Abstract
Background:
Previous analyses of mortality were conducted in a large cohort of ethylene oxide (EtO) exposed workers employed at 13 sterilization facilities throughout the U.S. and followed from the start of operation through 1998. Statistically significant elevated mortality was reported from hematopoietic cancer in men and breast cancer in women compared to the general population. Possible healthy worker survivor bias was not addressed.
Methods:
To examine survivor bias in this cohort, employment termination was analyzed with statistical models stratified on sex and race that included age, employment duration, and cumulative EtO exposure. To reduce survivor bias employment duration was included in Poisson regression model specifications for estimating standardized mortality ratios for several cancer outcomes.
Results:
Strong statistically significant effects of unlagged cumulative EtO exposure were observed on rate of employment termination, indicating potential healthy worker survivor effect bias. Adjustment for employment duration in analyses of mortality resulted in statistically significant and stronger associations between cumulative EtO exposure and lung cancer, female breast cancer and hematopoietic cancer. There was a striking reduction in nonmalignant respiratory disease mortality risk with increasing employment duration with a further (nonsignificant) reduction with cumulative EtO, suggesting that EtO itself is driving termination of workers with respiratory morbidity even though the average EtO exposures in this population were generally far below odor and acute irritancy thresholds.
Conclusions:
Important survivor bias was present in this EtO cohort and may be present in many occupational settings involving irritant exposures.
Keywords: breast cancer, employment duration, lung cancer, survivor bias
1 |. INTRODUCTION
The healthy worker survivor effect (HWSE) has long been recognized as an important source of bias in occupational epidemiology,1,2 for example in studies of diesel exhaust, metalworking fluids and for occupational asthma.3–6 The causes of these effects may include depletion by exposure of high-susceptible subpopulations, and selective removal from exposure of individuals with unaccounted-for confounding exposures such as smokers, or individuals experiencing early symptoms. If smoking were a risk factor for leaving employment, the surviving workforce would have less cumulative smoking history but higher cumulative workplace exposures. Attenuation of exposure-response with higher cumulative exposures appears to be a manifestation of HWSE quite generally.7 Respiratory disease endpoints are particularly vulnerable to the healthy worker effect (HWE), reflecting selection at hire, and presumably also to HWSE.8
Chronic industrial exposure to reactive, irritant or sensitizing agents would be expected to exhibit a potentially strong HWSE. Ethylene oxide (EtO) is a reactive gas used to sterilize medical instruments, other manufactured products and even food materials like spices. In this analysis a previously studied cohort of workers exposed to EtO was examined with regard to HWSE for several mortality outcomes. Duration of employment was used as a means to adjust for HWSE in contrast to approaches using g-estimation and related methods.2
2 |. METHODS
2.1 |. Original data set
The study population has been previously compiled by NIOSH investigators and described,9-11 consisting of all workers in 14 sterilization facilities with 3 or more months since first exposure to EtO. Use of EtO began as early as 1938 but nine of the facilities first used EtO after 1960. Follow-up of this cohort began with 3 months of EtO exposure and continued until 31 December 1998.10 An exposure matrix was constructed by these investigators based on over 2350 time-weighted exposure values from 18 plants and on modeling using a subset of samples for which detailed process information was available (relating to product type, sterilizer volume, ventilation, and aeration).12 The result was estimated exposure levels in eight process categories (sterilizer operator, chamber area, maintenance, production, etc) over time which could be mapped to the work history available as department and/or job classifications.
The outcomes examined included those reported to be associated with EtO exposure by the previous investigators (all lymphopoietic cancer (ICD-9: 200-208) and breast cancer [ICD-9: 174]) as well as lung cancer (ICD-9: 162) and nonmalignant respiratory diseases (ICD-9: 460519). The previous analyses did not address possible survivor bias.
2.2 |. Statistical analysis
Employment duration, defined as the period from date of first hire until last date of observation or date of final termination, whichever occurred first, was analyzed with Poisson regression. Follow-up in the employment duration model began 3 months after first EtO exposure and continued until the earlier of 5 years before death, 3 months before a plant closing, or 31 December 1998. Rates of termination were modeled using Poisson regression in SAS13 (see Appendix for modeling code) with a table constructed to jointly classify all observation time in demographic levels as well as detailed levels of time-dependent employment duration, EtO exposure duration, and unlagged EtO cumulative exposures. The unit of observation was one person-day but observations were collapsed to a smaller file using fine stratification of the continuous variables. By definition in this model specification the employment duration variable remains fixed, after termination of employment, at the worker’s final duration. For rate of employment termination the following model was specified and applied separately for each demographic group:
where, PY = person-years; quits = number of terminations in PY; dur = employment duration, in years from hire; cumEto = cumulative EtO, lagged or unlagged, in ppm-yr. In this model, exp(a0) is the predicted annual termination rate in 40-year-old workers with no EtO exposure (and no duration).
Mortality analyses also utilized Poisson regression (see Appendix for model detail). A classification table was constructed for all observation time on demographic variables, time-dependent EtO exposure and employment duration metrics, and outcomes based on person-days and then collapsed using fine stratification. An offset was applied consisting of the expected number of cause-specific deaths in each classification cell based on U.S. national rates. Thus these were statistical models of standardized mortality ratios (SMR). Time-dependent cumulative exposure metrics, such as cumEtO, for EtO were calculated with a lag to account for the delay following the tumor initiation process and the resulting death. For nonmalignant respiratory disease, a 2.5 years lag was applied because developing respiratory impairment is often a cause of exposure termination.14
To address possible survivor effects, models of mortality rates for specific outcomes were fit in two specifications (see Appendix for detailed rationale). The first
applies employment duration as a multiplicative adjustment of baseline risk. To calculate the expected deaths, nexpected, U.S. age-, race-, sex-, and year-specific mortality rates15 were multiplied by PY in each cell. In these models, exp(a0) is the predicted SMR for workers with no EtO exposure and no duration over the period of follow-up. This model specification would be appropriate if population susceptibility was changing with employment duration. The second
applies employment duration as an additive adjustment of baseline risk. This specification would be appropriate if, for example, the selection on duration were the result of smokers generally terminating sooner than nonsmokers or if the observed EtO-associated excess mortality did not depend on other modifiers of the baseline risk that depend on duration.
On prior mechanistic grounds, when exposure is appropriately identified, measured and specified for modeling an outcome, employment duration by itself would not be a confounder (or causing collider bias). If there are other violations of assumptions such as effect-modification by age, then duration could appear as a confounder (in which case that interaction would need to be part of g-estimation as well). Similarly, If there is substantial exposure misclassification, those leaving might have had higher exposures on average than those staying who have the same assigned exposures. This could introduce survivor bias: those with long employment would have systematically less true exposure than assigned (and visa versa) which would likely distort the estimated exposure response.
3 |. RESULTS
3.1 |. Demographic aspects
There were 18 235 workers available for study from 14 plants but one plant lacked exposure history, leaving a population of 17 460. Further exclusions due to unknown vital status, foreign deaths and missing demographic information resulted in 17 185 workers (98.4%) for analysis. White women were the largest demographic group (n = 7878) followed by white men (n = 5858), black women (n = 1823) and black men (n = 1626) (Table 1). The employment and exposure history by the end of follow-up shows that workers were employed on average 8 to 9 years with black women having the longest duration, 9.1 year (Table 1). Workers’ exposure to EtO occurred over an average of 5 years with white women having the least (4.7 years) and black women the most (5.4 years) (Table 1). Women generally had lower estimated time-averaged EtO exposure concentrations and lower (lagged and unlagged) cumulative EtO exposure, by a factor of about two. Black women had a much smaller standard deviation for cumulative EtO indicating less diverse work activities. The overall time-averaged EtO exposure was 5.4 ppm (26.91/4.96, from Table 1).
TABLE 1.
Workers’ final durations, and assigned EtO exposures based on an exposure matrix,a by sex and race
| White men | Black men | White women | Black women | All | ||
|---|---|---|---|---|---|---|
| N | 5858 | 1626 | 7878 | 1823 | 17 185 | |
| Employment duration, y | Mean | 8.5 | 8.3 | 8.9 | 9.1 | 8.7 |
| SD | 9.5 | 8.6 | 9.5 | 8.9 | 9.4 | |
| Maximum | 63.1 | 43.3 | 52.8 | 39.7 | 63.1 | |
| Duration of EtO exposure, y | Mean | 5.0 | 5.5 | 4.7 | 5.4 | 5.0 |
| SD | 6.2 | 6.4 | 5.8 | 6.5 | 6.1 | |
| Maximum | 39.2 | 38.4 | 37.7 | 28.1 | 39.2 | |
| EtO exposure intensity,b ppm | Mean | 7.8 | 7.9 | 4.5 | 3.8 | 5.9 |
| SD | 11.9 | 10.9 | 8.9 | 3.3 | 10.0 | |
| Maximum | 328 | 144 | 296 | 31.2 | 328 | |
| Cumulative EtO exposure,c unlagged, | Mean | 37.2 | 40.0 | 18.7 | 17.7 | 26.9 |
| ppm-yr | SD | 88.7 | 82.9 | 41.0 | 25.4 | 65.3 |
| Maximum | 1219 | 1351 | 694 | 225 | 1351 | |
| Cumulative EtO exposure, lagged | Mean | 34.6 | 38.2 | 18.1 | 17.3 | 25.6 |
| 10 y, ppm-yrs | SD | 84.2 | 77.0 | 40.1 | 25.2 | 62.1 |
| Maximum | 1219 | 1351 | 694 | 225 | 1351 |
Abbreviation: EtO, ethylene oxide.
Greife.12
Mean, across population, of career mean EtO concentration more than 0 over observation time.
Cumulative exposure as of end of employment.
3.2 |. Employment termination models
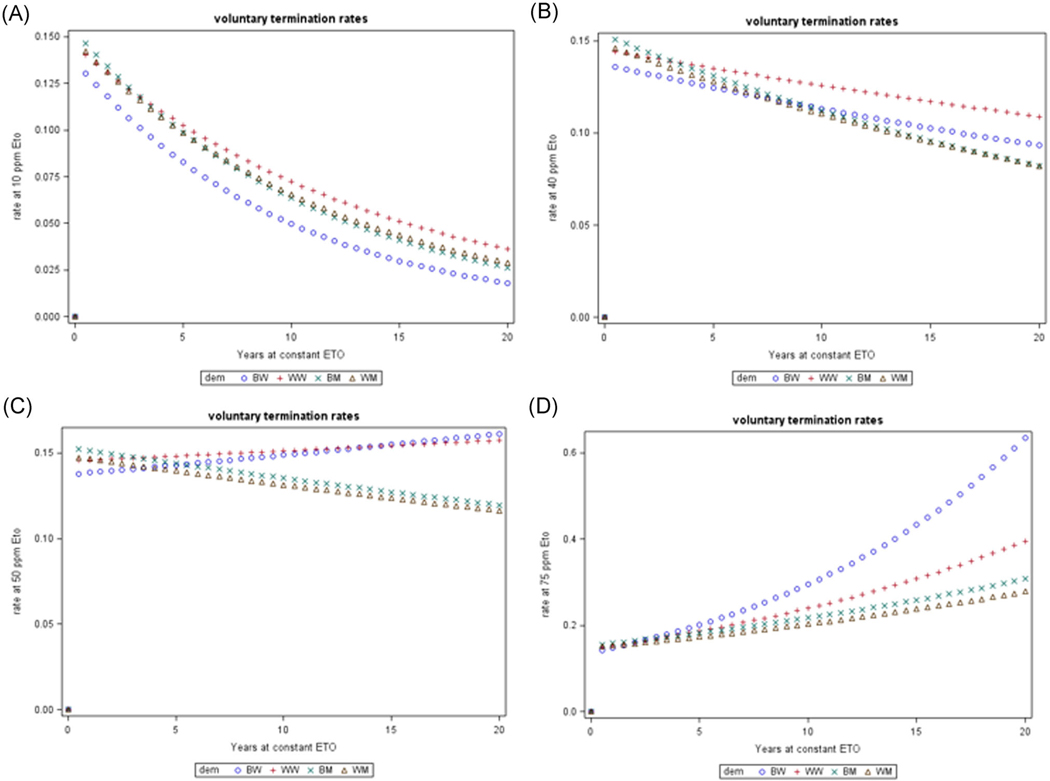
Out of the 17 185 workers, 14 314 met the criterion of voluntary or discretionary termination before end of follow-up for mortality. Poisson regression models of employment termination as functions of age, employment duration and (unlagged) cumulative EtO exposure in each of the demographic groups reveal a highly statistically significant negative effect of duration (diminishing rate of leaving with increasing time on job) and positive effects of EtO cumulative exposure that are highly significant for all but the smaller work group of black women (Table 2). All demographic groups have similar initial rates of termination of about 15% per year (exp[−1.9], at age 40) but black women show the lowest rate of termination at low EtO exposures and progressively advance to the highest termination rate at higher EtO exposures (Figure 1). White women show a less pronounced progression and men (white and black) show a smaller dependence on EtO exposures.
TABLE 2.
Models of rate of employment termination (quits per person-year) on duration and cumulative EtO exposure (unlagged), controlling for age by Poisson regression, stratifying on sex and race
| β | SEβ | 95% CLa | ||
|---|---|---|---|---|
| 1 | White men | |||
| intercept | −1.91 | 0.03 | −1.97, −1.85 | |
| Duration | −.099 | 0.004 | −0.11, −0.09 | |
| Cum Eto unlagged | .0017 | 0.0002 | 0.001, 0.002 | |
| 2 | Black men | |||
| intercept | −1.88 | 0.06 | −1.99, −1.76 | |
| Duration | −.107 | 0.008 | −0.12, −0.09 | |
| Cum Eto unlagged | .0019 | 0.0004 | 0.001, 0.003 | |
| 3 | White women | |||
| intercept | −1.93 | 0.02 | −1.97, −1.89 | |
| Duration | −.088 | 0.0033 | −0.094, −0.081 | |
| Cum Eto unlagged | .0018 | 0.0004 | 0.001, 0.003 | |
| 4 | Black women | |||
| intercept | −1.98 | 0.05 | −2.09, −1.88 | |
| Duration | −.129 | 0.0097 | −0.15, −0.11 | |
| Cum Eto unlagged | .0027 | 0.0018 | −0.001, 0.006 |
Note: Model: quits = exp(a0 + a1 × (age-40) + a2 × (age-40)2 + b3 × dur + b4 × cumEto) × PY.
where, in each classification cell: PY, person-years; quits, number of terminations; dur, mean employment duration, in years from hire to observation; cumEto, cumulative EtO, unlagged, in ppm-yr; follow-up until first of: 5 year before (death or end of study follow-up), 3 months before plant closings and date of termination; separate model for each demographic group.
CL, Wald confidence limits.
FIGURE 1.
Predicted rates of employment termination with employment duration in four demographic groups at fixed ethylene oxide exposure concentrations: A, 10 ppm, B, 40 ppm, C, 50 ppm, D, 75 ppm
3.3 |. Mortality from nonmalignant respiratory disease
For 131 workers nonmalignant respiratory disease was the underlying cause of death. The standardized mortality ratio (SMR) for nonmalignant respiratory disease (NMRD) in this population was 0.93 for white men, rising to 1.25 for black women (Table 3; model 1), which are elevated compared to healthy industrial populations.8 There appeared to be a substantial healthy worker effect (hiring) for black men (SMR = 0.63 = exp(−0.064 − 0.392); Table 3, model 1). When modeled with Poisson regression, there was a further elevated intercept corresponding to SMR = 1.75 (exp(0.56)) and a highly significant decline with duration of employment (Table 3; model 2): 6% for each year of employment (1-exp(−0.062); 95%CL: 4.0, 8.1%). Addition of the term for unlagged EtO cumulative exposure resulted in no improvement in model fit (Table 3; model 4).
TABLE 3.
Standardized mortality ratio for nonmalignant respiratory disease (by Poisson regression, as log(SMR)) on sex, race, employment duration, and cumulative EtO (NMRD deaths: 131)
| Demographic groups with Duration or cumulative EtO | β | SEβ | 95% CLa | SMR(0)b (% loss)c | Model −2lnL | |
|---|---|---|---|---|---|---|
| 1 | White men (intcp) | −.064 | 0.140 | −0.34, 0.21 | 0.93 | 2696.848 |
| iBlack mend | −.397 | 0.403 | −1.19, 0.39 | 0.63 | ||
| iWhite womend | .052 | 0.185 | −0.31, 0.42 | 0.99 | ||
| iBlack womend | .234 | 0.614 | −0.97, 1.44 | 1.25 | ||
| 2 | White men (intcp) | .560 | 0.164 | 0.24, 0.88 | 1.75 | 2659.238 |
| iBlack men | −.457 | 0.403 | −1.25, 0.33 | 1.11 | ||
| iWhite women | .019 | 0.185 | −0.34, 0.38 | 1.78 | ||
| iBlack women | .157 | 0.614 | −1.05, 1.36 | 1.32 | ||
| Duration | −.0617 | 0.0113 | −0.08, −0.04 | (6.0%) | ||
| 3 | White men (intcp) | .146 | 0.150 | −0.15, 0.44 | 1.16 | 2686.478 |
| iBlack men | −.363 | 0.404 | −1.15, 0.43 | 0.80 | ||
| iWhite women | −.046 | 0.186 | −0.41, 0.32 | 1.11 | ||
| iBlack women | .180 | 0.615 | −1.02, 1.38 | 1.14 | ||
| Cum Eto | −.0045 | 0.0017 | −0.01, −0.001 | (0.45%) | ||
| 4 | White men (intcp) | .574 | 0.165 | 0.25, 0.90 | 1.77 | 2658.862 |
| iBlack men | −.452 | 0.403 | −1.24, 0.34 | 1.13 | ||
| iWhite women | .000 | 0.188 | −0.37, 0.37 | 1.77 | ||
| iBlack women | .154 | 0.614 | −1.05, 1.36 | 1.32 | ||
| Duration | −.0584 | 0.0124 | −0.08, −0.03 | (5.7%) | ||
| Cum Eto | −.0010 | 0.0017 | −0.004, 0.002 | (0.10%) |
Note: Model: deaths = exp(a0 + a1 × rac0 + a2 × sex0 + a3 × rac0 × sex0 + b1 × dur + c1 × cumEto) × nexpected.where, intcp, intercept; duration – time-dependent employment duration, in years from hire; cumEto–cumulative EtO, lagged 2.5 y, ppm-yrs; rac0: 0 =white, 1 = nonwhite; sex0: 0 = men 1 = women; nexpected–number of deaths expected based on U.S. rates; four separate models. Abbreviations: EtO, ethylene oxide; NMRD, nonmalignant respiratory disease; SMR, standardized mortality ratio.
CL – Wald confidence limits.
SMR(0) – estimated baseline SMR; adjusted for cumEto or employment duration (models 2-4).
% reduction in NMRD mortality per year (duration) or per ppm-year (cumulative EtO).
incremental increase in log (SMR): for black men vs white men (iBlack men); for white women vs men (iWhite women); for black women vs. white women and men (iBlack women).
3.4 |. Lung cancer mortality
Lung cancer was the underlying cause of death for 247 workers with SMR = 1.06. In contrast to NMRD, EtO was associated with excess lung cancer (Table 4). With terms only for 10-year lagged cumulative EtO in all men, white women and black women, the associations were not statistically significant (χ2 from likelihood ratio test (LRT) (3 df) = 3.5; P = .3; model 1). However, including a multiplicative adjustment to baseline rate based on employment duration improved the model fit (χ2 (3 df) = 4.6; P = .2; model 2), and with the additive adjustment to baseline there was further improvement (χ2 (3 df) = 6.9; P = .07; model 3). Limiting the exposure terms to female workers produced a significant result (χ2 (2 df) = 6.13; P = .047). Testing the significance of the sex difference in EtO effect using the interaction term yielded a P-value .18 (data not shown).
TABLE 4.
Standardized mortality ratio for lung cancer (by Poisson regression) on sex, race, employment duration, and cumulative EtO (lung cancer deaths: 247)
| Duration and cumulative EtO (lag = 10 y) | β | SEβ | 95% CLa | Δ-2lnLbX2 (df) | Pb | |
|---|---|---|---|---|---|---|
| 1 | Model: deaths = exp(a0 + a1 × rac0 + a2 × sex0 + a3 × rac0 × sex0) × (1 + c1 × cumEto + c3 × Sceto + c4*RSceto) × nexpected | |||||
| intercept | .0890 | 3.47 (3) | .32 | |||
| Cum EtO All men | −.0002 | 0.0007 | −0.002, 0.001 | |||
| Cum EtO iWhite womenc | .0013 | 0.0024 | −0.003, 0.006 | |||
| Cum EtO iBlack womenc | .0241 | 0.0347 | −0.044, 0.092 | |||
| 2 | Model: deaths = exp(a0 + a1 × rac0 + a2 × sex0 + a3 × rac0 × sex0 + b1 × dur) × (1 + c1 × cumEto + c3 × Sceto + c4 × RSceto) × nexpected | |||||
| intercept | .2721 | 4.60 (3) | .20 | |||
| Duration | −.0206 | 0.0077 | −0.036, −0.006 | |||
| Cum EtO All men | .0008 | 0.0012 | −0.002, 0.003 | |||
| Cum EtO iWhite women | .0025 | 0.0035 | −0.004, 0.009 | |||
| Cum EtO iBlack women | .0308 | 0.0433 | −0.054, 0.116 | |||
| 3 | Model: deaths = exp(a0 + a1 × rac0 + a2 × sex0 + a3 × rac0 × sex0) × (exp(b1 × dur) + c1 × cumEto + c3 × Sceto + c4 × RSceto) × nexpected | |||||
| intercept | .3251 | 6.98 (3) | .07 | |||
| Duration | −.0264 | 0.0086 | −0.043, −0.010 | |||
| Cum EtO All men | .0005 | 0.0006 | −0.001, 0.002 | |||
| Cum EtO iWhite women | .0025 | 0.0022 | −0.002, 0.007 | |||
| Cum EtO iBlack women | .0215 | 0.0293 | −0.036, 0.079 | |||
| 4 | Model: deaths = exp(a0 + a1 × rac0 + a2 × sex0 + a3 × rac0 × sex0) × (exp(b1 × dur) + c3 × Sceto + c4 × RSceto) × nexpected | |||||
| intercept | .3270 | 6.13 (2) | .047 | |||
| Duration | −.0228 | 0.0074 | −0.037, −0.008 | |||
| Cum EtO iWhite women | .0028 | 0.0021 | −0.001, 0.007 | |||
| Cum EtO iBlack women | .0217 | 0.0299 | −0.037, 0.080 | |||
Note: nexpected, number of deaths expected based on U.S. rates; rac0–race coded as 0 = white, 1 = nonwhite; sex0, sex coded as 0 = men, 1 = women; duration, time-dependent employment duration, yrs; Cum EtO, cumulative EtO exposure, lagged 10 y, as ppm-yrs EtO; Sceto, cumEto×sex0; RSceto, Sceto×rac0.
CL – Wald confidence limits.
Change in 2ln(Likelihood) with addition of cumulative EtO terms and associated p.
incremental increase in log(SMR): for white women vs men (iWhite women); for black women vs white women and men (iBlack women).
3.5 |. Mortality from cancer of the female breast
For the 102 deaths from female breast cancer, there was no statistically significant difference in mortality on cumulative EtO exposure with a 10-year lag by race, black women having a slightly smaller association (Table 5; models 1 and 2). Adjustment for baseline risk, both additive and multiplicative, produced insignificant improvement in model fit; in the better fitting model, the contribution of (lagged) cumulative EtO exposure was not significant (χ2 (1 df) = 1.55; P = .21) (Table 5, models 3 and 4). This analysis lacked important risk factor information such as parity or age at first live birth. However, with a 20-year lag, the contribution of cumulative EtO was significant (LRT: P = .04) and improved considerably with the multiplicative adjustment for baseline risk (LRT: P = .01) (Table 5; models 5-8).
TABLE 5.
Standardized mortality ratio for female breast cancer (by Poisson regression) on sex, race, employment duration, and cumulative EtO (breast cancer deaths: 102)
| Cumulative Eto (lag = 10 y) | β | SEβ | 95% CIa | Δ-2lnLX2 (df)b | Pb | |
|---|---|---|---|---|---|---|
| With lag=10 y | ||||||
| 1 | Model: deaths = exp(a0 + a1 × rac0) × (1 + c3 × Sceto + c4 × RSceto) × nexpected | |||||
| intercept | −.0630 | 0.97 (2) | 0.61 | |||
| Cum EtO White women | .0025 | 0.0032 | −0.0037, 0.0087 | |||
| Cum EtO iBlack womenc | −.0035 | 0.0110 | −0.025, 0.018 | |||
| 2 | Model: deaths = exp(a0 + a1 × rac0) × (1 + c3 × Sceto) × nexpected | |||||
| intercept | −.0616 | 0.93 (1) | .33 | |||
| Cum EtO All women | .0024 | 0.0031 | −0.0036, 0.0084 | |||
| 3 | Model: deaths = exp(a0 + a1 × rac0 + b1×dur) × (1 + c3 × Sceto) × nexpected | |||||
| intercept | .0239 | 1.47 (1) | .23 | |||
| Duration | −.0105 | 0.0135 | −0.037, 0.016 | |||
| Cum EtO All women | .0030 | 0.0030 | −0.0028, 0.0087 | |||
| 4 | Model: deaths = exp(a0 + a1 × rac0) × (exp(b1 × dur) + c3 × Sceto) × nexpected | |||||
| intercept | .0212 | 1.55 (1) | .21 | |||
| Duration | −.0107 | 0.0128 | −0.036, 0.014 | |||
| Cum EtO All women | .0039 | 0.0043 | −0.0045, 0.012 | |||
| With lag=20 y | ||||||
| 5 | Model: deaths = exp(a0 + a1 × rac0) × (1 + c3 × Sceto + c4 × RSceto) × nexpected | |||||
| intercept | −.1067 | 4.17 (2) | .12 | |||
| Cum EtO White women | .0099 | 0.0062 | −0.0022, 0.022 | |||
| Cum EtO iBlack women | −.0101 | 0.0234 | −0.056, 0.036 | |||
| 6 | Model: deaths = exp(a0 + a1 × rac0) × (1 + c3 × Sceto) × nexpected | |||||
| intercept | −.1031 | 4.06 (1) | 0.04 | |||
| Cum EtO All women | .0094 | 0.0059 | −0.0022, 0.021 | |||
| 7 | Model: deaths = exp(a0 + a1 × rac0 + b1 × dur) × (1 + c3 × Sceto) × nexpected | |||||
| intercept | .0311 | 6.04 (1) | 0.014 | |||
| Duration | −.0181 | 0.0125 | −0.043, 0.0063 | |||
| Cum EtO All women | .0166 | 0.0096 | −0.0023, 0.035 | |||
| 8 | Model: deaths = exp(a0 + a1 × rac0) × (exp(b1 × dur) + c3 × Sceto) × nexpected | |||||
| intercept | .0242 | 5.29 (1) | 0.02 | |||
| Duration | −.0151 | 0.0137 | −0.042, 0.012 | |||
| Cum EtO All women | .0097 | 0.0053 | −0.0006, 0.020 | |||
Note: nexpected, number of deaths expected based on U.S. rates; rac0, race coded as 0 = white, 1 = nonwhite; sex0, sex coded as 0 =men, 1 = women; duration–time-dependent employment duration, yrs; cumEto, cumulative EtO exposure, lagged 10 y, as ppm-yrs EtO; Sceto, cumEto×sex0; RSceto, Sceto×rac0.
CL – Wald confidence limits.
Change in 2lnL with addition of cumulative EtO terms and associated p.
iBlack women – incremental increase in log(SMR) for black women vs white women.
3.6 |. Mortality from all lymphopoietic cancer
The 73 lymphopoietic cancer deaths did not represent an overall excess (SMR = 0.96; 95% CI, 0.76-1.20), based on U.S. rates, particularly for white men (SMR = 0.92), and white women (SMR = 0.85), but among black workers, there was a statistically significant increase in SMR with cumulative EtO exposure (lagged 2.5 years; LRT: P = .011) (Table 6; model 1). A multiplicative adjustment to baseline risk with employment duration resulted in a modest improvement in model fit for the association with EtO exposure (for EtO effect; LRT: P = .007) (Table 6; model 2); the additive adjustment had slightly less effect (for EtO effect; P = .008) (Table 6; model 3).
TABLE 6.
Standardized mortality ratio for all lymphopoietic cancer (by Poisson regression) on sex, race, employment duration, and cumulative EtO (cancer deaths: 73)
| Cumulative EtO (lag = 10 y) | β | SEβ | 95% CLa | Δ-2lnLbX2 (1 df) | Pb | |
|---|---|---|---|---|---|---|
| 1 | Model: deaths = exp(a0 + a2 × sex0) × (1 + c2*Rceto) × nexpected | |||||
| intercept | −.113 | 0.1831 | −0.47, 0.25 | 6.53 | .011 | |
| Cum EtO all Black workers | .0129 | 0.0079 | −0.003, 0.028 | |||
| 2 | Model: deaths = exp(a0 + a2 × sex0 + b1 × dur) × (1 + c2*Rceto) × nexpected | |||||
| intercept | −.0007 | 0.2160 | −0.42, 0.42 | 7.27 | .007 | |
| Duration | −.0116 | 0.0125 | −0.036, 0.013 | |||
| Cum EtO all Black workers | .0153 | 0.0093 | −0.003, 0.034 | |||
| 3 | Model: deaths = exp(a0 + a2 × sex0) × (exp(b1 × dur) + c2*Rceto) × nexpected | |||||
| intercept | −.0063 | 0.2203 | −0.44, 0.43 | 7.06 | .008 | |
| Duration | −.0105 | 0.0131 | −0.036, 0.015 | |||
| Cum EtO all Black workers | .0118 | 0.0071 | −0.002, 0.026 | |||
Note: nexpected, number of deaths expected based on U.S. rates; rac0, race coded as 0 = white, 1 = nonwhite; sex0, sex coded as 0 = men; 1 = women; duration, time-dependent employment duration, yrs; cumEto, cumulative EtO exposure, lagged 2.5 y, as ppm-yrs EtO; Rceto, cumEto×rac0.
CL, Wald confidence limits.
Change in 2lnL with addition of cumulative EtO terms.
4 |. DISCUSSION
4.1 |. Findings
With EtO, there appears not to be an overall association with NMRD mortality although an SMR of 0.93 is somewhat high for typical industrial populations free of respiratory threats, where SMRs range 0.5-0.8.8 An earlier mortality analysis of this cohort with follow-up through 1987 observed an NMRD SMR of 0.80.11 In the present analysis of the EtO cohort, during follow-up with a small duration of employment (both during and following termination of employment), the predicted mortality rate was considerably elevated (from intercept, SMR = 1.75) (Table 3; model 2); after about 9 years of EtO exposure, the predicted NMRD deaths would be about as expected from U.S. rates (exp(0.56-9 × 0.062)); at less than 9 years, there would be more NMRD deaths than expected without addressing the survivor effect and at greater than 9 years, fewer than expected. If not accounted for, this distortion in the baseline SMRs related to survival would confound a toxic effect of EtO on NMRD mortality, if present. The observation that EtO is a strong determinant of employment termination and employment duration is a strong predictor of declining NMRD risk but EtO itself is a weak (and negative) risk factor for NMRD (Table 3; model 4) suggests that EtO may be simultaneously modifying the baseline rate (by promoting selective termination of high-risk individuals) as well as increased risk for NMRD. The EtO example presents an unusual opportunity to observe potential survivor bias related to occult health effects.
In other EtO studies lung cancer has been reported not to have significantly elevated incidence16 or mortality,17 although a strong HWE bias was evident in the mortality study (all-cause SMR = 0.79; 95%CI: 0.71-0.78; all-cancer SMR = 0.86, 95%CI: 0.71-1.04).17 The previous study of mortality in the present cohort10 did not report analyses of lung cancer mortality beyond the modest overall association (SMR = 1.05; 95% CI, 0.95-1.17). Because duration is contributing to any cumulative exposure metric, this method of controlling HWSE may fail if exposure intensity is relatively uniform across jobs and time, resulting in high collinearity. However, observing positive exposure effects in the same models with negative duration effects indicates that collinearity is not the problem, rather, that survivor bias is present violating underlying model assumptions. This method would fail if an extreme survivor bias is present, possibly driven by EtO exposure levels, which may have been the case in this cohort affecting NMRD mortality. Across all outcomes analyzed in the present work, the estimates for the duration effects were negative but statistically significant only for lung cancer (the outcome most associated with smoking).
In the present analysis cumulative EtO exposure was significantly associated with lung cancer death only in women, with a suggestion of substantially higher risk in black women. This brings up the possibility of important exposure misclassification. If within the defined broad exposure groups there was considerable sex/race-dependent differential assignment of job tasks such that men had lower exposures than average and women higher exposures, misclassification would result. This could happen, for example, if the tasks of unloading and processing sterilized materials were preferentially assigned to women, and the highest exposed jobs assigned to black women. The observation that black women were the least likely to terminate employment at low exposure but showed the strongest termination rate dependence on EtO supports the existence of such misclassification. Differential susceptibility by sex would be another possible explanation for the stronger EtO effect in women. However, the adjustment specification addressing susceptibility (Table 4; model 2) fit less well than the specification relating to selection on other risk factors, like smoking (Table 4; model 3).
Previous analyses of female breast cancer in EtO populations have observed positive, nonlinear associations with lagged cumulative EtO exposures. There was a statistically significant, apparently supralinear, association of incidence of breast cancer with EtO in a categorical, internally standardized analysis of Swedish workers16 based on unlagged cumulative exposures. In the present U.S. cohort, with a 20 year lag, Steenland et al10 using Cox regression, reported essentially uniformly elevated breast cancer mortality across three lower strata of cumulative exposure and a significantly higher odds ratio in the highest exposure strata (OR = 3.76; 95% CI, 1.03-13.6). Reproductive history was not available for this Swedish or U.S study. After identification of more than 200 additional, incident cases of breast cancer in the U.S. cohort by questionnaire survey,18 analyses of incidence exposure-response, with Cox regression and a 15-year lag and controlling for reproductive parity, produced significant trends on both cum. EtO and log(cum. EtO) metrics (P = .02); a stronger association was observed with duration of EtO exposure itself (lagged 15 years; P = .006). All of the Cox regression models assumed a log-linear relationship: log(OR) = a+bX which would under-estimate response at low exposures for a carcinogen which actually has a linear exposure-response. Observing here the improvement with a 20-yr vs. 10-yr lag suggests that early exposures may have been important, possibly related to reproductive status such as at the age of first live birth. However, an EtO cumulative exposure metric restricted to exposures occurring up to age 35, with a 10-yr lag, did not predict risk well for premenopausal or all breast cancer (data not shown).
For all haematopoietic cancer incidence,16 Mikoczy et al (17 incident cases) found no significant increases using unlagged cumulative EtO exposure. Steenland et al10 in the present mortality cohort (with Cox regression and a 15 year lag)) observed increasing odds ratios with log(cum. EtO) but only in men (LRT: P = .02); stratification of analyses by race was not reported.
4.2 |. Limitations
This study demonstrates a method of adjustment for survivor effects, a need that was suggested long ago by Gilbert19 and has been applied using employment duration in other studies.20–23 As applied here, the method constrains the baseline risk in the form of an exponential decline based on employment duration. The risk factors contributing to this decline are not known nor is the optimum form of this duration-association. It offers a partial correction and appears to increase statistical power for outcomes affected by survivor bias. Other selection effects related to employment status or health effects causing changes in exposure status, may be poorly or inappropriately controlled using employment duration. Some have posited that If work status is both a mediator and a confounder (or proxy thereof) of the exposure-outcome relation, then adjustment by employment duration (as done here) may result in a biased estimate of the dose-response.2,24,25 Therefore, more sophisticated methods (eg, g-estimation) may be required. Methods based on directed acyclic graph-derived regression structures and g-estimation of failure time attempt to address these issues but face similar unknowns, and have difficulties accommodating continuous time-dependent exposures.2,6 A parallel treatment of the EtO data by such methods would be informative.
5 |. CONCLUSIONS
Many occupational exposures may play a role in the termination of employment because of irritant or other adverse health effects whether or not manifest as symptoms and whether or not perceived as work-related. In the case of EtO, the threshold for odor is quite high (about 250 ppm), and acute symptoms occur above 200 ppm, exposures far higher than in most of the jobs in this study (Table 1). Besides EtO, examples might include toluene di-isocyanate and other isocyanates (sensitizers), formaldehyde, glutaraldehyde, and other aldehydes, diacetyl, and other α-diketones, acrylamide, silica, irritant metal compounds, for example, hexavalent chromium, titanium dioxide, and innumerable other agents with irritancy potential. If the population affected and leaving employment or avoiding exposure differs from those remaining on unmeasured risk factors or modifiers of determinants for the end-point understudy, important bias could result. Thus survivors in exposure might have lower inherent susceptibility and should be compared to similar populations without exposure. If smokers are differentially affected (more likely or less likely to leave) that could have a large impact of estimates of exposure effects for any smoking-related disease. If the rate of smokers leaving employment is enhanced by exposure, then even stronger bias would result. As suggested here for NMRD, the magnitude of survivor bias can be very large, potentially obscuring important exposure risks.
ACKNOWLEDGMENTS
Leslie T. Stayner, David B. Richardson, Jonathan Chevrier, and Robert D. Daniels provided helpful comments and recommendations for this work. The human data analyzed in this work was collected in previous published studies that were approved under NIOSH guidelines and procedures for protection of human subjects. The data made available for this work was de-identified and none of the reported findings can be linked to individual study subjects.
APPENDIX A
Example of SAS proc countreg model specification for employment termination rate model
[This model does not allow duration effect to depend on race or sex.}
| proc countreg data=etoaf32_f2; class IA; |
| model cNQ= IA SEX RAC RS aCNLDUR aCNLETO Rceto Sceto RSceto/offset=lnPY; |
| run; |
where: cNQ: no. of quits in classification cell (usually 0); IA: age strata 1 to 9; SEX: 0 =men 1 =women; RAC: 0 =white, 1 =nonwhite; RS = SEX*RAC; aCNLDUR = duration of employment; aCNLETO = unlagged cumulative EtO exposure; Rceto = aCNLETO×RAC; Sceto = aCNLETO×SEX; RSceto = aCNLETO×RS; lnPY = log(person-years)
(durations and cumulative exposures as p-yr-weighted cell means)
| Parameter estimates |
Approx Pr >|t| |
||||
|---|---|---|---|---|---|
| Parameter | DF | Estimate | SE | t Value | |
| Intercept | 1 | −1.482120 | 0.071386 | −20.76 | <.0001 |
| IA 1 | 1 | 0.026156 | 0.072656 | 0.36 | 0.7188 |
| IA 2 | 1 | −0.077633 | 0.072023 | −1.08 | 0.2811 |
| IA 3 | 1 | −0.337052 | 0.073389 | −4.59 | <.0001 |
| IA 4 | 1 | −0.475046 | 0.074432 | −6.38 | <.0001 |
| IA 5 | 1 | −0.474857 | 0.075147 | −6.32 | <.0001 |
| IA 6 | 1 | −0.386475 | 0.075789 | −5.10 | <.0001 |
| IA 7 | 1 | −0.313229 | 0.078456 | −3.99 | <.0001 |
| IA 8 | 1 | −0.252783 | 0.083565 | −3.03 | 0.0025 |
| IA 9 | 0 | 0 | . | . | . |
| sex | 1 | 0.044432 | 0.020833 | 2.13 | 0.0329 |
| rac | 1 | −0.057120 | 0.034405 | −1.66 | 0.0969 |
| RS | 1 | −0.133694 | 0.049346 | −2.71 | 0.0067 |
| aCNLDUR | 1 | −0.095984 | 0.002352 | −40.81 | <.0001 |
| aCNLETO | 1 | 0.001659 | 0.000190 | 8.75 | <.0001 |
| Rceto | 1 | −0.000028954 | 0.000388 | −0.07 | 0.9404 |
| Sceto | 1 | 0.000611 | 0.000374 | 1.63 | 0.1022 |
| RSceto | 1 | −0.003591 | 0.001511 | −2.38 | 0.0174 |
Example SAS proc nlin model specification for employment termination rate model
Model specific for each demographic group sex, race:
[These models allow duration effect to depend on race or sex.}
| proc nlin data=eto.etoaf32_f1 nohalve method=gauss eformat sigsq=1; where rac=1 and sex=1; |
| parameters a0 = 0.25 a1 = 0 a2 = 0 b3 = 0 b4 = 0; |
| age40 = 5*(IA-5.5); * IA = 6 ~ age 40-44; |
| model.cNQ = exp(a0 + a1*age40 + a2*age40**2 + b3*aCNLDUR +b4*aCNLETO) * nPY; |
| _weight_ = 1/model.cNQ; |
| dev = Deviance(‘Poisson’, cNQ, Model.cNQ); |
| _loss_ = dev/_weight_; |
| * output out=fitdata (label = ‘Dataset containing additional results of the Poisson regression’) |
| Residual=Res Predicted=Predict_cNQ; |
| run; |
where: cNQ: no. of quits in classification cell (usually 0); age40 = age-40; strata 1 to 9; aCNLDUR = duration of employment; aCNLETO = unlagged cumulative EtO exposure; nPY = person-years
(durations and cumulative exposures as p-yr-weighted cell means)
| Parameter | Estimate | Approx. SE |
Approximate 95% Confidence Limits |
|
|---|---|---|---|---|
| a0 | −1.9848 | 0.0544 | −2.0915 | −1.8782 |
| a1 | 0.0155 | 0.00502 | 0.00562 | 0.0253 |
| a2 | 0.00172 | 0.000265 | 0.00120 | 0.00224 |
| b3 | −0.1290 | 0.00974 | −0.1481 | −0.1099 |
| b4 | 0.00274 | 0.00180 | −0.00080 | 0.00627 |
Example SAS proc nlin model specification for mortality outcome (lymphpoietic cancer death) with linear relative rate model for exposure to Eto in nonwhite workers
| proc nlin data=EtoAF45_fLPallR nohalve method=gauss eformat sigsq=1; |
| parameters a0 = 0.104 a2 = 0.01 a3 = −0.01 c2 = 0; |
| termA=exp(a0 + a2*sex0); |
| model.cD = termA * (exp(a3*aDure) + c2*Rceto) * expt; |
| _weight_ = 1/model.cD; |
| dev = Deviance(‘Poisson’, cD, Model.cD); |
| _loss_ = dev/_weight_;; |
| * output out=fitdata (label = ‘Dataset containing additional results of the Poisson regression’); |
| run; |
where: cD = number of observed deaths from lymphopoietic cancer in each classification cell;
Rceto = lagged cumulative EtO exposure in nonwhite workers; expt = expected number of deaths in each classification cell (based on national rates)
| Parameter | Estimate | Approx. SE | Approximate 95% Confidence Limits |
|
|---|---|---|---|---|
| a0 | −0.00630 | 0.2203 | −0.4381 | 0.4255 |
| a2 | −0.0153 | 0.2414 | −0.4885 | 0.4579 |
| a3 | −0.0105 | 0.0131 | −0.0361 | 0.0151 |
| c2 | 0.0118 | 0.00709 | −0.00207 | 0.0257 |
Rationale for model specifications regarding HWSE
Scenario 1
This specification assumes a population surviving in employment to dur has diminishing average susceptibility to Eto effect compared to population at dur= 0 (could be due, eg, to depletion of high risk individuals).
where b1 < 0, i.e. a declining background risk
Dividing by p-yrs:
Dividing by expected rate:
Scenario 2
This specification assumes a population surviving to dur has no diminishing susceptibility to Eto effect but that other causes of the outcome are diminishing resulting in a smaller contribution of nonattributable cases (by a factor exp(b1×dur) where b1 < 0). This could happen if smokers are leaving employment faster and the outcome is smoking-related.
Dividing by p-yrs:
Dividing by expected rate:
A typical study where HWSE is present may not be able to statistically distinguish these two options.
Footnotes
DISCLOSURE BY AJIM EDITOR OF RECORD
John D. Meyer declares that he has no conflict of interest in the review and publication decision regarding this article.
ETHICS APPROVAL AND INFORMED CONSENT
This work utilized deidentified data that had been previously collected, analyzed, and published under CDC/NIOSH policies for protection of human subjects.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Arrighi HM, Hertz-Piccioto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5:189–196. [DOI] [PubMed] [Google Scholar]
- 2.Buckley J, Keil A, McGrath L, Edwards J. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology. 2015;26:204–212. [DOI] [PubMed] [Google Scholar]
- 3.Garcia E, Picciotto S, Costello S, Bradshaw P, Eisen E. Assessment of the healthy worker survivor effect in cancer studies of the United Autoworkers-General Motors cohort. Occup Environ Med. 2017;74:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neophytou A, Picciotto S, Costello S, Eisen E. Occupational diesel exposure, duration of employment, and lung cancer: an application of the parametric G-formula. Epidemiology. 2016;27:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Moual N, Kauffmann F, Eisen EA, Kennedy SM. The healthy worker effect in asthma: work may cause asthma, but asthma may also influence work. Am J Respir Crit Care Med. 2008;177:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonathan Chevrier, Sally Picciotto, Ellen Eisen. A comparison of standard methods with g-estimation of accelerated failure-time models to address the healthy-worker survivor effect: application in a cohort of autoworkers exposed to metalworking fluids. Epidemiology. 2012;23:212–219. [DOI] [PubMed] [Google Scholar]
- 7.Stayner L, Steenland K, Dosemeci M, Hertz-Picciotto I. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health. 2003;29:317–324. [DOI] [PubMed] [Google Scholar]
- 8.Park RM, Neil AM, Punnett L, Moure-Eraso R, Silverstein MA. A comparison of PMRs and SMRs as estimators of occupational mortality. Epidemiology. 1991;2:49–59. [DOI] [PubMed] [Google Scholar]
- 9.Stayner L, Steenland K, Greife A, et al. Exposure-response analysis of cancer mortality in a cohort of workers exposed to ethylene oxide. Am J Epidemiol. 1993;138:787–798. [DOI] [PubMed] [Google Scholar]
- 10.Steenland K, Stayner L, Deddens J. Mortality analyses in a cohort of18 235 ethylene oxide exposed workers: follow up extended from 1987 to 1998. Occup Environ Med. 2004;61:2–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Steenland K, Stayner L, Greife A, et al. Mortality among workers exposed to ethylene oxide. N Engl J Med. 1991;324:1402–07. [DOI] [PubMed] [Google Scholar]
- 12.Greife AL. Development of a model for use in estimating exposure to ethylene oxide in a retrospective cohort mortality study. Scand J Work Environ Health. 1988;14:29. [PubMed] [Google Scholar]
- 13.SAS Institute, Inc. 2011. “SAS® Software version 9.3.” In. Cary, NC. [Google Scholar]
- 14.Park RM, Chen W. Silicosis exposure–response in a cohort of tin miners comparing alternate exposure metrics. Am J Ind Med. 2013;56:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubauer Berigan MK, Schubauerberigan M, Raudabaugh W, et al. LTAS.NET: a NIOSH life table analysis System for the windows environment. Ann Epidemiol. 2005;15:656–56. [Google Scholar]
- 16.Mikoczy Z, Tinnerberg H, Björk J, Albin M. Cancer incidence and mortality in Swedish sterilant workers exposed to ethylene oxide: updated cohort study findings 1972–2006. Int J Environ Res Public Health. 2011;8:2009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teta MJ, Sielken R, Flores CV. Ethylene oxide cancer risk assessment based on epidemiological data: application of revised regulatory guidelines. Risk Anal. 1999;19:1135–1155. [DOI] [PubMed] [Google Scholar]
- 18.Steenland K, Whelan E, Deddens J, Stayner L, Ward E. Ethylene oxide and breast cancer incidence in a cohort study of 7576 women (United States). Cancer Causes Control. 2003;14:531–539. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert ES. Some confounding factors in the study of mortality and occupational exposures. Am J Epidemiol. 1982;116:177–188. [DOI] [PubMed] [Google Scholar]
- 20.Brophy JT, Margaret MK, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case–control study. Environ Health. 2012;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garshick E, Laden F, Hart J, Davis M, Eisen E, Smith T. Lung cancer and elemental carbon exposure in trucking industry workers. Environ Health Perspect. 2012;120:1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garshick E, Laden F, Hart J, et al. ‘Lung cancer in railroad workers exposed to diesel exhaust’. Environ Health Perspect. 2004;112:1539–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park R The healthy worker survivor effect and mortality at two automotive engine manufacturing plants. Am J Ind Med. 1996;30:655–663. [DOI] [PubMed] [Google Scholar]
- 24.Robins J A graphical approach to the identification and estimation of causal parameters in mortality studies with sustained exposure periods. J Chronic Dis. 1987;40:139S–161SS. [DOI] [PubMed] [Google Scholar]
- 25.Naimi A, Cole S, Hudgens M, Brookhart MA, Richardson D. Assessingthe component associations of the healthy worker survivor bias: occupational asbestos exposure and lung cancer mortality. Ann Epidemiol. 2013;23:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]