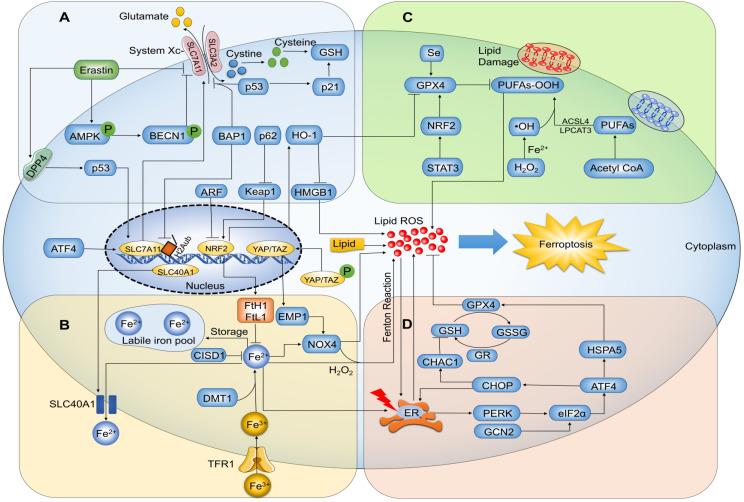
Figure 2.
Ferroptosis-related signaling molecules and signaling pathways. (A) Glutamate exchanges for cystine in a 1:1 ratio through the cystine/glutamate antiporter system Xc-, and inhibition of system Xc- by its core part SLC7A11 induces ferroptosis. (B) Ferric iron (Fe3+) bound to transferrin enters cells via membrane protein transferrin receptor 1 (TFR1) and localizes in endosomes, wherein the ferrireductase activity of STEAP3 reduces Fe3+ to redox-active iron (Fe2+). Finally, divalent metal transporter 1 (DMT1) releases Fe2+ from endosomes into a labile iron pool in the cytoplasm. In general, excess iron is stored in ferritin with ferritin heavy chain 1 (FtH1) and ferritin light chain 1 (FtL1). Under the action of H2O2, Fe2+ catalyzes the production of hydroxyl radical (HO∙) by Fenton reaction, triggering a chain reaction of radical lipid peroxidation and eventually leads to ferroptosis. (C) Ferroptosis is trigged by peroxidation (-OOH) of polyunsaturated fatty acids (PUFAs) and aberrant accumulation of lipid reactive oxygen species (ROS), resulting in membrane destabilization and rupture. Acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are necessary for ferroptosis to produce the target lipid pool containing arachidonic acid. GPX4 can hydrolyze lipid peroxides into non-toxic lipid alcohols (-OH). (D) Intracellular glutathione exists as oxidized glutathione (GSSG) and reduced glutathione (GSH); GPX4 requires GSH as a cofactor and reduces GSSG to GSH via glutathione reductase (GR). GPX4 inhibits the formation of Fe2+-dependent ROS by converting lipid hydroperoxides into lipid alcohols, and thus inhibits ferroptosis.