Abstract
Fibrosis is a common feature of several diseases, involves different organs, and results in significant morbidity and mortality. There are currently no effective therapies to halt the progression of fibrosis or reverse it. We have identified the highly water-soluble MMS-350, a novel bis-oxetanyl sulfoxide, as an antifibrotic agent. MMS-350 reduced the profibrotic phenotype induced in vitro in primary human fibroblasts and ameliorated bleomycin-induced pulmonary fibrosis in vivo. Furthermore, MMS-350 reversed fibrosis in human skin in organ culture. MMS-350 reduced levels of extracellular matrix proteins, the activation of fibroblasts, and the induction of pro-fibrotic factors. Similar effects at lower concentrations were observed with KRL507-031 and CL-613-091, two more lipophilic MMS-350 analogues. The fact that MMS-350 was effective at reducing pulmonary fibrosis induced by different triggers, the differential biological effects of its close structural analogues and its oral availability make it an attractive therapeutic candidate for organ fibrosis.
Keywords: Fibrosis, MMS-350, matrix proteins, TGFβ1
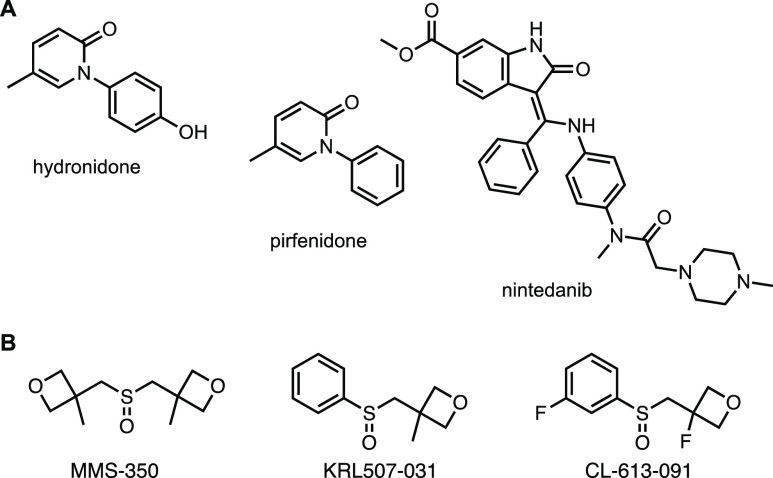
Fibrosis is a terminal complication of several diseases of unknown etiology such as systemic sclerosis (SSc), idiopathic pulmonary fibrosis, and conditions triggered by environmental agents or viral infections.1 Organ fibrosis is an irreversible end point of these morbidities, leading to organ failure and contributing to at least 45% of all deaths in industrialized countries.2 Systemic sclerosis, or scleroderma, is a connective tissue disease of unknown etiology. The hallmarks of SSc include fibrosis of the skin and internal organs. Lung involvement in SSc is currently the leading cause of death.3 No effective cures are available for scleroderma-associated and idiopathic pulmonary fibrosis, and the only option is transplantation. However, due to the substantial clinical need, drugs targeting fibrosis are intensely researched.4 Most commonly, extracellular factors, such as tumor necrosis factor (TNF), transforming growth factor (TGF)β signaling, or receptor tyrosine kinases are targeted by agents such as thalidomide, hydronidone and nintedanib, respectively. Other antifibrotic agents, such as pirfenidone, target intracellular TGFβ signaling or broader anti-inflammatory processes (Figure 1).
Figure 1.
(A) Antifibrotic drugs approved or in clinical trials and (B) experimental agents described in this report.
We recently developed a small organic compound, MMS-350, as a radiation protector and mitigator.5 MMS-350 is a novel, highly water-soluble bis-oxetanyl sulfoxide that can also serve as an additive to improve the aqueous solubility of hydrophobic organic molecules.6 Significantly, MMS-350 ameliorated ionizing radiation-induced lung fibrosis7 and profoundly reduced late-stage fibrosis in a thoracic irradiation model.8 While the molecular mechanism of action of MMS-350 is not yet known, we found that irradiated cells treated with MMS-350 experienced a drop in radiation-induced DNA strand breaks, reduced apoptotic cell death, and changes in radiation-induced G1/S- and G2/M cell cycle arrests.7
Our goal was to further examine the effects of MMS-350 and structurally related analogues in primary human fibroblasts treated with the most potent trigger of fibrosis, TGFβ1, in mice in which experimental fibrosis was induced using bleomycin, and in human skin in organ culture. Our findings suggest that MMS-350 and its analogues differentially ameliorate the fibrotic phenotype both in vitro and in vivo and, thus, this chemotype may provide viable candidates for the development of a therapy for pulmonary fibrosis.
MMS-350 Reduces Fibrotic Markers Induced by TGFβ1 In Vitro
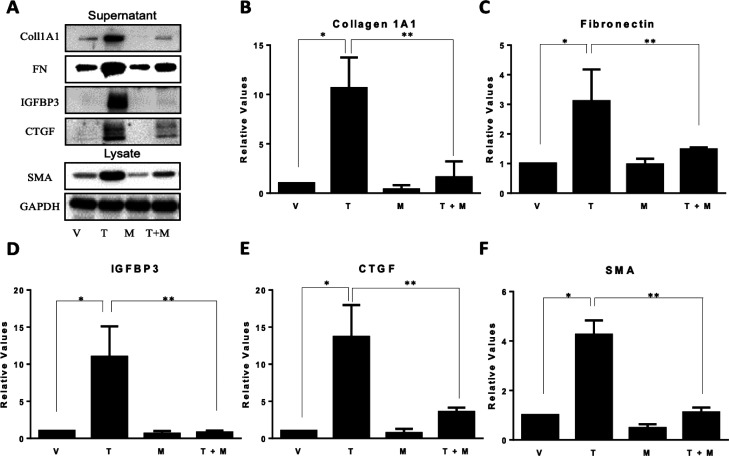
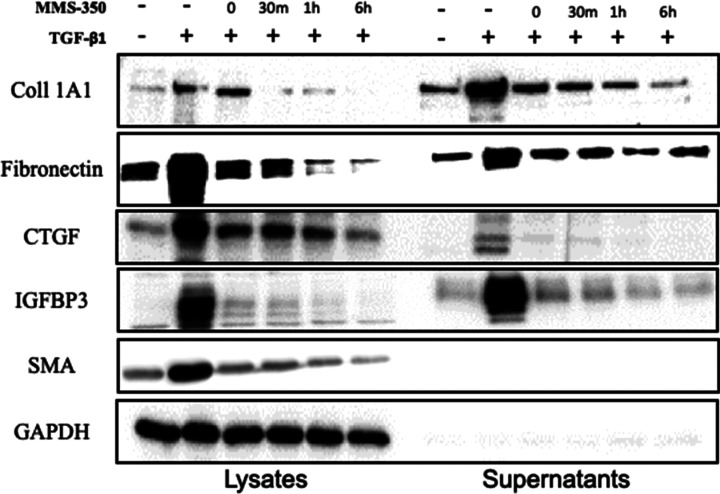
To determine if MMS-350 had an effect on fibrotic markers, normal human primary lung fibroblasts were treated with TGFβ1 and MMS-350 at the same time. MMS-350 displayed efficacy by decreasing protein concentrations of the extracellular matrix (ECM) components fibronectin (FN) and collagen type I (Coll1), secreted profibrotic factors insulin-like growth factor binding protein (IGFBP)-3 and connective tissue growth factor (CTGF), as well as the intracellular marker of fibroblast activation alpha-smooth muscle actin (α-SMA) (Figure 2). MMS-350 also significantly decreased the amount of these fibrotic factors when used with TGFβ1 compared to TGFβ1 alone (Figure 2). MMS-350 exerted therapeutic effects since its addition 30 min to 6 h after TGFβ1 still showed profound antifibrotic activity (Figure 3).
Figure 2.
MMS-350 decreases TGFβ1-induced fibrosis. (A) Immunoblotting with fibrotic factors from cell lysates and medium conditioned with primary human fibroblasts treated with 4 mM HCl/0.1% BSA as vehicle (V) for TGFβ1 and 1× PBS as vehicle for MMS-350 (V), 10 ng/mL TGFβ1 (T), 500 μg/mL MMS-350 (M), or TGFβ1 + MMS-350 (T+M) for 72 h. GAPDH was used as an internal control. (B–F) Graphical summary of densitometry data from (A) shown as mean relative change to vehicle control ± SDEV (B–E) or mean relative change to internal control ± SDEV (F). N = 3. P values are as follows: (B) *p = 0.002, **p = 0.005; (C) *p = 0.014, **p = 0.029; (D) *p = 0.007, **p = 0.006; (E) *p = 0.003, **p = 0.008; (F) *p = 0.0003, **p = 0.0004.
Figure 3.
MMS-350 exerts therapeutic effects. Immunoblotting of profibrotic factors in cells treated as indicated. MMS-350 (500 μg/mL) was added with 10 ng/mL TGFβ1 (0) or at 30 min to 6 h after TGFβ1.
MMS-350 Reduces Pulmonary Fibrosis in Vivo
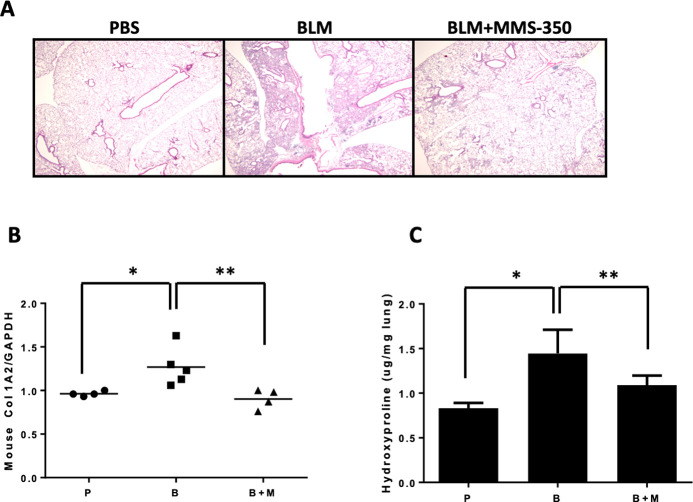
In bleomycin-induced lung fibrosis, MMS-350 ameliorated fibrosis histologically (Figure 4A). MMS-350 treatment reduced COL1A2 mRNA levels within 5 days of treatment (Figure 4B). These findings were confirmed by measurement of total collagen using hydroxyproline assays. MMS-350 resulted in a significant reduction of hydroxyproline levels (Figure 4C). Furthermore, mice treated with MMS-350 had less weight loss than mice treated with bleomycin alone, suggesting that MMS-350 reduced the extent of injury (data not shown). Overall survival was also improved in mice given bleomycin and MMS-350 compared to bleomycin-treated mice (87.5% vs 62.5%, respectively).
Figure 4.
MMS-350 improves pulmonary fibrosis in vivo. (A) Histologic appearance of lung tissues of mice treated with PBS (P), bleomycin (B), or bleomycin +2% MMS-350 (B+M) 14 days post-treatment. (B) Measurement of COL1A2 mRNA in lung tissues of mice treated as described in (A) for 5 days. Data are normalized to the housekeeping gene GAPDH. (C) Measurement of hydroxyproline in lungs of mice treated as described in (A) for 14 days. Graphs depict mean ± SD.
MMS-350 Reduces Dermal Fibrosis in Human Skin Ex Vivo
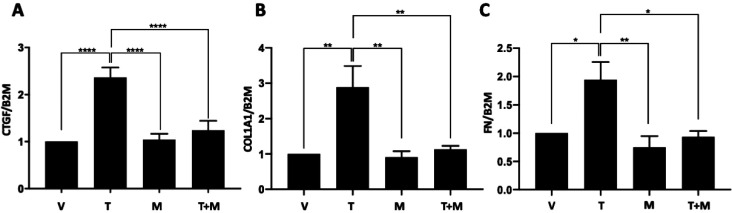
Since murine models of fibrosis do not completely recapitulate the human disease, we used human skin in organ culture to confirm that MMS-350 can reduce fibrosis in a human tissue. As shown in Figure 5, MMS-350 reversed the fibrotic phenotype in human tissue. Addition of MMS-350 24 h after triggering fibrosis with TGFβ resulted in a significant reduction of CTGF (Figure 5A), an early TGFβ response gene. Furthermore, addition of MMS-350 to TGFβ-treated skin punches significantly reduced COL1A1 (Figure 5B) and FN (Figure 5C) expression levels, restoring baseline levels of these molecules.
Figure 5.
MMS-350 reduces dermal fibrosis in human skin. Human skin was treated with vehicle (1× PBS) or 10 ng/mL TGFβ1 followed by 500 μg/mL MMS-350 after 24 h. (A) Expression of CTGF was measured in whole skin 24 h after the addition of MMS-350, ****p = <0.0001. (B) Expression of COL1A1 was measured in whole skin 72 h after the addition of MMS-350, **p = 0.0052, 0.0036, 0.0085. (C) Expression of FN was measured in whole skin 72 h after the addition of MMS-350, *p = 0.0205, 0.0135; **p = 0.0041. Expression was normalized to the housekeeping gene beta-2-microglobulin (B2M).
MMS-350 Analogues Exert Antifibrotic Effects
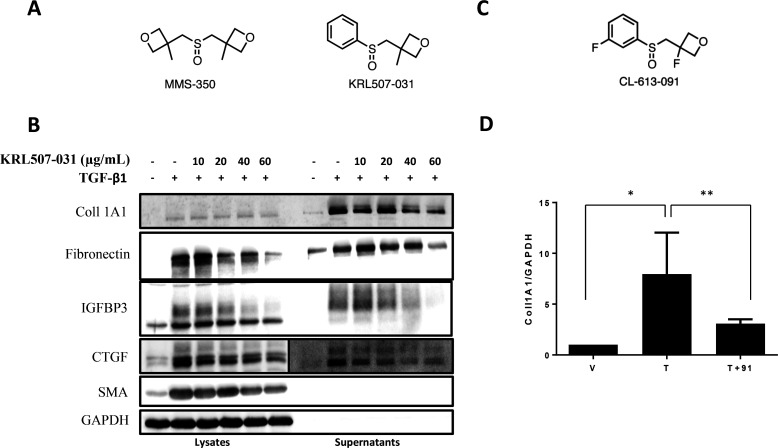
In order to increase the lipophilicity of MMS-350, and thereby potentially increase its potency, an analogue was synthesized that differed from MMS-350 (clogP −0.7) by the replacement of an oxetanyl methylene side chain with a phenyl ring (Figure 6A). KRL507-031 (clogP 1.1), tested as a racemate in these preliminary evaluations, showed similar antifibrotic activity to MMS-350, albeit at much lower concentrations (Figure 6B). Thus, KRL507-031 also ameliorated the fibrotic phenotype, suggesting that further structure–activity relationship (SAR) studies could be used to optimize the drug-like character of this chemotype.
Figure 6.
KRL507-031 and CL-613-091, two more lipophilic MMS-350 analogues, ameliorate fibrosis. (A) Structures of MMS-350 and KRL507-031. (B) Immunoblotting was used to detect fibrotic factors from cell lysates and medium conditioned by primary human fibroblasts treated with vehicle (DMSO), TGFβ1, or TGFβ1 + KRL507-031 at the indicated concentrations for 72 h. GAPDH was used as an internal control. (C) Structure of CL-613-091. (D) Primary fibroblasts were treated with TGFβ1 (T) with or without CL-613-091 (91, 500 μg/mL) for 48 h (V = vehicle). RNA was extracted and used in real time PCR to quantify COL1A1 levels. N = 4. *p = 0.007; **p = 0.027. Data are shown as mean ± SD.
To further define this SAR, a slightly more polar analogue was also synthesized that differed from MMS-350 and KRL507-031 by introducing fluorine atoms to the phenyl and oxetane rings of KRL507-031 (Figure 6C). Racemic CL-613-091 (clogP 1.0) also reduced COL1A1 expression in primary human fibroblasts (Figure 6D). Future studies will address the differential effects of enantiomerically pure KRL507-031 and CL-613-091 as new SAR leads. Additional modifications to MMS-350 that replaced both methyl substituents on the oxetane rings with fluorine atoms resulted in a significantly more hydrophilic and more polar compound, CL-613-092 (3,3′-(sulfinylbis(methylene))bis(3-fluorooxetane); clogP −1.2), that showed toxicity in vitro (data not shown).
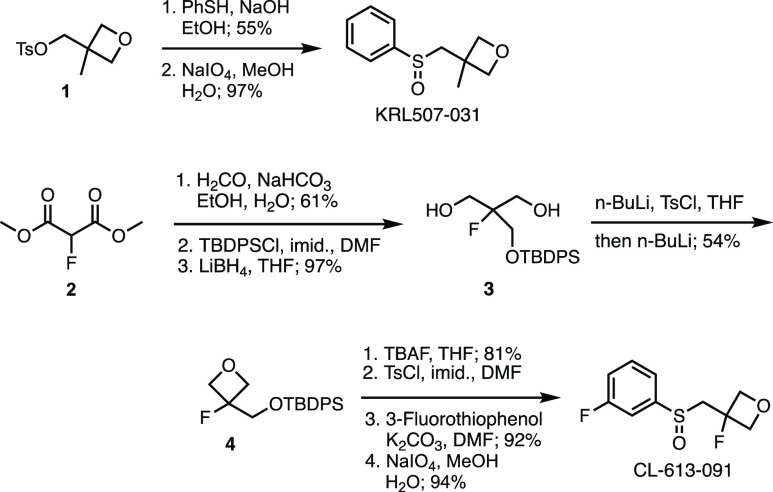
The synthesis of KRL507-031 and CL-613-091 is summarized in Scheme 1. Displacement of tosylate 1 with thiophenol and oxidation with sodium periodate provided KRL507-031 in 53% overall yield. For the preparation of CL-613-091, fluoromalonate 2 was hydroxymethylated with formaldehyde and converted to the silyl ether with tert-butyldiphenylchlorosilane (TBDPSCl). Reduction of both esters with lithium borohydride provided diol 3. Deprotonation with n-BuLi, in situ treatment with tosyl chloride, and addition of additional n-BuLi closed the oxetane ring in 54% yield to give 4. Finally, deprotection of the silyl ether, tosylation of the primary alcohol, displacement with 3-fluorothiophenol, and oxidation to the sulfoxide completed the synthesis of CL-613-091.
Scheme 1. Synthesis of KRL507-031 and CL-613-091.
The bifunctional sulfoxide MMS-350 was originally developed as a DMSO analogue to enhance the solubility of lipophilic small organic molecules6 and as a mild antioxidant for radiation mitigation.7 In contrast to DMSO, MMS-350 contains two oxetane side chains, which increase the lipophilicity of this analogue from a logP −1.4 (DMSO) to −0.7 (MMS-350), while preserving exquisite aqueous solubility.6 Oxetanes function as carbonyl group bioisosteres and can mediate specific protein/receptor interactions.9,10
Our results explain, at least in part, the mechanism by which MMS-350 ameliorates fibrosis. MMS-350 reduced activation of fibroblasts to myofibroblasts as detected by reduction of SMA levels. SMA is a marker of myofibroblasts and an indicator of the transition of fibroblasts to a contractile and synthetic state. Reduction of SMA is associated with reversal of the fibrotic phenotype of fibroblasts. MMS-350 also reduced levels of the profibrotic factors CTGF and IGFBP-3. CTGF is induced in response to fibrotic triggers such as TGFβ and bleomycin and mediates their profibrotic activity. Reduction of CTGF has been shown to block fibrosis in vitro and in vivo (reviewed in ref (11)). IGFBP-3 is also induced by TGFβ and has been shown to exert fibrotic effects on primary fibroblasts by increasing ECM production.12 IGFBP-3 also promotes fibrosis in human skin maintained in organ culture.13 Thus, by reducing the levels of two central pro-fibrotic mediators, MMS-350 is able to abrogate the development of a fibrotic phenotype.
MMS-350 has been shown to have additional protective effects. In one study, MMS-350 was identified as a mitigator of ionizing irradiation.5 More recently, MMS-350 was shown to ameliorate radiation-induced pulmonary fibrosis and increase survival of C57BL/6NHsd mice after total-body irradiation.7 Our findings now demonstrate that MMS-350 reduces profibrotic gene expression in primary human fibroblasts. The ability to reduce profibrotic gene expression by MMS-350 was also reported in endothelial and alveolar type II cells cultured from the lung tissues of mice following thoracic irradiation.8 The fact that MMS-350 reduces the expression of fibrotic genes in more than one cell type suggests that its mechanism of action is likely irrespective of cellular targets, and that its ability to ameliorate fibrosis is a general propensity of this chemotype and not restricted to one cell type.
Using a mouse model of irradiation-induced lung fibrosis, MMS-350 was shown to ameliorate fibrosis.7 In that study, MMS-350 was provided to mice in drinking water, making it difficult to accurately determine the amount of MMS-350 ingested by the mice. Our findings suggest that even lesser quantities of MMS-350 are effective at ameliorating fibrosis; in our study mice were given a limited amount of MMS-350 via oral gavage. Furthermore, by increasing the lipophilicity of the oxetanyl sulfoxide chemotype and replacing one of the side chains in MMS-350 with a phenyl group, the analogue KRL507-031 gained potency and was just as effective as the parent compound at 10 times lower levels in vitro.
In addition to the antifibrotic effects of MMS-350 in vitro and in vivo, we show that MMS-350 reduced fibrosis in human skin in organ culture. This suggests that MMS-350 is effective in human tissue and thus will likely be similarly effective in humans with fibrosis. The skin tissue model allows us to extend our findings and demonstrate direct relevance of the data to human disease.14
In summary, MMS-350 ameliorated fibrosis in two models where pulmonary fibrosis was induced using different triggers. MMS-350 was effective when given orally and showed no toxicity. MMS-350 was also effective at reducing TGFβ-induced pro-fibrotic factors including collagen, fibronectin, IGFBP-3, and CTGF. Since no effective or curative therapies exist yet for the fibrosis characteristic of scleroderma and related diseases such as idiopathic pulmonary fibrosis, the identification of MMS-350 as a novel antifibrotic agent could have significant impact on the treatment of fibrosis in scleroderma and related pathologies. Significantly, MMS-350 has shown low toxicity and high aqueous solubility, making it an attractive therapeutic option. Its oral administration renders it amenable to use by patients with scleroderma and other fibrosing diseases. Furthermore, due to the attractive physicochemical and biological properties of MMS-350, the compound could be used synergistically to enhance the absorption of other supportive treatments given to patients with fibrotic diseases. Preliminary SAR evaluations with the MMS-350 analogues KRL507-031 and CL-613-091 demonstrate considerable future opportunities for further modulation of the physiological properties of this new chemotype.
Acknowledgments
The authors thank T. S. Maskrey (University of Pittsburgh) for project management and technical assistance and Y. Skaf (University of Pittsburgh) for experimental compound purification and analysis.
Glossary
Abbreviations
- α-SMA
intracellular marker of fibroblast activation alpha-smooth muscle actin
- CL-613-091
(3-fluoro-3-(((3-fluorophenyl)sulfinyl)methyl)oxetane)
- Col1
collagen type I
- ECM
extracellular matrix
- CTGF
connective tissue growth factor
- FN
fibronectin
- GAPDH
glyceraldehyde phosphate dehydrogenase
- IGFBP
insulin-like growth factor binding protein
- KRL507-031
(3-methyl-3-((phenylsulfinyl)methyl)oxetane)
- MMS-350
3,3′-[sulfinylbis(methylene)]bis[3-methyl-oxetane
- SAR
structure–activity relationship
- SSc
systemic sclerosis
- TBDPS
tert-butyldiphenylchlorosilane
- TGFβ
transforming growth factor beta
- TNF
tumor necrosis factor
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00433.
Experimental details and 1H and 13C NMR spectra for new synthetic products; assay information (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported in part by grants K24AR060297 (CFB) and 2P50GM067082 (PW) from the National Institutes of Health. The authors have no relevant conflicts of interest to disclose.
The authors declare no competing financial interest.
Supplementary Material
References
- Leftheris K.; Zheng Y.; Machajewski T. T. Recent Advances in the Development of Small-Molecule Inhibitors for Idiopathic Pulmonary Fibrosis (IPF). Med. Chem. Rev. 2019, 54, 75–94. 10.29200/acsmedchemrev-v54.ch4. [DOI] [Google Scholar]
- Wynn T. A. Common and Unique Mechanisms Regulate Fibrosis in Various Fibroproliferative Diseases. J. Clin. Invest. 2007, 117, 524–529. 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen V. D.; Medsger T. A. Changes in Causes Of Death in Systemic Sclerosis, 1972–2002. Ann. Rheum. Dis. 2007, 66, 940–944. 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Zhu L.; Wang B.; Yuan M.; Zhu R. Drugs and Targets in Fibrosis. Front. Pharmacol. 2017, 8, 855. 10.3389/fphar.2017.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff J. P.; Shields D. S.; Wang H.; Skoda E. M.; Sprachman M. M.; Wipf P.; Garapati V. K.; Atkinson J.; London B.; Lazo J. S.; Kagan V.; Epperly M. W.; Greenberger J. S. Evaluation of Potential Ionizing Irradiation Protectors and Mitigators using Clonogenic Survival of Human Umbilical Cord Blood Hematopoietic Progenitor Cells. Exp. Hematol. 2013, 41, 957–966. 10.1016/j.exphem.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprachman M. M.; Wipf P. A Bifunctional Dimethylsulfoxide Substitute Enhances the Aqueous solubility of small organic molecules. Assay Drug Dev. Technol. 2012, 10, 269–277. 10.1089/adt.2011.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalash R.; Epperly M. W.; Goff J.; Dixon T.; Sprachman M. M.; Zhang X.; Shields D.; Cao S.; Franicola D.; Wipf P.; Berhane H.; Wang H.; Au J.; Greenberger J. S. Amelioration of Radiation-Induced Pulmonary Fibrosis by a Water-Soluble Bifunctional Sulfoxide Radiation Mitigator (MMS350). Radiat. Res. 2013, 180, 474–490. 10.1667/RR3233.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalash R.; Berhane H.; Goff J.; Houghton F.; Epperly M. W.; Dixon T.; Zhang X.; Sprachman M. M.; Wipf P.; Franicola D.; Wang H.; Greenberger J. S. Effects of Thoracic Irradiation on Pulmonary Endothelial Compared to Alveolar Type-II Cells in Fibrosis-Prone C57BL/6ntac Mice. In Vivo 2013, 27, 291–297. [PMC free article] [PubMed] [Google Scholar]
- Wuitschik G.; Rogers-Evans M.; Müller K.; Fischer H.; Wagner B.; Schuler F.; Polonchuk L.; Carreira E. M. Oxetanes as Promising Modules in Drug Discovery. Angew. Chem., Int. Ed. 2006, 45, 7736–7739. 10.1002/anie.200602343. [DOI] [PubMed] [Google Scholar]
- Burkhard J. A.; Wuitschik G.; Plancher J.-M.; Rogers-Evans M.; Carreira E. M. Synthesis and Stability of Oxetane Analogs of Thalidomide and Lenalidomide. Org. Lett. 2013, 15, 4312–4315. 10.1021/ol401705a. [DOI] [PubMed] [Google Scholar]
- Abraham D. J. TGF-β and SMAD Signalling in Fibrosis. Exp. Dermatol. 2008, 17, 887–890. 10.1111/j.1600-0625.2008.00789_7.x. [DOI] [Google Scholar]
- Pilewski J. M.; Liu L.; Henry A. C.; Knauer A. V.; Feghali-Bostwick C. A. Insulin-like Growth Factor Binding Proteins 3 and 5 Are Overexpressed in Idiopathic Pulmonary Fibrosis and Contribute to Extracellular Matrix Deposition. Am. J. Pathol. 2005, 166, 399–407. 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka H.; Larregina A. T.; Yamaguchi Y.; Feghali-Bostwick C. A. Human Skin Culture as an Ex Vivo Model for Assessing the Fibrotic Effects of Insulin-like Growth Factor Binding Proteins. Open Rheumatol. J. 2008, 2, 17–22. 10.2174/1874312900802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y.; Takihara T.; Chambers R. A.; Veraldi K. L.; Larregina A. T.; Feghali-Bostwick C. A. A Peptide Derived from Endostatin Ameliorates Organ Fibrosis. Sci. Transl. Med. 2012, 4, 136ra71. 10.1126/scitranslmed.3003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.