Abstract

Bromodomain-containing protein 4 (Brd4) plays a critical regulatory role in gene transcription that has been recently recognized as a promising strategy for cancer therapy. Based on the BRD4 protein containing two tandem bromodomain structures, BD1 and BD2, we designed and synthesized a series of 3,5-dimethylisoxazole derivative dimers targeting both bromodomains simultaneously to enhance protein binding potency. Among them, compound 22 significantly inhibited the proliferation of colorectal cancer cells HCT116 (IC50 = 162 nM), with a 20-fold increase in antiproliferative activity compared to inhibitor 14. The results of WesternBlot showed that compound 22 could down-regulate c-MYC protein levels and up-regulate HEXIM1 expression and modulate apoptosis through intrinsic pathways. In addition, compound 22 exhibited outstanding antitumor efficacy in the CT-26 tumor mouse model with a tumor suppression rate of 56.1%. Taken together, 3,5-dimethylisoxazole derivative dimer 22 has remarkable protein inhibitory effect and antitumor activity in vitro and in vivo. A protein binding model of compound 22 is being further analyzed, which will facilitate the development of bivalent BRD4 inhibitors and probe the biological function of BRD4.
Keywords: BRD4 inhibitors, colorectal cancer, bivalent ligands, epigenetics
The bromodomain (BRD)-containing protein plays a critical role in recognizing epigenetic acetylated lysine marks on histones.1 In humans, 46 proteins contain bromodomains segregated into eight groups.2 Among them, the bromodomain and extra-terminal domain (BET) family of proteins, consisting of BRD2, BRD3, BRD4, and testis-specific BRDT members share a common domain architecture, two N-terminal bromodomains and an extra-terminal domain.3−5 It has been proposed that BET proteins are crucial for cancer pathogenesis via modulating diverse transcriptional programs.6−8
BRD4 has been identified highly enriched in large clusters of enhancers that drive the expression of genes involved in the pathogenesis of cancer.9,10 Recent studies have shown that BRD4 directly regulates the expression of MYC genes as a therapeutic target in cancer.11,12 Further, BRD4 protein plays an essential role in recruiting the positive transcription elongation factor p-TEFb complex (CDK9 and cyclin T1), which is required for RNA polymerase II-dependent transcription elongation.13−15 Recently, a number of BRD4 inhibitors which target BET family proteins for cancer therapy have been reported, including JQ1 (1), OTX015 (2), ABBV075 (8), etc. (Figure 1).16−19 To our knowledge, most of the reported BRD4 inhibitors do not show selectivity for individual family members and bind BRD4 protein in a monovalent fashion.20 Almost at the same time, Tanaka and Waring disclosed the first bivalent BRD4 molecule inhibitor, MT1 (6) and AZD5153 (7), respectively, exhibiting promising biological activities in vitro and in vivo.17,20 MT1 is an intramolecular bivalent BRD4 binder with more potent proliferation activity than the corresponding monovalent antagonist, JQ1.17 However, AZD5153, a clinical candidate bivalent BET inhibitor, is a single inhibitor molecule containing two acetyl lysine mimicking moieties simultaneously engaging both bromodomains.21 Given that no bivalent BRD4 inhibitors are currently available in clinics or in the market, developing bivalent BRD4 inhibitors with diverse core structures and excellent pharmacological profile is essential for therapeutic studies.
Figure 1.
Selected examples of known BET inhibitors.
Here we detailed the identification and characterization of a new class of bivalent BET inhibitors containing the 3,5-dimethylisoxazole motif. We found that compound 22 was a highly potent and efficacious bivalent inhibitor against colorectal cancer, which was also stronger than its monovalent counterparts.
Our previously reported quinoxalinone BRD4 inhibitor, compound 5i, effectively inhibited BRD4 protein (IC50 = 73 nM).22 The docking model showed that the ethyl in compound 5i extended into the solvent region outside the pocket. Compound 5i had no room for optimization and was not suitable for further development as a lead compound (Figure 2). We used the skeleton hopping technique to replace the quinoxalinone with a quinazolinone motif to obtain compound 14 with BRD4 protein activity IC50 = 32 nM, which was remarkably higher than that of compound 5i. Molecular docking models implied that the 3-nitrogen atom of compound 14 faced the extrapocket solvent region, which provided an advantage for further design of bivalent ligand (Figure 3). Therefore, the above results suggested that compound 14 deserved further development for the design of dimerized BRD4 inhibitors.
Figure 2.
Design and modification strategy of our lead compound 14.
Figure 3.

Docking pose for compound 14 without specific chirality (yellow, A) in BRD4(1) protein. (B) Compounds 14 without specific chirality (yellow) and 5i (green) were superimposed in the docking models (PDB code 2YEL).
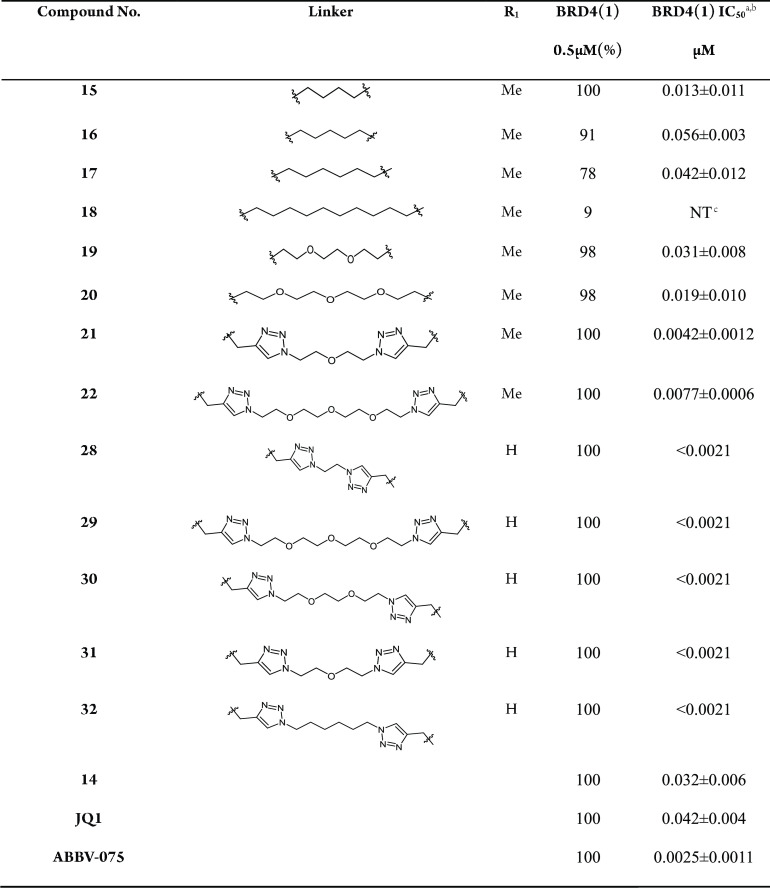
First, we selected carbon chains, polyethylene glycol chains, and triazole polyethylene glycol chains as the linkers and obtained dimeric BRD4 inhibitors 15–22 by connecting two molecules of compounds 14. Interestingly, compounds containing triazole as linker (21, 22) exhibited outstanding activities against BRD4 protein, which was greatly superior to that of other types of dimer inhibitors. Subsequently, compounds 28–32 were designed and synthesized by further optimization, including the removal of methyl group, the introduction of carbon chains, etc. It was noteworthy that, compared to compounds 21 and 22, compounds 28–32 showed considerably more potent BRD4 inhibition activities (IC50 < 2.1 nM).
Compounds 15–22 were synthesized as shown in Scheme 1. Bromination of the starting material 2-aminobenzophenone, followed by amine reduction with tert-butyl subsulfinate, led to the formation of intermediate 10. Then, compound 10 was reduced using sodium borohydride to give 11, and subsequent treatment with triphosgene afforded the quinazolinone 12, which was then subjected to methylation to give the key intermediate compound 13. Finally, the target compound 14 was prepared by Suzuki cross-coupling reaction to introduce the 3,5-dimethylisoxazole group. Compound 14 was treated with 1,4-dibromobutane, 1,5-dibromopentane, 1,6-dibromohexane, and 1,10-dibromodecane, respectively, to gain compounds 15–18. In addition, compound 14 reacted with triethylene glycol ditoluenesulfonate or tetraethylene glycol ditoluenesulfonate to obtain compounds 19–20. Compounds 21–22 were yielded by the reaction of compound 14 with 3-bromopropyne, followed by a click reaction with N3-PEG1-N3 or N3-PEG3-N3.
Scheme 1.
Reagents and conditions: (a) NBS, DCM, 0 °C; (b) tert-butanesulfinamide, tetraethyl titanate, THF, reflux; (c) NaBH4, THF, H2O, r.t.; (d) bis(trichloromethyl) carbonate, THF, r.t.; (e) dimethylsulfate: Cs2CO3, acetonitrile, r.t.; (f) phenylboronic acids, Pd(P(Ph)3)4, Na2CO3, N2, H2O/EtOH/toluene, 80 °C; (g) diverse linkers, NaH, DMF, 0 °C to r.t.; (h) 3-bromopropyne, Cs2CO3, DMF, 0 °C to r.t.; (i) diverse linkers, CuSO4, sodium erythorbate, MeOH, H2O, r.t.
The synthesis of compounds 28–32 was described in Scheme 2. Compound 9 was used as the starting material to obtain compound 23 via Suzuki cross-coupling reaction, which was treated with tert-butylenesulfinate to produce intermediates 24. Then, compound 24 was reduced by sodium borohydride to produce compound 25, followed by substitution via 3-bromopropyne to generate intermediate 26. The key intermediate 27 was prepared by treatment of compound 26 with triphosgene. Lastly, intermediate 27 was reacted with different linkers to obtain the target compounds 28–32 via click reaction.
Scheme 2.

Reagents and conditions: (a) phenylboronic acids, Pd(P(Ph)3)4, Na2CO3, N2, H2O/EtOH/toluene, 80 °C; (b) tert-butanesulfinamide, tetraethyl titanate, THF, reflux; (c) NaBH4, THF, H2O, r.t.; (d) 3-bromopropyne, NaH, DMF, 0 °C to r.t.; (e) bis(trichloromethyl)carbonate, THF, r.t.; (f) diverse linkers, CuSO4, sodium erythorbate, MeOH, H2O, r.t.
In the initial phase of our study, compounds 15–22 were designed and synthesized using compound 14 as the lead compound. The results of Alphascreen BRD4 assay demonstrated that most of the compounds showed more potent activities than the lead compound 14. Among them, the compounds (21, 22) containing triazoles displayed prominent inhibitory activities with BRD4(1) IC50 = 4.2 nM and 7.7 nM, respectively. The BRD4 inhibitory activities of compounds 19 and 20 using polyethylene glycol as the linker were more valid than that of analogs with other linkers. Furthermore, compounds 15–18 with carbon chains as the linker exhibited relatively weak inhibitory activities, except for compound 15 (Table 1).
Table 1. BRD4(1) Inhibitory Activities of Compounds 14–22 and 28–32.
Data are expressed as the mean ± SD from the dose–response curves of at least three independent experiments.
IC50 values are shown as mean values of at least three determinations unless specified otherwise.
Not tested.
Based on the above activity results, we further optimized the bivalent inhibitors (21, 22) using linkers containing a triazole group. The methyl on the nitrogen atom was also removed, and the polyethylene glycol chain was replaced by a carbon chain to produce compounds 28–32. The BRD4(1) protein activities of compounds 28–32 (BRD4(1) IC50 < 2.1 nM) were much superior to those of other bivalent compounds (Table 1).
The binding model of compound 14 and BRD4BD1 protein was predicted, and the molecular docking model showed that the 3,5-dimethylisoxazole group in compound 14 could bind to the Kac pocket of the BRD4 bromodomain as a Kac mimic. The nitrogen atom in 3,5-dimethylisoxazole formed a hydrogen bond to the key amino acid ASN140 located in the Kac pocket, anchoring compound 14 in the Kac pocket. The benzene ring at position 4 extended into the WPF hydrophobic pocket formed by Trp81, Pro82, and Phe83. In addition, the nitrogen atom at the 3-position of compound 14 faced the solvent region outside the pocket, providing a modification site for postmodification optimization. Compound 14 overlapped perfectly with our previously announced compound 5i. The monomer moiety (compound 14) of bivalent ligand 22 could manage to bind to the BRD4Kac pocket, which was similar to the binding pattern of single-molecule compound 14. 3,5-Dimethylisoxazole filled the Kac pocket forming a hydrogen bond with Asn140, and the 4-position benzene ring extended into the WPF hydrophobic pocket formed by Trp81, Pro82, and Phe83. In addition, the triazole moiety formed weak π–π stacking interactions with the 4-position benzene ring located in the WPF shelf and Trp81. Another monomer moiety connected with nitrogen atoms at the 3-position via a linker extended into the outer solvent region of the BRD4Kac pocket (Figure 4). Moreover, we speculated that ligand 22 could bind to another bromodomain in tandem according to a previous report, enhancing protein binding activity.17
Figure 4.

Docking pose for compound 22 without specific chirality (purple, A) in BRD4(1) protein. Compounds 14 without specific chirality (yellow) and 22 without specific chirality (purple) were superimposed in the docking models (PDB code 2YEL).
Considering that the above compounds have noticeable BRD4 protein inhibitory activities, we evaluated the antiproliferative activities of all compounds 14–22 and 28–32 at the cellular level. Primary screening tests were performed on pancreatic cancer cells BxPc3, Mia-capa2, and colorectal cancer cells HCT116, respectively.23,24 As a result, it was found that both pancreatic and colon cancer cells were sensitive to most compounds.
In particular, compounds 17 and 22 exhibited exceptional activities against all three tumor cell lines, with an ideal inhibition rate of 59.7% and 83.6% at 2 μM against colon cancer cell HCT116, respectively. However, compound 14 only exhibited a 14% inhibition rate against HCT116 at 2 μM. Therefore, antiproliferative activities of compounds 17 and 22 against HCT116 cells were further tested at gradient concentrations. The results indicated that compounds 17 and 22 had antiproliferative potency with an IC50 value of 1.1 μM and 0.16 μM against HCT116 cells, respectively (Table 2). Both compounds 17 and 22 exhibited significantly more potent antiproliferative activities than the lead compound 14, and particularly the inhibitory activity of compound 22 was 20-fold more than that of compound 14. In addition, the antiproliferative activity of compound 22 was better than that of the positive compounds JQ1 and ABBV-075. Thus, our next task was to further investigate the biological mechanisms of compound 22.
Table 2. Anti-proliferation Activities of Compounds 14–22 and 28–32.
| Compound No. | BxPc3 2.5 μM (%) | Mia-capa2 2.5 μM (%) | HCT116 2.0 μM (%) | HCT116 IC50 (μM)ab |
|---|---|---|---|---|
| 15 | 46.4 | 68.2 | 59.3 | NTc |
| 16 | 27.9 | 71.3 | 52.0 | NT |
| 17 | 44.0 | 70.9 | 59.7 | 1.101 ± 0.023 |
| 18 | 45.2 | 65.2 | 48.8 | NT |
| 19 | 7.1 | 67.3 | 43.4 | NT |
| 20 | 42.1 | 73.4 | 35.3 | NT |
| 21 | 23.2 | 64.0 | 49.6 | NT |
| 22 | 61.3 | 68.4 | 83.6 | 0.162 ± 0.012 |
| 28 | 53.0 | 62.2 | 60.3 | NT |
| 29 | 42.2 | 66.4 | 60.4 | NT |
| 30 | 21.8 | 63.2 | 60.5 | NT |
| 31 | 52.1 | 54.4 | 55.6 | NT |
| 32 | 37.1 | 57.2 | 60.1 | NT |
| 14 | 11.2 | 59.1 | 14.0 | 3.341 ± 0.011 |
| JQ1 | 9.1 | 56.2 | 23.2 | 4.212 ± 0.015 |
| ABBV-075 | 36.8 | 64.3 | 69.5 | 0.550 ± 0.009 |
Data are expressed as the mean ± SD from the dose–response curves of at least three independent experiments.
IC50 values are shown as mean values of at least three determinations unless specified otherwise.
Not tested.
The cell cycle arrest potency of compound 22 was evaluated in HCT116 cells. Cell cycle arrest experiments revealed that compound 22 dramatically increased the percentage of HCT116 cells in the G0/G1 phase at 0.16 μM for 48 h. The G0/G1 phase arrest effect of compound 22 was significantly more than that of the lead compound 14 (Figure 5).
Figure 5.

Effects of compounds 22 and 14 on the cell cycle arrest of HCT116 cells.
We further explored the potency of compound 22 on clone-forming against HCT116 cells. The results showed that 22 significantly inhibited the proliferation of HCT116 cells in a concentration-dependent manner for 12 days. In addition, the effect of compound 22 on suppressing the proliferation of HCT116 cells notably exceeded the lead compound 14 (Figure 6).
Figure 6.

Plate clone-forming effects of compounds 22 and 14 in HCT116 cell lines.
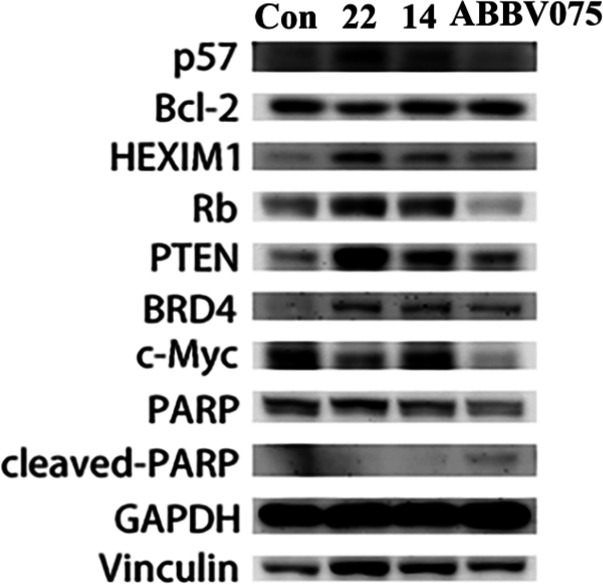
Because compound 22 exhibited excellent biological activity at both protein and cellular levels, we moved on to understand its intrinsic mechanism of inducing apoptosis in HCT116 cells. After treatment of HCT116 cells with compound 22 for 48 h, the contents of relevant proteins such as Bcl-2, HEXIM1, BRD4, and c-Myc were determined by WesternBlot. The results showed that compound 22 was able to significantly down-regulate c-Myc protein expression and decreased Bcl-2 protein expression in HCT116 cells compared to lead compound 14. In addition, compound 22 remarkably up-regulated the expression level of HEXIM1 protein and enriched BRD4 protein, consistent with the findings reported in previous literature (Figure 7).25,26 Moreover, compound 22 could activate downstream apoptotic effectors such as caspase 3 (Figure S1). Mechanism studies indicated that compound 22 promoted apoptosis in HCT116 cells via an intrinsic pathway, which was conducive to further study of the mechanism of BET protein in cells.
Figure 7.
Effects of compounds 22 (1 μM), 14 (1 μM), and ABBV-075 (1 μM) on protein expression in HCT116 cancer cells.
Based on the inhibitory activity of 22 against human colorectal cancer cell line HCT116, the effect of compound 22 on tumor growth inhibition in vivo was further investigated in the CT-26 (mouse colorectal cancer cell line) model mouse. The results showed that the intraperitoneal administration of compound 22 at 20 mg/kg for 14 days prominently inhibited tumor growth in CT-26 model mice, and the tumor suppression rate was 56.1%, significantly superior to 11.5% of the lead compound 14 (Figures 8 and S2). There were also no serious toxic side effects in the tumor mice and no obvious weight loss in the rats, confirming the feasibility of bivalent BET inhibitors. In addition, we conducted immunohistochemical analysis of tumor tissue in sections. The results demonstrated that compound 22 notably promoted the expression of HEXIM1 and decreased the expression of c-Myc and KI67.27−29 Meanwhile, HE staining implied the promotion of morphological changes in cell necrosis (Figure 9). In addition, the pharmacokinetic parameters of compound 22 were predicted using SwissADME and Discovery Studio 3.0, which indicated that compound 22 showed poor absorption and bioavailability (Figure S3 and Table S1).30,31
Figure 8.
Anticancer effects of compound 22 in CT-26 xenograft model of colorectal cancer.
Figure 9.
Immunohistochemistry of CT-26 tumor tissues treated with compound 22.
Taken together, based on the BRD4 inhibitor compound 5i previously reported by our group, we designed and synthesized the lead compound 14 utilizing the skeleton-hopping principle, and the BRD4(1) protein activity of compound 14 significantly increased with an IC50 value of 32 nM. Since the structural characteristics of BRD4 contained two tandem bromodomains, we designed and synthesized a series of bivalent BRD4 inhibitors by using different types and lengths of linkers for connecting two molecules 14. Among them, compound 22 exhibited excellent BRD4 protein inhibitory activity with an IC50 value of 7.7 nM and showed significant antiproliferative activity with an IC50 value of 162 nM against HCT116 cells. Meanwhile, compound 22 resulted in cell cycle arrest at the G0/G1 phase. WesternBlot results indicated that compound 22 interfered with apoptosis via intrinsic pathways. CT26 mouse model in vivo experiments implied that compound 22 effectively inhibited tumor growth in vivo with a tumor suppression rate of 56.1%. In addition, immunohistochemistry results showed that compound 22 achieved antitumor effects in vivo by inhibiting the BRD4 signaling pathway and thus might be a powerful chemical probe for further investigation of BRD4 bromodomains.
Glossary
Abbreviations
- Brd4
bromodomain-containing protein 4
- HEXIM1
hexamethylene bis-acetamide inducible protein
- MYC
myelocytomatosis oncogene
- pTEFb
positive transcription elongation factor b
- BET
bromodomain and extraterminal domain
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00294.
Compound synthesis, spectroscopic characterization, and biology experimental procedures (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This study was supported by the Natural Science Foundation of Jiangsu Province (No. BK 20141349), the China National Key HiTech Innovation Project for the R&D of Novel Drugs (No. 2013ZX09301303-002), and China Scholarship Council (201907060014).
The authors declare no competing financial interest.
Supplementary Material
References
- Liu Z.; Wang P.; Chen H.; Wold E. A.; Tian B.; Brasier A. R.; Zhou J. Drug Discovery Targeting Bromodomain-Containing Protein 4. J. Med. Chem. 2017, 60, 4533–4558. 10.1021/acs.jmedchem.6b01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C. A. Pathogenesis of NUT midline carcinoma. Annu. Rev. Pathol.: Mech. Dis. 2012, 7, 247–65. 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- Wyce A.; Ganji G.; Smitheman K. N.; Chung C. W.; Korenchuk S.; Bai Y.; Barbash O.; Le B.; Craggs P. D.; McCabe M. T.; Kennedy-Wilson K. M.; Sanchez L. V.; Gosmini R. L.; Parr N.; McHugh C. F.; Dhanak D.; Prinjha R. K.; Auger K. R.; Tummino P. J. BET inhibition silences expression of MYCN and BCL2 and induces cytotoxicity in neuroblastoma tumor models. PLoS One 2013, 8, e72967 10.1371/journal.pone.0072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez R.; Meslamani J.; Zhou M. M. The bromodomain: from epigenome reader to druggable target. Biochim. Biophys. Acta, Gene Regul. Mech. 2014, 1839, 676–85. 10.1016/j.bbagrm.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure M.; Crews C. M. Small-Molecule PROTACS: New Approaches to Protein Degradation. Angew. Chem., Int. Ed. 2016, 55, 1966–73. 10.1002/anie.201507978. [DOI] [PubMed] [Google Scholar]
- Chapuy B.; McKeown M. R.; Lin C. Y.; Monti S.; Roemer M. G.; Qi J.; Rahl P. B.; Sun H. H.; Yeda K. T.; Doench J. G.; Reichert E.; Kung A. L.; Rodig S. J.; Young R. A.; Shipp M. A.; Bradner J. E. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell 2013, 24, 777–90. 10.1016/j.ccr.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Wang Y.; Zeng L.; Wu Y.; Deng J.; Zhang Q.; Lin Y.; Li J.; Kang T.; Tao M.; Rusinova E.; Zhang G.; Wang C.; Zhu H.; Yao J.; Zeng Y. X.; Evers B. M.; Zhou M. M.; Zhou B. P. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 2014, 25, 210–25. 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmore J. E.; Issa G. C.; Lemieux M. E.; Rahl P. B.; Shi J.; Jacobs H. M.; Kastritis E.; Gilpatrick T.; Paranal R. M.; Qi J.; Chesi M.; Schinzel A. C.; McKeown M. R.; Heffernan T. P.; Vakoc C. R.; Bergsagel P. L.; Ghobrial I. M.; Richardson P. G.; Young R. A.; Hahn W. C.; Anderson K. C.; Kung A. L.; Bradner J. E.; Mitsiades C. S. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–17. 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. A.; Conery A. R.; Bryant B. M.; Sandy P.; Balasubramanian S.; Mele D. A.; Bergeron L.; Sims R. J. 3rd. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16669–74. 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A.; Rodger E. J.; Eccles M. R. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol. 2018, 51, 149–159. 10.1016/j.semcancer.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Hogg S. J.; Vervoort S. J.; Deswal S.; Ott C. J.; Li J.; Cluse L. A.; Beavis P. A.; Darcy P. K.; Martin B. P.; Spencer A.; Traunbauer A. K.; Sadovnik I.; Bauer K.; Valent P.; Bradner J. E.; Zuber J.; Shortt J.; Johnstone R. W. BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1. Cell Rep. 2017, 18, 2162–2174. 10.1016/j.celrep.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Qian Y.; Altieri M.; Dong H.; Wang J.; Raina K.; Hines J.; Winkler J. D.; Crew A. P.; Coleman K.; Crews C. M. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem. Biol. 2015, 22, 755–63. 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Smith S. G.; Zhou M. M. Discovery of Chemical Inhibitors of Human Bromodomains. Chem. Rev. 2015, 115, 11625–68. 10.1021/acs.chemrev.5b00205. [DOI] [PubMed] [Google Scholar]
- Boi M.; Gaudio E.; Bonetti P.; Kwee I.; Bernasconi E.; Tarantelli C.; Rinaldi A.; Testoni M.; Cascione L.; Ponzoni M.; Mensah A. A.; Stathis A.; Stussi G.; Riveiro M. E.; Herait P.; Inghirami G.; Cvitkovic E.; Zucca E.; Bertoni F. The BET Bromodomain Inhibitor OTX015 Affects Pathogenetic Pathways in Preclinical B-cell Tumor Models and Synergizes with Targeted Drugs. Clin. Cancer Res. 2015, 21, 1628–38. 10.1158/1078-0432.CCR-14-1561. [DOI] [PubMed] [Google Scholar]
- Li Y.; Sabari B. R.; Panchenko T.; Wen H.; Zhao D.; Guan H.; Wan L.; Huang H.; Tang Z.; Zhao Y.; Roeder R. G.; Shi X.; Allis C. D.; Li H. Molecular Coupling of Histone Crotonylation and Active Transcription by AF9 YEATS Domain. Mol. Cell 2016, 62, 181–193. 10.1016/j.molcel.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyasen G. W.; Hattersley M. M.; Yao Y.; Dulak A.; Wang W.; Petteruti P.; Dale I. L.; Boiko S.; Cheung T.; Zhang J.; Wen S.; Castriotta L.; Lawson D.; Collins M.; Bao L.; Ahdesmaki M. J.; Walker G.; O’Connor G.; Yeh T. C.; Rabow A. A.; Dry J. R.; Reimer C.; Lyne P.; Mills G. B.; Fawell S. E.; Waring M. J.; Zinda M.; Clark E.; Chen H. AZD5153: A Novel Bivalent BET Bromodomain Inhibitor Highly Active against Hematologic Malignancies. Mol. Cancer Ther. 2016, 15, 2563–2574. 10.1158/1535-7163.MCT-16-0141. [DOI] [PubMed] [Google Scholar]
- Tanaka M.; Roberts J. M.; Seo H. S.; Souza A.; Paulk J.; Scott T. G.; DeAngelo S. L.; Dhe-Paganon S.; Bradner J. E. Design and characterization of bivalent BET inhibitors. Nat. Chem. Biol. 2016, 12, 1089–1096. 10.1038/nchembio.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLure K. G.; Gesner E. M.; Tsujikawa L.; Kharenko O. A.; Attwell S.; Campeau E.; Wasiak S.; Stein A.; White A.; Fontano E.; Suto R. K.; Wong N. C.; Wagner G. S.; Hansen H. C.; Young P. R. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One 2013, 8, e83190 10.1371/journal.pone.0083190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P.; Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat. Rev. Drug Discovery 2014, 13, 337–56. 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- Waring M. J.; Chen H.; Rabow A. A.; Walker G.; Bobby R. Potent and selective bivalent inhibitors of BET bromodomains. Nat. Chem. Biol. 2016, 12, 1097–1104. 10.1038/nchembio.2210. [DOI] [PubMed] [Google Scholar]
- Bradbury R. H.; Callis R.; Carr G. R.; Chen H.; Clark E.; Feron L.; Glossop S.; Graham M. A.; Hattersley M.; Jones C.; Lamont S. G.; Ouvry G.; Patel A.; Patel J.; Rabow A. A.; Roberts C. A.; Stokes S.; Stratton N.; Walker G. E.; Ward L.; Whalley D.; Whittaker D.; Wrigley G.; Waring M. J. Optimization of a Series of Bivalent Triazolopyridazine Based Bromodomain and Extraterminal Inhibitors: The Discovery of (3R)-4-[2-[4-[1-(3-Methoxy-[1,2,4]triazolo[4,3-b]pyridazin-6-yl)-4-piperidyl]phenoxy]ethyl]-1,3-dimethyl-piperazin-2-one (AZD5153). J. Med. Chem. 2016, 59, 7801–17. 10.1021/acs.jmedchem.6b00070. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhao L.; Xu B.; Yang L.; Zhang J.; Zhang H.; Zhou J. Design, synthesis and biological evaluation of dihydroquinoxalinone derivatives as BRD4 inhibitors. Bioorg. Chem. 2016, 68, 236–44. 10.1016/j.bioorg.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Li R.; Xiao H.; Liu W.; Zeng X.; Xie G.; Yang W.; Shi L.; Yin Y.; Tao K. BRD4 Inhibitor AZD5153 Suppresses the Proliferation of Colorectal Cancer Cells and Sensitizes the Anticancer Effect of PARP Inhibitor. Int. J. Biol. Sci. 2019, 15, 1942–1954. 10.7150/ijbs.34162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.; Huang Z.; Long D.; Jin W. BET inhibitor bromosporine enhances 5-FU effect in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2020, 521, 840–845. 10.1016/j.bbrc.2019.11.009. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Chen P.; Zhao L.; Zhang F.; Zhang B.; Xu C.; Zhang H.; Zhou J. Exploiting the 7-methylimidazo[1,5-a]pyrazin-8(7H)-one scaffold for the development of novel chemical inhibitors for Bromodomain and Extraterminal Domain (BET) family. Bioorg. Chem. 2019, 90, 103044. 10.1016/j.bioorg.2019.103044. [DOI] [PubMed] [Google Scholar]
- Chen P.; Yang Y.; Yang L.; Tian J.; Zhang F.; Zhou J.; Zhang H. 3-Hydroxyisoindolin-1-one derivates: Synthesis by palladium-catalyzed CH activation as BRD4 inhibitors against human acute myeloid leukemia (AML) cells. Bioorg. Chem. 2019, 86, 119–125. 10.1016/j.bioorg.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Ba M.; Long H.; Yan Z.; Wang S.; Wu Y.; Tu Y.; Gong Y.; Cui S. BRD4 promotes gastric cancer progression through the transcriptional and epigenetic regulation of c-MYC. J. Cell. Biochem. 2018, 119, 973–982. 10.1002/jcb.26264. [DOI] [PubMed] [Google Scholar]
- Bowry A.; Piberger A. L.; Rojas P.; Saponaro M.; Petermann E. BET Inhibition Induces HEXIM1- and RAD51-Dependent Conflicts between Transcription and Replication. Cell Rep. 2018, 25, 2061–2069. (e4) 10.1016/j.celrep.2018.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. J.; Stubbs M.; Liu P.; Ruggeri B.; Khabele D. The BET inhibitor INCB054329 reduces homologous recombination efficiency and augments PARP inhibitor activity in ovarian cancer. Gynecol. Oncol. 2018, 149, 575–584. 10.1016/j.ygyno.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Shi C. Y.; Xie J.; Dai J. H.; He S. L. Identification of Potential Dipeptidyl Peptidase (DPP)-IV Inhibitors among Moringa oleifera Phytochemicals by Virtual Screening, Molecular Docking Analysis, ADME/T-Based Prediction, and In Vitro Analyses. Molecules 2020, 25, 189. 10.3390/molecules25010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.