Abstract
Analgesics with no abuse liability are highly demanded in the market. KOR agonists have been proved to be strong analgesics without MOR agonist-related side effects, such as respiratory depression, tolerance, and dependence liability; however, activation of KOR in the central nervous system (CNS) may cause sedation and anxiety. It has been reported that peripheral KOR activation produces comparable bioactivity without CNS-related side effects. Herein, we designed and synthesized a novel tetrapeptide (SHR0687), which was shown to be a highly potent KOR agonist with excellent selectivity over other opioid receptors, such as MOR and DOR. In addition, SHR0687 displayed favorable PK profiles across species, as well as robust in vivo efficacy in a rat carrageenan-induced pain model. Notably, SHR0687 exhibited negligible blood–brain barrier penetration, which was meaningful in minimizing CNS-related side effects.
Keywords: Opioid receptors, kappa, analgesics, peripheral nervous system, BBB penetration, SHR0687
Opioid receptors from family A of the G-protein-coupled receptors include three major subtypes (mu [MOR], kappa [KOR], and delta [DOR]), which are widely distributed in the brain, spinal cord, and digestive tract.1−3 As well-known targets for pain treatment, opioid receptors are involved in the physiologic process of pain-related perception and modulation.3 In the clinic setting, MOR agonists, such as morphine, are the most widely used powerful analgesics.4 However, activation of MOR often leads to serious side effects, such as respiratory depression, tolerance, and dependence liability.5 DOR agonists are less antinociceptive with notable convulsions, which have limited therapeutic development.6,3 As a potential alternative analgesic, KOR agonists have a strong antinociceptive effect with minimal respiratory depression and drug dependence.7 Additionally, KOR agonists have been shown to be useful for treating pruritus, opiate dependence, and depression.8
Further research has verified that KOR agonists in the central nervous system (CNS) may cause sedation and anxiety,9 whereas peripheral KOR activation produces comparable bioactivity without CNS-related side effects.10 These inspiring results have spurred considerable interest of discovering novel KOR agonists with high region selectivity in the peripheral nervous system (PNS).
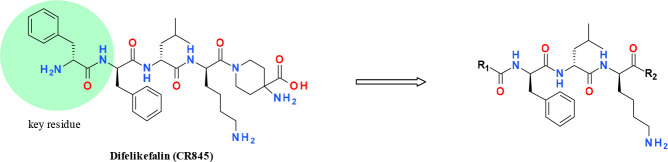
Recently, two peripherally selective KOR agonists were reported in clinical development at phase III. Asimadoline (Figure 1) has been shown to have reasonable efficacy for treatment of visceral pain.11 Another peptide-based molecule, difelikefalin (CR845), is undergoing clinical trials for treating chronic and postoperative pain.12,13 Considering the huge demand for analgesics without abuse liability, there has been considerable interest in developing diverse high-potency KOR agonists without CNS-related side effects. Our program focuses on the structure–activity relation (SAR) exploration of peptide oligomers as KOR agonists with high topologic polar surface areas (TPSAs), which presumably have limited ability to passively cross the brain–blood barrier (BBB).
Figure 1.

Structures of asimadoline and difelikefalin (CR845).
Based on previous publications, the N-terminal d-phenylamine (Phe) functional group of CR-845 is a key pharmacophore and this area is very sensitive for the binding activity of KOR.14−16 Herein, we focused on exploring alternative novel unnatural amino acids to mimic the key Phe group (Table 1). The initial idea was to introduce cyclized phenylamine tetrahydroisoquinoline 1, but unfortunately the potency decreased completely. Then, the open-chained benzyl amine 2 was shown to have no KOR activity. Subsequently, a cyclization ring afforded 2,3-dihydro-1H-inden-2-amine 3, which was still inactive (EC50 > 1000 pM). Interestingly, the phenethyl amine 4 exhibited high potency by extension of one more carbon chain from compound 2. Further extending the chain to afford 5 with an EC50 = 45 pM satisfied KOR potency. We preferred to select compound 4 considering it had a less flexible side chain that might be better for physicochemical properties. As shown in Table 1, the R2 group with either morphine or amino acid displayed the same level of potency (compounds 6 and 4). Inspired by these results, additional compounds with mono and bis substitutions between the amide group and phenylpropyl amine linker (7 and 8) were designed. Unfortunately, the potency dropped completely. It was speculated that the steric effect close to the amide most likely affected the binding affinity. As a result, a new strategy was adjusted to the other side of the N atom close to the phenyl group. Inserting cyclopropane into the benzyl amine afforded compound 9 to maintain the potency compared with 6. Thus, more effort was focused on the study of substitution groups (10–15). It was found that disubstituted groups, such as cyclopropane or dimethyl (10 and 11), were well-tolerated and the potency was comparable to compound 6. Unexpectedly, introducing the racemic ethyl substitution improved potency (compound 12). This result indicated that the less steric group was more favorable for potency. Subsequently, two mono methyl with R and S configuration compounds were investigated. Interestingly, the R group 14 was more potent than the S configuration 13. Next, the R configuration isopropyl 15 was shown to decrease potency compared with 14. This result further confirmed the impact of steric effects on potency. Finally, the tail group R2 was optimized and it was found that the methyl urea group yielded compound 16 to exhibit the highest potency in this series.
Table 1. Human KOR Agonist in Vitro Activitya.
EC50 values are means of two or more runs with CR845 as positive control for each batch (CR845 KOR EC50 = 2.62 ± 0.75 pM, n = 5).
This optimized pharmacophore residue was also supported by computational docking studies. Based on the overlay of the agonist-bound KOR protein structure (PDB code: 6B73) and the 3D-bound ligand conformation of the tetrapeptide agonist (DIPP-NH2) in DOR co-Xray crystal structure (PDB code: 4RWA), we modeled the bound conformation of compound 16 to KOR, as depicted in Figure 2. The basic nitrogen in the phenethyl amine group makes strong salt bridge interaction with ASP138 and hydrogen bond with TYR320. The phenyl group of phenethyl amine is enclosed by three aromatic and hydrophobic side chains of HIS291, TYR139, and ILE294. This pocket helped the gain of potency of R isomer 14 compared with compound 13 which might be due to a more favorable binding position. The peptidic backbone amide groups make hydrogen bonds with ASP138, GLN115, and TYR312, respectively. The lysine group of compound 16 is within the salt bridge distance to GLU209, while the tail urea motif is largely exposed toward the solvent. Additionally, the methyl urea group seemed to make two interactions in the binding pocket of KOR. One is an intramolecular hydrogen bond with lysine NH, and the other is a dipole interaction with Arg202. These two interactions possibly enhanced the potency.
Figure 2.

Modeled binding pose of compound 16 with agonist bound KOR protein structure.
As an interesting result, compound 16 (SHR0687) was selected for further in vitro and in vivo evaluations. As shown in Table 2, this tetrapeptide displayed excellent selectivity over other opioid receptors, such as MOR and DOR, whose EC50 was >50000 nM in cAMP accumulation assays.
Table 2. In Vitro Potency of SHR0687a.
| Compound ID | SHR0687 |
|---|---|
| MW/AlogP/PSA | 720.9/2.06/186.8 |
| hKOR EC50 (pM) | 0.53 |
| hMOR EC50 (nM) | >50000 |
| hDOR EC50 (nM) | >50000 |
The positive control for hMOR and hDOR test was morphine (hMOR EC50 = 79.12 ± 9.91 nM, n = 3; hDOR EC50 = 203.97 ± 33.51 nM, n = 3).
In addition, SHR0687 was stable in in vitro liver microsomes across species, such as rats, mice, dogs, and humans (Table 3), which correlated well with the in vivo PK results (Table 5). Moreover, the cytochrome P450 inhibition of SHR0687 was investigated to assess the potential likelihood of drug–drug interactions (DDIs) with major CYP isoforms, including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. SHR0687 was found with no obvious CYP450 inhibition with all CYP isoform IC50 values >30 μM. The hERG IC50 of SHR0687 was >30 μM, indicating a higher cardiac safety margin.
Table 3. LMS, CYP, and hERG data of SHR0687.
| Compound ID | SHR0687 |
|---|---|
| LMS t1/2 (min)a | 40 (h); >268 (r); 115 (m); 216 (d) |
| hCYPs IC50 (μM) | All >30 |
| 1A2/2C9/2D6/3A4(m)a/3A4(t)c2C19 | |
| hERG IC50 (patchliner, μM) | >30 |
h = humans, r = rats, m = mice, d = dogs.
m: midazolam.
t: testosterone (FDA recommended 3A4 substrates).
Table 5. Intravenous PK Data of SHR0687a,b.
| Species | Mouse | Rat | Dog |
|---|---|---|---|
| AUC0–t (ng/mL·h) | 1656 | 1553 | 2619 |
| t1/2 (h) | 0.34 | 4.78 | 4.45 |
| CL (mL/min/kg) | 10.1 | 10.5 | 3.1 |
| Vz (mL/kg) | 297 | 4515 | 1236 |
CL is clearance; t1/2 is the half-life of the compound exposure in plasma; AUC is the area under the curve.
The dosage for mouse and rat was 1 mg/kg and for dog was 0.5 mg/kg.
Considering that our goal was discovery of a KOR agonist with good PNS selectivity and avoiding CNS side effects, we investigated the brain permeability of SHR0687 in a rat BBB model using the Kpu,u value for evaluation of CNS penetration.17,18 As summarized in Table 4, the Kpu,u value of SHR0687 was 0.006, suggesting that the BBB penetration capacity of SHR0687 was very limited.
Table 4. BBB Penetration Data of SHR0687 on SD Rat (iv, 1 mg/kg).
| Cmpd | fu_rat plasma | fu_brain tissue | plasma (ng/mL) | Brain tissue (ng/g) | Kpu, u |
|---|---|---|---|---|---|
| SHR0687 | 21.1% | 2.58% | 1144 | 57.2 | 0.006a |
t test (P = 0.007).
As summarized in Table 5, SHR0687 has a favorable PK profile across species with low clearance and good in vivo exposure (AUC = 1656 ng/mL·h on mice; AUC = 1553 ng/mL·h on rats; AUC = 2619 ng/mL·h on dogs). The adequate exposure was helpful for the following rat in vivo pain model study at a relatively lower dosage.
Furthermore, SHR0687 was investigated in a rat carrageenan-induced pain model to evaluate the therapeutic effect.19 In agreement with the high in vitro potency, SHR0687 displayed remarkable efficacious characteristics, even at a 0.03 mg/kg dosage. A clear dose-dependent effect was observed from 0.03 to 0.3 mg/kg (28.9%–66.7%; Figure 3). It is worth mentioning that SHR0687 exhibited comparable or slightly better efficacy at the same dosage (66.7% vs 61.9% @ 0.3 mg/kg) with CR-845 employed as a control group in the same study.
Figure 3.

Efficacy study of SHR0687 on a rat carrageenan-induced pain model. N = 8 rats in each group. Statistical comparisons were performed using the Excel software t test. The data between the model and control groups were analyzed and compared to determine whether there was a significant statistical significance. *P < 0.05 indicates that there is a significant difference between the model and control groups, **P < 0.01 indicates that there is a highly significant difference between the model and control groups, #P < 0.05 indicates that there is a significant difference between the model and control groups, ##P < 0.01 indicates that there is a highly significant difference between the model and the administration groups.
The synthesis of SHR0687 was straightforward, as shown in Scheme 1. Our strategy was to synthesize N and C terminal scaffolds in parallel and then to couple two pieces at the late stage. The N terminal scaffold of methyl urea intermediate 20 was prepared in four steps. Initially, commercially available primary amine 17 was treated with methylcarbamic chloride, followed by deprotection of the Boc group to afford urea substrate 18. Then, 18 was coupled with carboxylic acid 19, followed by removal of the Fmoc protecting group to achieve N terminal substrate 20 in a decent yield. The C terminal scaffold synthesis began with known intermediate 21 (see Supporting Information). Treating intermediate 21 with chloroacetyl chloride yielded compound 22, which was coupled by (R)-2-phenylpropan-1-amine 23 and then followed by Boc protection. Subsequently the benzyl ether group was removed via hydrogenation which afforded carboxylic acid 24 in an overall yield of 82% for four steps. The last two steps were coupling intermediates 24 and 20, followed by removal of the Boc group, with successful production of compound 16 (SHR0687).
Scheme 1. Synthetic Route of SHR0687.
Reagents and conditions: (a) methylcarbamic chloride, DIPEA, DCM, r.t. 2 h; (b) HCl/dioxane, DCM, r.t. 2 h; (c) 19, HATU, Et3N, DMF, r.t. 4 h; (d) Et3N, DMF, r.t. 12 h, 32% four steps; (e) 2-chloroacetyl chloride, Et3N, DCM, r.t. 12 h; (f) 23, KI, K2CO3, DMF, 60 °C 12 h; (g) (Boc)2O, Et3N, DCM, r.t. 12 h; (h) Pd/C, H2, MeOH, r.t. 12 h, 82% four steps; (i) 20, HATU, Et3N, DMF, r.t. 12 h; (j) HCl/dioxane, DCM, r.t. 12 h, 50% two steps.
In summary, SHR0687 exhibited a highly potent KOR agonist with excellent selectivity over other opioid receptors, such as MOR and DOR. This novel tetrapeptide displayed favorable PK profiles across species, as well as robust in vivo efficacy in a rat carrageenan-induced pain model. Notably, SHR0687 showed minimal BBB penetration with an extremely low Kpu,u value, thus indicating marginal CNS-related side effects for the potential therapeutic treatment of pain.
Acknowledgments
We thank Guimei Yang, Tao Liu, Dongdong Bai, Zhendong Xue, Yuchang Mao, Lilin Liu, Anle Zhang, Wenjian Qian, and the entire KOR project team for their contributions.
Glossary
Abbreviations
- MOR
mu-opioid receptor
- KOR
kappa-opioid receptor
- DOR
delta-opioid receptor
- PNS
peripheral nervous system
- CNS
central-nervous system
- BBB
blood–brain barrier
- DCM
dichloromethane
- hERG
human ether-a-go-go related gene
- CYP
cytochrome P450 enzymes
- PK
pharmacokinetics
- HATU
2-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- Boc
t-Butyloxy carbonyl
- DIPEA
N,N-Diisopropylethylamine
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00287.
Synthetic procedures, analytical data, in vitro assay protocols, in vivo pharmacokinetic studies, and in vivo efficacy model details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pasternak G. W. Opiate pharmacology and relief of pain. J. Clin. Oncol. 2014, 32 (16), 1655–1661. 10.1200/JCO.2013.53.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M.; Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci. Res. 1995, 23, 121–145. 10.1016/0168-0102(95)00933-K. [DOI] [PubMed] [Google Scholar]
- Stein C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W.; Pan Y. X. Mu opioids and their receptors: evolution of a concept. Pharmacol. Rev. 2013, 65 (4), 1257–1317. 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea M.; Asim M. F.; Wolber G.; Schmidhammer H. The μ opioid receptor and ligands acting at the μ opioid receptor, as therapeutics and potential therapeutics. Curr. Pharm. Des. 2014, 19 (42), 7415–7434. 10.2174/13816128113199990362. [DOI] [PubMed] [Google Scholar]
- Coop A.; Rice K. C. Role of δ-opioid receptors in biological processes. Drug News Perspect 2000, 13, 481–487. [PubMed] [Google Scholar]
- Cahill C. M.; Taylor A. M.; Cook C.; Ong E.; Morón J. A.; Evans C. J. Does the kappa opioid receptor system contribute to pain aversion?. Front. Pharmacol. 2014, 5, 253. 10.3389/fphar.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H.; Ebata T.; Takamori K.; Miyasato K.; Muramatsu T.; Nakamoto H.; Kurihara M.; Yanagita T.; Suzuki H. Efficacy and safety of a novel κ-agonist for managing intractable pruritus in dialysis patients. Am. J. Nephrol. 2012, 36 (2), 175–183. 10.1159/000341268. [DOI] [PubMed] [Google Scholar]
- Stein C.; Machelska H. Modulation of peripheral sensory neurons by the immune system: Implications for pain therapy. Pharmacol. Rev. 2011, 63, 860–881. 10.1124/pr.110.003145. [DOI] [PubMed] [Google Scholar]
- Albert-Vartanian A.; Boyd M. R.; Hall A. L.; Morgado S. J.; Nguyen E.; Nguyen V. P. H.; Patel S. P.; Russo L. J.; Shao A. J.; Raffa R. B. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential?. J. Clin. Pharm. Ther. 2016, 41 (4), 371–382. 10.1111/jcpt.12404. [DOI] [PubMed] [Google Scholar]
- Mangel A. W.; Williams V. S. Asimadoline in the treatment of irritable bowel syndrome. Expert Opin. Invest. Drugs 2010, 19, 1257–1264. 10.1517/13543784.2010.515209. [DOI] [PubMed] [Google Scholar]
- Vanderah T. W.; Schteingart C. D.; Trojnar J.; Junien J. L.; Lai J.; Rivier P. J.M. Fe200041 (D-Phe-D-Phe-D-Nle-D-Arg-NH2): A peripheral efficacious κ opioid agonist with unprecedented selectivity. J. Pharmacol. Exp. Ther. 2004, 310, 326–333. 10.1124/jpet.104.065391. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. https://clinicaltrials.gov/ (accessed on April 20, 2020), Identifier: NCT01361568, NCT00877799, NCT01789476, NCT02944448.
- Dooley C. T.; Ny P.; Bidlack J. M.; Houghten R. A. Selective ligands for the μ, δ, and κ opioid receptors identified from a single mixture based tetrapeptide positional scanning combinatorial library. J. Biol. Chem. 1998, 273, 18848–18856. 10.1074/jbc.273.30.18848. [DOI] [PubMed] [Google Scholar]
- Chavkin C.; Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc. Natl. Acad. Sci. U. S. A. 1981, 78 (10), 6543–6547. 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung F. T.; Meyer J. P.; Li G.; Lou B. S.; Stropova D.; Davis P.; Yamamura H. I.; Porreca F.; Hruby V. J. Highly κ receptor-selective Dynorphin A analogues with modifications in position 3 of Dynorphin A(1 - 11)-NH2. J. Med. Chem. 1995, 38, 585–586. 10.1021/jm00004a002. [DOI] [PubMed] [Google Scholar]
- Wan H.; Rehngren M.; Giordanetto F.; Bergström F.; Tunek A. High-throughput screening of drug brain tissue binding and in silico prediction for assessment of CNS drug delivery. J. Med. Chem. 2007, 50, 4606–4615. 10.1021/jm070375w. [DOI] [PubMed] [Google Scholar]
- Liu H.; Dong K.; Zhang W.; Summerfield S. G.; Terstappen G. C. Prediction of brain: blood unbound concentration ratios in CNS drug discovery employing in silico and in vitro model systems. Drug Discovery Today 2018, 23 (7), 1357–1372. 10.1016/j.drudis.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Barrot M. Tests and models of nociception and pain in rodents. Neuroscience 2012, 211, 39–50. 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.