Google Scholar]
- 175.Garcia-Montojo M, De Las Heras V, Dominguez-Mozo M, Bartolome M, Garcia-Martinez MA, Arroyo R, Alvarez-Lafuente R, HHV-6 and Multiple Sclerosis Study Group. 2011. Human herpesvirus 6 and effectiveness of interferon beta1b in multiple sclerosis patients. Eur J Neurol 18:1027–1035. doi: 10.1111/j.1468-1331.2011.03410.x. [DOI] [PubMed] [Google Scholar]
- 176.Alvarez-Lafuente R, de las Heras V, García-Montojo M, Bartolomé M, Arroyo R. 2007. Human herpesvirus-6 and multiple sclerosis: relapsing-remitting versus secondary progressive. Mult Scler 13:578–583. doi: 10.1177/1352458506072667. [DOI] [PubMed] [Google Scholar]
- 177.Dominguez-Mozo MI, Garcia-Montojo M, De Las Heras V, Garcia-Martinez A, Arias-Leal AM, Casanova I, Arroyo R, Alvarez-Lafuente R. 2012. MHC2TA mRNA levels and human herpesvirus 6 in multiple sclerosis patients treated with interferon beta along two-year follow-up. BMC Neurol 12:107. doi: 10.1186/1471-2377-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alvarez-Lafuente R, De las Heras V, Bartolome M, Picazo JJ, Arroyo R. 2004. Relapsing-remitting multiple sclerosis and human herpesvirus 6 active infection. Arch Neurol 61:1523–1527. doi: 10.1001/archneur.61.10.1523. [DOI] [PubMed] [Google Scholar]
- 179.Pormohammad A, Azimi T, Falah F, Faghihloo E. 2018. Relationship of human herpes virus 6 and multiple sclerosis: a systematic review and meta-analysis. J Cell Physiol 233:2850–2862. doi: 10.1002/jcp.26000. [DOI] [PubMed] [Google Scholar]
- 180.Wilborn F, Schmidt CA, Brinkmann V, Jendroska K, Oettle H, Siegert W. 1994. A potential role for human herpesvirus type 6 in nervous system disease. J Neuroimmunol 49:213–214. doi: 10.1016/0165-5728(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 181.Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, Calabresi PA, Brennan MB, Maloni HW, McFarland HF, Lin HC, Patnaik M, Jacobson S. 1997. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med 3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 182.Ablashi DV, Eastman HB, Owen CB, Roman MM, Friedman J, Zabriskie JB, Peterson DL, Pearson GR, Whitman JE. 2000. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J Clin Virol 16:179–191. doi: 10.1016/s1386-6532(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 183.Villoslada P, Juste C, Tintore M, Llorenc V, Codina G, Pozo-Rosich P, Montalban X. 2003. The immune response against herpesvirus is more prominent in the early stages of MS. Neurology 60:1944–1948. doi: 10.1212/01.wnl.0000069461.53733.f7. [DOI] [PubMed] [Google Scholar]
- 184.Riverol M, Sepulcre J, Fernandez-Diez B, Villoslada P, Fernandez-Alonso M, Rubio M, Rodriguez A, Uccelli A, Brieva L. 2007. Antibodies against Epstein-Barr virus and herpesvirus type 6 are associated with the early phases of multiple sclerosis. J Neuroimmunol 192:184–185. doi: 10.1016/j.jneuroim.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 185.Sola P, Merelli E, Marasca R, Poggi M, Luppi M, Montorsi M, Torelli G. 1993. Human herpesvirus 6 and multiple sclerosis: survey of anti-HHV-6 antibodies by immunofluorescence analysis and of viral sequences by polymerase chain reaction. J Neurol Neurosurg Psychiatry 56:917–919. doi: 10.1136/jnnp.56.8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Khaki M, Ghazavi A, Ghasami K, Rafiei M, Payani MA, Ghaznavi-Rad E, Mosayebi G. 2011. Evaluation of viral antibodies in Iranian multiple sclerosis patients. Neurosciences (Riyadh) 16:224–228. [PubMed] [Google Scholar]
- 187.Enbom M, Wang FZ, Fredrikson S, Martin C, Dahl H, Linde A. 1999. Similar humoral and cellular immunological reactivities to human herpesvirus 6 in patients with multiple sclerosis and controls. Clin Diagn Lab Immunol 6:545–549. doi: 10.1128/CDLI.6.4.545-549.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Comabella M, Montalban X, Horga A, Messmer B, Kakalacheva K, Strowig T, Caballero E, Münz C, Lünemann J. 2010. Antiviral immune response in patients with multiple sclerosis and healthy siblings. Mult Scler 16:355–358. doi: 10.1177/1352458509357066. [DOI] [PubMed] [Google Scholar]
- 189.Ortega-Madueno I, Garcia-Montojo M, Dominguez-Mozo MI, Garcia-Martinez A, Arias-Leal AM, Casanova I, Arroyo R, Alvarez-Lafuente R. 2014. Anti-human herpesvirus 6A/B IgG correlates with relapses and progression in multiple sclerosis. PLoS One 9:e104836. doi: 10.1371/journal.pone.0104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Campbell A, Hogestyn JM, Folts CJ, Lopez B, Proschel C, Mock D, Mayer-Proschel M. 2017. Expression of the human herpesvirus 6A latency-associated transcript U94A disrupts human oligodendrocyte progenitor migration. Sci Rep 7:3978. doi: 10.1038/s41598-017-04432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Engdahl E, Gustafsson R, Huang J, Bistrom M, Lima Bomfim I, Stridh P, Khademi M, Brenner N, Butt J, Michel A, Jons D, Hortlund M, Alonso-Magdalena L, Hedstrom AK, Flamand L, Ihira M, Yoshikawa T, Andersen O, Hillert J, Alfredsson L, Waterboer T, Sundstrom P, Olsson T, Kockum I, Fogdell-Hahn A. 2019. Increased serological response against human herpesvirus 6A is associated with risk for multiple sclerosis. Front Immunol 10:2715. doi: 10.3389/fimmu.2019.02715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chapenko S, Millers A, Nora Z, Logina I, Kukaine R, Murovska M. 2003. Correlation between HHV-6 reactivation and multiple sclerosis disease activity. J Med Virol 69:111–117. doi: 10.1002/jmv.10258. [DOI] [PubMed] [Google Scholar]
- 193.Simpson S Jr, Taylor B, Dwyer DE, Taylor J, Blizzard L, Ponsonby AL, Pittas F, Dwyer T, van der Mei I. 2012. Anti-HHV-6 IgG titer significantly predicts subsequent relapse risk in multiple sclerosis. Mult Scler 18:799–806. doi: 10.1177/1352458511428081. [DOI] [PubMed] [Google Scholar]
- 194.Simpson S Jr, Taylor B, Burrows J, Burrows S, Dwyer DE, Taylor J, Ponsonby AL, Blizzard L, Dwyer T, Pittas F, van der Mei I. 2014. EBV & HHV6 reactivation is infrequent and not associated with MS clinical course. Acta Neurol Scand 130:328–337. doi: 10.1111/ane.12268. [DOI] [PubMed] [Google Scholar]
- 195.Caselli E, Boni M, Bracci A, Rotola A, Cermelli C, Castellazzi M, Di Luca D, Cassai E. 2002. Detection of antibodies directed against human herpesvirus 6 U94/REP in sera of patients affected by multiple sclerosis. J Clin Microbiol 40:4131–4137. doi: 10.1128/jcm.40.11.4131-4137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wuest SC, Mexhitaj I, Chai NR, Romm E, Scheffel J, Xu B, Lane K, Wu T, Bielekova B. 2014. A complex role of herpes viruses in the disease process of multiple sclerosis. PLoS One 9:e105434. doi: 10.1371/journal.pone.0105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brudek T, Luhdorf P, Christensen T, Hansen HJ, Moller-Larsen A. 2007. Activation of endogenous retrovirus reverse transcriptase in multiple sclerosis patient lymphocytes by inactivated HSV-1, HHV-6 and VZV. J Neuroimmunol 187:147–155. doi: 10.1016/j.jneuroim.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 198.Soldan SS, Leist TP, Juhng KN, McFarland HF, Jacobson S. 2000. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol 47:306–313. doi:. [DOI] [PubMed] [Google Scholar]
- 199.Cirone M, Cuomo L, Zompetta C, Ruggieri S, Frati L, Faggioni A, Ragona G. 2002. Human herpesvirus 6 and multiple sclerosis: a study of T cell cross-reactivity to viral and myelin basic protein antigens. J Med Virol 68:268–272. doi: 10.1002/jmv.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Derfuss T, Hohlfeld R, Meinl E. 2005. Intrathecal antibody (IgG) production against human herpesvirus type 6 occurs in about 20% of multiple sclerosis patients and might be linked to a polyspecific B-cell response. J Neurol 252:968–971. doi: 10.1007/s00415-005-0794-z. [DOI] [PubMed] [Google Scholar]
- 201.Virtanen JO, Pietiläinen-Nicklén J, Uotila L, Färkkilä M, Vaheri A, Koskiniemi M. 2011. Intrathecal human herpesvirus 6 antibodies in multiple sclerosis and other demyelinating diseases presenting as oligoclonal bands in cerebrospinal fluid. J Neuroimmunol 237:93–97. doi: 10.1016/j.jneuroim.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 202.Pietilainen-Nicklen J, Virtanen JO, Uotila L, Salonen O, Farkkila M, Koskiniemi M. 2014. HHV-6-positivity in diseases with demyelination. J Clin Virol 61:216–219. doi: 10.1016/j.jcv.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 203.Rizzo R, Gentili V, Casetta I, Caselli E, De Gennaro R, Granieri E, Cassai E, Di Luca D, Rotola A. 2012. Altered natural killer cells' response to herpes virus infection in multiple sclerosis involves KIR2DL2 expression. J Neuroimmunol 251:55–64. doi: 10.1016/j.jneuroim.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 204.Rizzo R, Bortolotti D, Gentili V, Rotola A, Bolzani S, Caselli E, Tola MR, Di Luca D. 2019. KIR2DS2/KIR2DL2/HLA-C1 haplotype is associated with Alzheimer's disease: implication for the role of herpesvirus infections. J Alzheimers Dis 67:1379–1389. doi: 10.3233/JAD-180777. [DOI] [PubMed] [Google Scholar]
- 205.Keyvani H, Zahednasab H, Aljanabi HAA, Asadi M, Mirzaei R, Esghaei M, Karampoor S. 2020. The role of human herpesvirus-6 and inflammatory markers in the pathogenesis of multiple sclerosis. J Neuroimmunol 346:577313. doi: 10.1016/j.jneuroim.2020.577313. [DOI] [PubMed] [Google Scholar]
- 206.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ. 2003. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann Neurol 53:189–197. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 207.Cheng W, Ma Y, Gong F, Hu C, Qian L, Huang Q, Yu Q, Zhang J, Chen S, Liu Z, Chen X, Zhou T, Zhang D. 2012. Cross-reactivity of autoreactive T cells with MBP and viral antigens in patients with MS. Front Biosci (Landmark Ed) 17:1648–1658. doi: 10.2741/4010. [DOI] [PubMed] [Google Scholar]
- 208.Soldan SS, Fogdell-Hahn A, Brennan MB, Mittleman BB, Ballerini C, Massacesi L, Seya T, McFarland HF, Jacobson S. 2001. Elevated serum and cerebrospinal fluid levels of soluble human herpesvirus type 6 cellular receptor, membrane cofactor protein, in patients with multiple sclerosis. Ann Neurol 50:486–493. doi: 10.1002/ana.1135. [DOI] [PubMed] [Google Scholar]
- 209.Fogdell-Hahn A, Soldan SS, Shue S, Akhyani N, Refai H, Ahlqvist J, Jacobson S. 2005. Co-purification of soluble membrane cofactor protein (CD46) and human herpesvirus 6 variant A genome in serum from multiple sclerosis patients. Virus Res 110:57–63. doi: 10.1016/j.virusres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 210.Kong H, Baerbig Q, Duncan L, Shepel N, Mayne M. 2003. Human herpesvirus type 6 indirectly enhances oligodendrocyte cell death. J Neurovirol 9:539–550. doi: 10.1080/13550280390241241. [DOI] [PubMed] [Google Scholar]
- 211.Pan R, Zhang Q, Anthony SM, Zhou Y, Zou X, Cassell M, Perlman S. 2020. Oligodendrocytes that survive acute coronavirus infection induce prolonged inflammatory responses in the CNS. Proc Natl Acad Sci U S A 117:15902–15910. doi: 10.1073/pnas.2003432117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gardell JL, Dazin P, Islar J, Menge T, Genain CP, Lalive PH. 2006. Apoptotic effects of human herpesvirus-6A on glia and neurons as potential triggers for central nervous system autoimmunity. J Clin Virol 37:S11–S16. doi: 10.1016/S1386-6532(06)70005-1. [DOI] [PubMed] [Google Scholar]
- 213.Leibovitch EC, Caruso B, Ha SK, Schindler MK, Lee NJ, Luciano NJ, Billioux BJ, Guy JR, Yen C, Sati P, Silva AC, Reich DS, Jacobson S. 2018. Herpesvirus trigger accelerates neuroinflammation in a nonhuman primate model of multiple sclerosis. Proc Natl Acad Sci U S A 115:11292–11297. doi: 10.1073/pnas.1811974115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ascherio A, Munger KL. 2016. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol 36:103–114. doi: 10.1055/s-0036-1579693. [DOI] [PubMed] [Google Scholar]
- 215.Flamand L, Stefanescu I, Ablashi DV, Menezes J. 1993. Activation of the Epstein-Barr virus replicative cycle by human herpesvirus 6. J Virol 67:6768–6777. doi: 10.1128/JVI.67.11.6768-6777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Charvet B, Reynaud JM, Gourru-Lesimple G, Perron H, Marche PN, Horvat B. 2018. Induction of proinflammatory multiple sclerosis-associated retrovirus envelope protein by human herpesvirus-6A and CD46 receptor engagement. Front Immunol 9:2803. doi: 10.3389/fimmu.2018.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Munger KL, Hongell K, Cortese M, Aivo J, Soilu-Hanninen M, Surcel HM, Ascherio A. 2019. Epstein-Barr virus and multiple sclerosis risk in the Finnish maternity cohort. Ann Neurol 86:436–442. doi: 10.1002/ana.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, Cross AH, de Seze J, Leppert D, Montalban X, Selmaj K, Wiendl H, Kerloeguen C, Willi R, Li B, Kakarieka A, Tomic D, Goodyear A, Ingili R, Haring DA, Ramanathan K, Merschhemke M, Kappos L, ASCLEPIOS I and ASCLEPIOS II Trial Groups. 2020. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 383:546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 219.Kuerten S, Lanz TV, Lingampalli N, Lahey LJ, Kleinschnitz C, Maurer M, Schroeter M, Braune S, Ziemssen T, Ho PP, Robinson WH, Steinman L. 2020. Autoantibodies against central nervous system antigens in a subset of B cell-dominant multiple sclerosis patients. Proc Natl Acad Sci U S A 117:21512–21518. doi: 10.1073/pnas.2011249117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ramesh A, Schubert RD, Greenfield AL, Dandekar R, Loudermilk R, Sabatino JJ, Koelzer MT, Tran EB, Koshal K, Kim K, Pröbstel A-K, Banerji D, Guo C-Y, Green AJ, Bove RM, DeRisi JL, Gelfand JM, Cree BAC, Zamvil SS, Baranzini SE, Hauser SL, Wilson MR. 2020. A pathogenic and clonally expanded B cell transcriptome in active multiple sclerosis. Proc Natl Acad Sci U S A 117:22932–22943. doi: 10.1073/pnas.2008523117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hauser SL. 2020. Progress in multiple sclerosis research: an example of bedside to bench. JAMA 324:841–842. doi: 10.1001/jama.2020.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Selkoe DJ, Hardy J. 2016. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Welikovitch LA, Do Carmo S, Maglóczky Z, Malcolm JC, Lőke J, Klein WL, Freund T, Cuello AC. 2020. Early intraneuronal amyloid triggers neuron-derived inflammatory signaling in APP transgenic rats and human brain. Proc Natl Acad Sci U S A 117:6844–6854. doi: 10.1073/pnas.1914593117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Madore C, Yin Z, Leibowitz J, Butovsky O. 2020. Microglia, lifestyle stress, and neurodegeneration. Immunity 52:222–240. doi: 10.1016/j.immuni.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. 2000. Inflammation and Alzheimer's disease. Neurobiol Aging 21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Karch CM, Goate AM. 2015. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hammond TR, Marsh SE, Stevens B. 2019. Immune signaling in neurodegeneration. Immunity 50:955–974. doi: 10.1016/j.immuni.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Salter MW, Stevens B. 2017. Microglia emerge as central players in brain disease. Nat Med 23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 229.Albright AV, Lavi E, Black JB, Goldberg S, O'Connor MJ, González-Scarano F. 1998. The effect of human herpesvirus-6 (HHV-6) on cultured human neural cells: oligodendrocytes and microglia. J Neurovirol 4:486–494. doi: 10.3109/13550289809113493. [DOI] [PubMed] [Google Scholar]
- 230.Reynaud JM, Horvat B. 2013. Human herpesvirus 6 and neuroinflammation. ISRN Virology 2013:1–11. doi: 10.5402/2013/834890. [DOI] [Google Scholar]
- 231.Donati D, Martinelli E, Cassiani-Ingoni R, Ahlqvist J, Hou J, Major EO, Jacobson S. 2005. Variant-specific tropism of human herpesvirus 6 in human astrocytes. J Virol 79:9439–9448. doi: 10.1128/JVI.79.15.9439-9448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.De Filippis L, Foglieni C, Silva S, Vescovi A, Lusso P, Malnati MS. 2006. Differentiated human neural stem cells: a new ex vivo model to study HHV-6 infection of the central nervous system. J Clin Virol 37:S27–S32. doi: 10.1016/S1386-6532(06)70008-7. [DOI] [PubMed] [Google Scholar]
- 233.Liddelow SA, Marsh SE, Stevens B. 2020. Microglia and astrocytes in disease: dynamic duo or partners in crime? Trends Immunol 41:820–835. doi: 10.1016/j.it.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 234.Urban SL, Jensen IJ, Shan Q, Pewe LL, Xue HH, Badovinac VP, Harty JT. 2020. Peripherally induced brain tissue-resident memory CD8(+) T cells mediate protection against CNS infection. Nat Immunol 21:938–949. doi: 10.1038/s41590-020-0711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Poon DC, Ho YS, Chiu K, Wong HL, Chang RC. 2015. Sickness: from the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci Biobehav Rev 57:30–45. doi: 10.1016/j.neubiorev.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 236.Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, Xie Z, Chu X, Yang J, Wang H, Chang S, Gong Y, Ruan L, Zhang G, Yan S, Lian W, Du C, Yang D, Zhang Q, Lin F, Liu J, Zhang H, Ge C, Xiao S, Ding J, Geng M. 2019. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res 29:787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Itzhaki RF. 2018. Corroboration of a major role for herpes simplex virus type 1 in Alzheimer's disease. Front Aging Neurosci 10:324. doi: 10.3389/fnagi.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kennedy PGE, Grinfeld E, Gow JW. 1998. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci U S A 95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Levin MJ, Cai G-Y, Manchak MD, Pizer LI. 2003. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol 77:6979–6987. doi: 10.1128/jvi.77.12.6979-6987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Frost GR, Li YM. 2017. The role of astrocytes in amyloid production and Alzheimer's disease. Open Biol 7:170228. doi: 10.1098/rsob.170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Licastro F, Carbone I, Ianni M, Porcellini E. 2011. Gene signature in Alzheimer's disease and environmental factors: the virus chronicle. J Alzheimers Dis 27:809–817. doi: 10.3233/JAD-2011-110755. [DOI] [PubMed] [Google Scholar]
- 242.Carter CJ. 2013. Susceptibility genes are enriched in those of the herpes simplex virus 1/host interactome in psychiatric and neurological disorders. Pathog Dis 69:240–261. doi: 10.1111/2049-632X.12077. [DOI] [PubMed] [Google Scholar]
- 243.Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, Sano M, Liang WS, Beckmann ND, Price ND, Reiman EM, Schadt EE, Ehrlich ME, Gandy S, Dudley JT. 2018. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 99:64–82. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Costa Sa AC, Madsen H, Brown JR. 2019. Shared molecular signatures across neurodegenerative diseases and herpes virus infections highlights potential mechanisms for maladaptive innate immune responses. Sci Rep 9:8795. doi: 10.1038/s41598-019-45129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chiu WC, Tsan YT, Tsai SL, Chang CJ, Wang JD, Chen PC, HDAiTR G, Health Data Analysis in Taiwan (hDATa) Research Group. 2014. Hepatitis C viral infection and the risk of dementia. Eur J Neurol 21:1068-e59. doi: 10.1111/ene.12317. [DOI] [PubMed] [Google Scholar]
- 246.Zendehdel A, Roham M. 2020. Role of Helicobacter pylori infection in the manifestation of old age-related diseases. Mol Genet Genomic Med 8:e1157. doi: 10.1002/mgg3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J. 2019. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5:eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Singhrao SK, Olsen I. 2019. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer's disease. J Oral Microbiol 11:1563405. doi: 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Haditsch U, Roth T, Rodriguez L, Hancock S, Cecere T, Nguyen M, Arastu-Kapur S, Broce S, Raha D, Lynch CC, Holsinger LJ, Dominy SS, Ermini F. 2020. Alzheimer's disease-like neurodegeneration in Porphyromonas gingivalis infected neurons with persistent expression of active gingipains. J Alzheimers Dis 75:1361–1376. doi: 10.3233/JAD-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Balin BJ, Hammond CJ, Little CS, Hingley ST, Al-Atrache Z, Appelt DM, Whittum-Hudson JA, Hudson AP. 2018. Chlamydia pneumoniae: an etiologic agent for late-onset dementia. Front Aging Neurosci 10:302. doi: 10.3389/fnagi.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Woods JJ, Skelding KA, Martin KL, Aryal R, Sontag E, Johnstone DM, Horvat JC, Hansbro PM, Milward EA. 2020. Assessment of evidence for or against contributions of Chlamydia pneumoniae infections to Alzheimer's disease etiology. Brain Behav Immun 83:22–32. doi: 10.1016/j.bbi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 252.Alonso R, Pisa D, Aguado B, Carrasco L. 2017. Identification of fungal species in brain tissue from Alzheimer's disease by next-generation sequencing. J Alzheimers Dis 58:55–67. doi: 10.3233/JAD-170058. [DOI] [PubMed] [Google Scholar]
- 253.Miklossy J. 2011. Alzheimer's disease—a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. J Neuroinflammation 8:90. doi: 10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. 2010. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Spitzer P, Condic M, Herrmann M, Oberstein TJ, Scharin-Mehlmann M, Gilbert DF, Friedrich O, Gromer T, Kornhuber J, Lang R, Maler JM. 2016. Amyloidogenic amyloid-beta-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci Rep 6:32228. doi: 10.1038/srep32228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Miklossy J, McGeer PL. 2016. Common mechanisms involved in Alzheimer's disease and type 2 diabetes: a key role of chronic bacterial infection and inflammation. Aging (Albany NY) 8:575–588. doi: 10.18632/aging.100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Moir RD, Lathe R, Tanzi RE. 2018. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimers Dement 14:1602–1614. doi: 10.1016/j.jalz.2018.06.3040. [DOI] [PubMed] [Google Scholar]
- 258.Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, Gyorgy B, Breakefield XO, Tanzi RE, Moir RD. 2018. Alzheimer's disease-associated beta-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99:56–63. doi: 10.1016/j.neuron.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, Frost EH, Fülöp T Jr. 2015. β-Amyloid peptides display protective activity against the human Alzheimer's disease-associated herpes simplex virus-1. Biogerontology 16:85–98. doi: 10.1007/s10522-014-9538-8. [DOI] [PubMed] [Google Scholar]
- 260.White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J, Hartshorn KL. 2014. Alzheimer's associated beta-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One 9:e101364. doi: 10.1371/journal.pone.0101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. 2016. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med 8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ezzat K, Pernemalm M, Pålsson S, Roberts TC, Järver P, Dondalska A, Bestas B, Sobkowiak MJ, Levänen B, Sköld M, Thompson EA, Saher O, Kari OK, Lajunen T, Sverremark Ekström E, Nilsson C, Ishchenko Y, Malm T, Wood MJA, Power UF, Masich S, Lindén A, Sandberg JK, Lehtiö J, Spetz A-L, El Andaloussi S. 2019. The viral protein corona directs viral pathogenesis and amyloid aggregation. Nat Commun 10:2331. doi: 10.1038/s41467-019-10192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wozniak MA, Frost AL, Itzhaki RF. 2009. Alzheimer's disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis 16:341–350. doi: 10.3233/JAD-2009-0963. [DOI] [PubMed] [Google Scholar]
- 264.Itzhaki RF, Golde TE, Heneka MT, Readhead B. 2020. Do infections have a role in the pathogenesis of Alzheimer disease? Nat Rev Neurol 16:193–197. doi: 10.1038/s41582-020-0323-9. [DOI] [PubMed] [Google Scholar]
- 265.Jamieson GA, Maitland NJ, Craske J, Wilcock GK, Itzhaki RF. 1991. Detection of herpes simplex virus type 1 DNA sequences in normal and Alzheimer's disease brain using polymerase chain reaction. Biochem Soc Trans 19:122S. doi: 10.1042/bst019122s. [DOI] [PubMed] [Google Scholar]
- 266.Itzhaki RF, Lin W-R, Shang D, Wilcock GK, Faragher B, Jamieson GA. 1997. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349:241–244. doi: 10.1016/S0140-6736(96)10149-5. [DOI] [PubMed] [Google Scholar]
- 267.Wozniak MA, Mee AP, Itzhaki RF. 2009. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol 217:131–138. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 268.Piacentini R, Civitelli L, Ripoli C, Marcocci ME, De Chiara G, Garaci E, Azzena GB, Palamara AT, Grassi C. 2011. HSV-1 promotes Ca2+-mediated APP phosphorylation and Abeta accumulation in rat cortical neurons. Neurobiol Aging 32:2323.e13–2323.e26. doi: 10.1016/j.neurobiolaging.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 269.Wozniak MA, Frost AL, Preston CM, Itzhaki RF. 2011. Antivirals reduce the formation of key Alzheimer's disease molecules in cell cultures acutely infected with herpes simplex virus type 1. PLoS One 6:e25152. doi: 10.1371/journal.pone.0025152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zare-Shahabadi A, Masliah E, Johnson GVW, Rezaei N. 2015. Autophagy in Alzheimer’s disease. Rev Neurosci 26:385–395. doi: 10.1515/revneuro-2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Remondelli P, Renna M. 2017. The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front Mol Neurosci 10:187. doi: 10.3389/fnmol.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhou F, van Laar T, Huang H, Zhang L. 2011. APP and APLP1 are degraded through autophagy in response to proteasome inhibition in neuronal cells. Protein Cell 2:377–383. doi: 10.1007/s13238-011-1047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Itzhaki RF, Wozniak MA. 2012. Could antivirals be used to treat Alzheimer's disease? Future Microbiol 7:307–309. doi: 10.2217/fmb.12.10. [DOI] [PubMed] [Google Scholar]
- 274.Wang Y, Mandelkow E. 2012. Degradation of tau protein by autophagy and proteasomal pathways. Biochem Soc Trans 40:644–652. doi: 10.1042/BST20120071. [DOI] [PubMed] [Google Scholar]
- 275.Granato M, Santarelli R, Farina A, Gonnella R, Lotti LV, Faggioni A, Cirone M. 2014. Epstein-Barr virus blocks the autophagic flux and appropriates the autophagic machinery to enhance viral replication. J Virol 88:12715–12726. doi: 10.1128/JVI.02199-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Granato M, Santarelli R, Filardi M, Gonnella R, Farina A, Torrisi MR, Faggioni A, Cirone M. 2015. The activation of KSHV lytic cycle blocks autophagy in PEL cells. Autophagy 11:1978–1986. doi: 10.1080/15548627.2015.1091911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Santarelli R, Granato M, Pentassuglia G, Lacconi V, Gilardini Montani MS, Gonnella R, Tafani M, Torrisi MR, Faggioni A, Cirone M. 2016. KSHV reduces autophagy in THP-1 cells and in differentiating monocytes by decreasing CAST/calpastatin and ATG5 expression. Autophagy 12:2311–2325. doi: 10.1080/15548627.2016.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. 2017. Mitophagy and Alzheimer's disease: cellular and molecular mechanisms. Trends Neurosci 40:151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cirone M. 2018. EBV and KSHV infection dysregulates autophagy to optimize viral replication, prevent immune recognition and promote tumorigenesis. Viruses 10:599. doi: 10.3390/v10110599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, Bergantin LB, Abdel-Daim MM, Stankiewicz AM. 2018. Autophagy and Alzheimer's disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci 10:04. doi: 10.3389/fnagi.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Romeo MA, Faggioni A, Cirone M. 2019. Could autophagy dysregulation link neurotropic viruses to Alzheimer’s disease? Neural Regen Res 14:1503–1506. doi: 10.4103/1673-5374.253508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Romeo MA, Masuelli L, Gaeta A, Nazzari C, Granato M, Gilardini Montani MS, Faggioni A, Cirone M. 2019. Impact of HHV-6A and HHV-6B lytic infection on autophagy and endoplasmic reticulum stress. J Gen Virol 100:89–98. doi: 10.1099/jgv.0.001176. [DOI] [PubMed] [Google Scholar]
- 283.Lovheim H, Norman T, Weidung B, Olsson J, Josefsson M, Adolfsson R, Nyberg L, Elgh F. 2019. Herpes simplex virus, APOE epsilon4, and cognitive decline in old age: results from the Betula Cohort Study. J Alzheimers Dis 67:211–220. doi: 10.3233/JAD-171162. [DOI] [PubMed] [Google Scholar]
- 284.Letenneur L, Peres K, Fleury H, Garrigue I, Barberger-Gateau P, Helmer C, Orgogozo JM, Gauthier S, Dartigues JF. 2008. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS One 3:e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Jayasuriya AN, Itzhaki RF, Wozniak MA, Patel R, Smit EJ, Noone R, Gilleran G, Taylor S, White DJ. 2008. Apolipoprotein E-epsilon 4 and recurrent genital herpes in individuals co-infected with herpes simplex virus type 2 and HIV. Sex Transm Infect 84:516–517. doi: 10.1136/sti.2008.032367. [DOI] [PubMed] [Google Scholar]
- 286.Huang C, Yan B, Lei D, Si Y, Li H, Chen MW, Li L, Chen F, Zhou Q, Zhou D, Li JM. 2015. Apolipoprotein 4 may increase viral load and seizure frequency in mesial temporal lobe epilepsy patients with positive human herpes virus 6B. Neurosci Lett 593:29–34. doi: 10.1016/j.neulet.2014.12.063. [DOI] [PubMed] [Google Scholar]
- 287.Kuo C-L, Pilling LC, Atkins JL, Masoli JAH, Delgado J, Kuchel GA, Melzer D. 2020. APOE e4 genotype predicts severe COVID-19 in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci 75:2231–2232. doi: 10.1093/gerona/glaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cairns DM, Rouleau N, Parker RN, Walsh KG, Gehrke L, Kaplan DL. 2020. A 3D human brain-like tissue model of herpes-induced Alzheimer's disease. Sci Adv 6:eaay8828. doi: 10.1126/sciadv.aay8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Itzhaki RF. 2014. Herpes simplex virus type 1 and Alzheimer's disease: increasing evidence for a major role of the virus. Front Aging Neurosci 6:3216–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tzeng NS, Chung CH, Lin FH, Chiang CP, Yeh CB, Huang SY, Lu RB, Chang HA, Kao YC, Yeh HW, Chiang WS, Chou YC, Tsao CH, Wu YF, Chien WC. 2018. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections—a nationwide, population-based cohort study in Taiwan. Neurotherapeutics 15:417–429. doi: 10.1007/s13311-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen VC, Wu SI, Huang KY, Yang YH, Kuo TY, Liang HY, Huang KL, Gossop M. 2018. Herpes zoster and dementia: a nationwide population-based cohort study. J Clin Psychiatry 79:16m11312. doi: 10.4088/JCP.16m11312. [DOI] [PubMed] [Google Scholar]
- 292.Tsai MC, Cheng WL, Sheu JJ, Huang CC, Shia BC, Kao LT, Lin HC. 2017. Increased risk of dementia following herpes zoster ophthalmicus. PLoS One 12:e0188490. doi: 10.1371/journal.pone.0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Agostini S, Mancuso R, Baglio F, Cabinio M, Hernis A, Guerini FR, Calabrese E, Nemni R, Clerici M. 2016. Lack of evidence for a role of HHV-6 in the pathogenesis of Alzheimer's disease. J Alzheimers Dis 49:229–235. doi: 10.3233/JAD-150464. [DOI] [PubMed] [Google Scholar]
- 294.Westman G, Blomberg J, Yun Z, Lannfelt L, Ingelsson M, Eriksson BM. 2017. Decreased HHV-6 IgG in Alzheimer's disease. Front Neurol 8:40. doi: 10.3389/fneur.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bortolotti D, Gentili V, Rotola A, Caselli E, Rizzo R. 2019. HHV-6A infection induces amyloid-beta expression and activation of microglial cells. Alzheimers Res Ther 11:104. doi: 10.1186/s13195-019-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Romeo MA, Gilardini Montani MS, Gaeta A, D'Orazi G, Faggioni A, Cirone M. 2020. HHV-6A infection dysregulates autophagy/UPR interplay increasing beta amyloid production and tau phosphorylation in astrocytoma cells as well as in primary neurons, possible molecular mechanisms linking viral infection to Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis 1866:165647. doi: 10.1016/j.bbadis.2019.165647. [DOI] [PubMed] [Google Scholar]
- 297.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. 2016. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy 12:225–244. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Romeo MA, Gilardini Montani MS, Falcinelli L, Gaeta A, Nazzari C, Faggioni A, Cirone M. 2019. HHV-6B reduces autophagy and induces ER stress in primary monocytes impairing their survival and differentiation into dendritic cells. Virus Res 273:197757. doi: 10.1016/j.virusres.2019.197757. [DOI] [PubMed] [Google Scholar]
- 299.Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. 2002. Herpesviruses in brain and Alzheimer's disease. J Pathol 197:395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- 300.Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, Masliah E, Gabrielli L, Licastro F. 2014. Herpes virus in Alzheimer's disease: relation to progression of the disease. Neurobiol Aging 35:122–129. doi: 10.1016/j.neurobiolaging.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 301.Allnutt MA, Johnson K, Bennett DA, Connor SM, Troncoso JC, Pletnikova O, Albert MS, Resnick SM, Scholz SW, De Jager PL, Jacobson S. 2020. Human herpesvirus 6 detection in Alzheimer's disease cases and controls across multiple cohorts. Neuron 105:1027–1035. doi: 10.1016/j.neuron.2019.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Leibovitch EC, Brunetto GS, Caruso B, Fenton K, Ohayon J, Reich DS, Jacobson S. 2014. Coinfection of human herpesviruses 6A (HHV-6A) and HHV-6B as demonstrated by novel digital droplet PCR assay. PLoS One 9:e92328. doi: 10.1371/journal.pone.0092328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jeong HH, Liu Z. 2019. Are HHV-6A and HHV-7 really more abundant in Alzheimer's disease? Neuron 104:1034–1035. doi: 10.1016/j.neuron.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 304.Chorlton SD. 2020. Reanalysis of Alzheimer's brain sequencing data reveals absence of purported HHV-6A and HHV-7. J Bioinform Comput Biol 18:2050012. doi: 10.1142/S0219720020500122. [DOI] [PubMed] [Google Scholar]
- 305.Readhead B, Haure-Mirande JV, Ehrlich ME, Gandy S, Dudley JT. 2019. Clarifying the potential role of microbes in Alzheimer's disease. Neuron 104:1036–1037. doi: 10.1016/j.neuron.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Flamand L, Gravel A, Boutolleau D, Alvarez-Lafuente R, Jacobson S, Malnati MS, Kohn D, Tang YW, Yoshikawa T, Ablashi D. 2008. Multicenter comparison of PCR assays for detection of human herpesvirus 6 DNA in serum. J Clin Microbiol 46:2700–2706. doi: 10.1128/JCM.00370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Dietrich J, Blumberg BM, Roshal M, Baker JV, Hurley SD, Mayer-Proschel M, Mock DJ. 2004. Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties. J Neurosci 24:4875–4883. doi: 10.1523/JNEUROSCI.5584-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ahlqvist J, Fotheringham J, Akhyani N, Yao K, Fogdell-Hahn A, Jacobson S. 2005. Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes. J Neurovirol 11:384–394. doi: 10.1080/13550280591002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Prusty BK, Gulve N, Govind S, Krueger GRF, Feichtinger J, Larcombe L, Aspinall R, Ablashi DV, Toro CT. 2018. Active HHV-6 infection of cerebellar Purkinje cells in mood disorders. Front Microbiol 9:1955. doi: 10.3389/fmicb.2018.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]