To the Editor:
The coronavirus disease (COVID-19) pandemic has triggered precipitous entry of multiple novel therapeutic candidates into clinical trials often without control groups, randomization, or adequate statistical power. To this long list can be added a repurposing of existing therapeutic strategies used for other inflammatory or viral illnesses. A search of clinicaltrials.gov on July 3, 2020, identified 1,366 registered trials, of which 279 were randomized controlled trials (RCTs) assessing immunomodulatory therapies. These include targets against 39 different immune pathways and 90 different drugs or therapies (Figure 1). A cure may be stumbled on fortuitously among the various heterogenous study designs and interventions, whereas 14 of the 279 RCTs would generate a statistically significant outcome at the 5% level (albeit in either direction) by chance alone, assuming they were all adequately powered.
Figure 1.
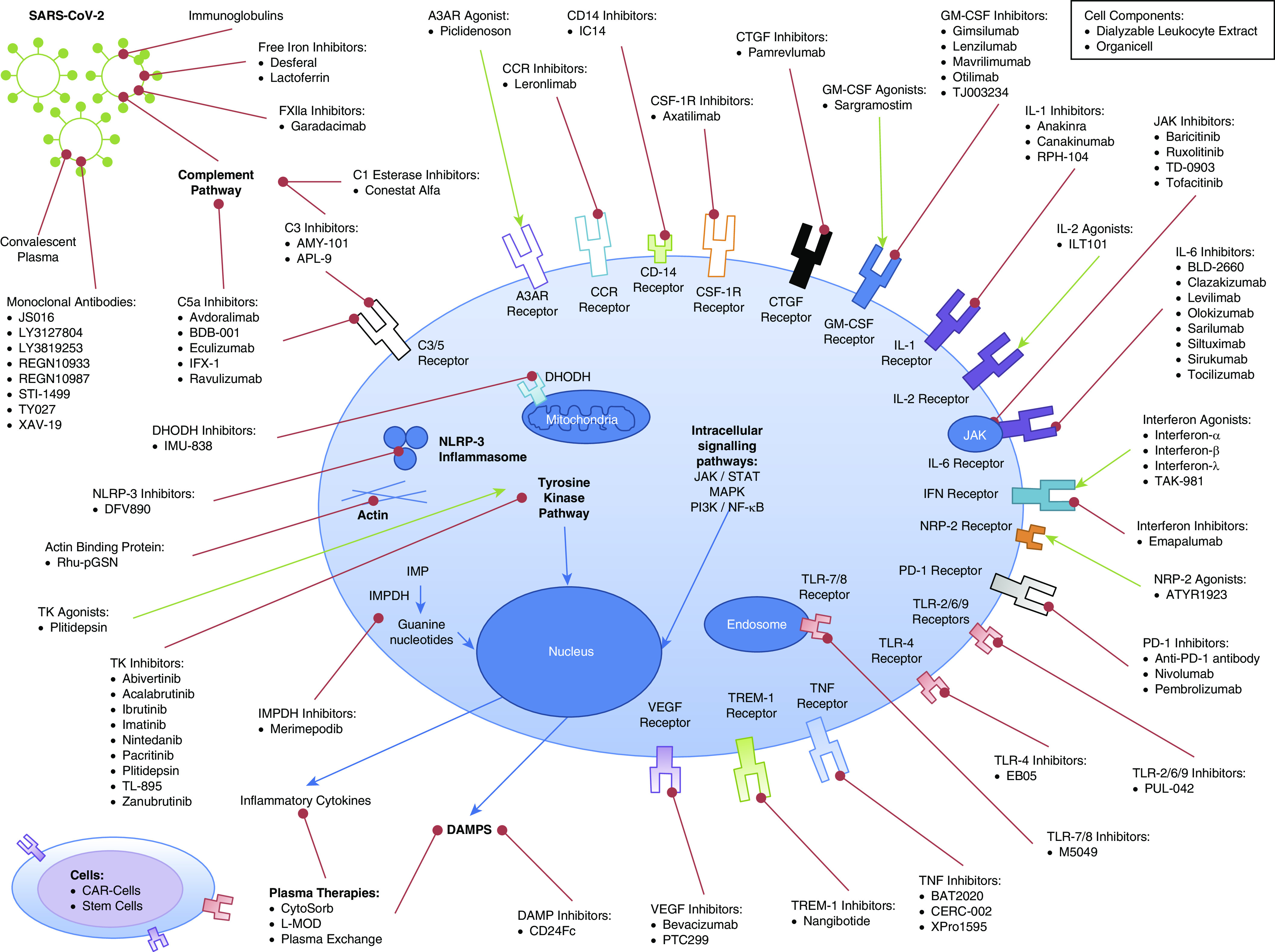
Summary of biological therapies undergoing randomized controlled trials in coronavirus disease (COVID-19). A3AR = adenosine A3 receptor; CAR = chimeric antigen receptor; CCR = C-C chemokine receptor; CSF-1R = colony stimulating factor 1 receptor; CTGF = connective tissue growth factor; DAMP = damage-associated molecular patterns; DHODH = dihydroorotate dehydrogenase; GM-CSF = granulocyte–macrophage colony–stimulating factor; IMP = inosine-5′-monophosphate; IMPDH = inosine-5′-monophosphate dehydrogenase; JAK = Janus kinase; L-MOD = leukocyte modulator hemoperfusion; MAPK = mitogen-activated protein kinase; NF-κB = nuclear factor-κB; NLRP-3 = NOD-, LRR-, and pyrin domain-containing protein 3; NRP-2 = neuropilin 2; PD-1 = programmed cell death protein 1; PI3K = phosphoinositide 3-kinase; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; STAT = signal transducer and activator of transcription; TREM-1 = triggering receptor expressed on myeloid cells-1; VEGF = vascular endothelial growth factor.
Our still incomplete understanding of the COVID-19 disease process, including temporal change, has driven arguably inappropriate, ill-timed, or ill-judged interventions, either within trials or compassionate use. The description of the “cytokine storm” epithet to COVID-19 has driven the application of immunosuppressive therapies. At the time of writing, 47 registered RCTs were evaluating inhibition of IL-6, mostly recruiting on clinical criteria alone without incorporating measurement of circulating IL-6 concentrations. Although circulating IL-6 concentrations are higher among nonsurvivors of COVID-19 compared with survivors (1, 2), circulating IL-6 concentrations in COVID-19 are often 1–2 log-orders lower than those in other causes of acute respiratory distress syndrome or viral influenza (3). Although there may indeed be benefit from inhibiting IL-6, timing, dosing, and patient selection are key. Outcome improvements in some subsets may be diluted or counterbalanced by lack of effect or harm in others. An acceptable toxicity profile for use in other inflammatory conditions does not necessarily translate to COVID-19, especially in the critically ill subset, in whom both the severity of the disease process and multiple iatrogenic factors magnify immunosuppression and the risk of secondary nosocomial infection. A single dose of the IL-6 inhibitor tociluzimab can significantly dampen any C-reactive protein and temperature response for 1 week (4). Apart from a potential increased risk of infection, traditional clinical signs may be masked with resulting delays in identification and treatment. The same risk–benefit balance holds for other immunomodulators.
As a further example of scientific uncertainty, therapeutic approaches with directly opposing actions are being promulgated. As an example, with granulocyte–macrophage colony–stimulating factor, both direct activation and inhibition are being targeted. If modulation in one direction proves successful, the counter approach may well harm. A further possibility is that both are efficacious, albeit at different time points in the disease process; to our knowledge, the critical issue of timing is not being addressed. Although the scientific merits behind these contrasting approaches have been eloquently argued, the challenge lies in determining the Goldilocks effect (5). The intricacy behind the pleiotropic biology of these drug targets and the unknown trade-offs between advantage and detriment in a complex multisystem disease cannot be underestimated.
Publication bias for positive results in small case series may also provide a false reassurance of the safety and efficacy of an experimental intervention. Similar issues arise at the other end of the spectrum. Although buoyed by the impressive outcome improvements achieved by low-dose dexamethasone within the large-scale RECOVERY (Randomised Evaluation of COVID-19 Therapy) study, the explanation for many unexplained findings in this study remained unresolved such as the disparate effects depending on sex, age, illness severity, and timing of intervention (6).
Well-meaning attempts to intervene should not take priority over understanding of the pathogenic mechanisms underlying impaired viral clearance and the development of organ failure. The use of theranostic biomarkers may identify patients most likely to benefit and to subsequently monitor for treatment effects. Risk stratification can also be performed using routinely collected clinical parameters (7). This will enable trial enrichment, targeting patients most likely to benefit and not exposing those patients unlikely to benefit to potential detriment.
Decades of sepsis research exploring immunomodulatory therapies have fallen short of expectation and, in some cases, resulted in harm (8). It has been convenient to blame the intervention rather than acknowledging flaws in the underlying scientific rationale or study design. We fear that COVID-19 may be a case of déjà vu and argue for a measured approach based on sound science.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202008-3148LE on September 14, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElvaney OJ, McEvoy N, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. doi: 10.1001/jamainternmed.2020.3313. [online ahead of print] 30 Jun 2020; DOI: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 4.Morena V, Milazzo L, Oreni L, Bestetti G, Fossali T, Bassoli C, et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang FM, Lee KM, Teijaro JR, Becher B, Hamilton JA. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. [online ahead of print] 17 Jul 2020; DOI: 10.1056/NEJMoa2021436. [Google Scholar]
- 7.Manson J, Crooks C, Naja M, Ledlie A, Goulden B, Liddle T. et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. doi: 10.1016/S2665-9913(20)30275-7. [online ahead of print] 21 Aug 2020; DOI: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink MP, Warren HS. Strategies to improve drug development for sepsis. Nat Rev Drug Discov. 2014;13:741–758. doi: 10.1038/nrd4368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


