Pulmonary arterial hypertension (PAH) is a progressive disease characterized by increased pulmonary arterial pressure and pulmonary vascular resistance, ultimately leading to right heart failure and death. This increased vascular resistance leads to pulmonary vascular wall thickening and remodeling via phenotypic changes in proliferation and apoptosis in pulmonary arterial smooth muscle cells (PASMCs), pulmonary arterial endothelial cells (PAECs), pericytes, and fibroblasts (1). Over the past decade, appreciation has increased regarding the pervasive importance of noncoding RNA biology in controlling pulmonary vascular function and the pathogenic progression to PAH (2). Though studies of microRNAs in PAH have dominated the literature, the biologic roles of long noncoding RNAs (lncRNAs) increasingly are emerging as pathogenic hubs of disease (3).
TYKRIL and lncRNA Biology
Tens of thousands of lncRNA transcripts are encoded by the human genome. They are transcripts over 200 nucleotides long without predicted protein-coding potential. lncRNAs typically bind either proteins or other RNA molecules to enact epigenetic, transcriptional, and posttranscriptional regulation of gene expression, affecting a wide range of biological processes ranging from cell proliferation, apoptosis, and differentiation (4). lncRNAs have dynamic and specific expression patterns, are expressed in both the nucleus and cytoplasm, and are released at detectable and reproducible quantities into the circulating plasma (5). A crucial challenge in the study of these molecules is their poor sequence conservation across mammalian species, thus making analysis of their in vivo mechanisms of action particularly challenging.
Though a number of lncRNAs have been reported as dysregulated in tissue and plasma of subjects with PAH, the actions of only a few lncRNAs thus far have been implicated in pulmonary vascular pathophysiology. In this issue of the Journal, Zehendner and colleagues (pp. 1445–1457) screened the landscape of lncRNAs in human PAH lung tissue; they characterized the novel lncRNA TYKRIL (tyrosine kinase receptor–inducing lncRNA) in pulmonary vascular remodeling and suggest it as a new therapeutic target (6). The team began by conducting RNA-sequencing analysis of PASMCs and lung pericytes exposed to hypoxia and derived from patients with idiopathic PAH. This global screening approach allowed for the identification of numerous dynamically altered lncRNAs, including TYKRIL. In cultured PASMCs and pericytes and in lung slices from patients with PAH, the team demonstrated that TYKRIL regulates tyrosine kinase signaling by binding the tumor suppressor p53 and facilitating the transcription of platelet-derived growth factor receptor PDGFRβ, thus promoting the hyperproliferative and apoptosis-resistant phenotypes of these cells in PAH (Figure 1A).
Figure 1.
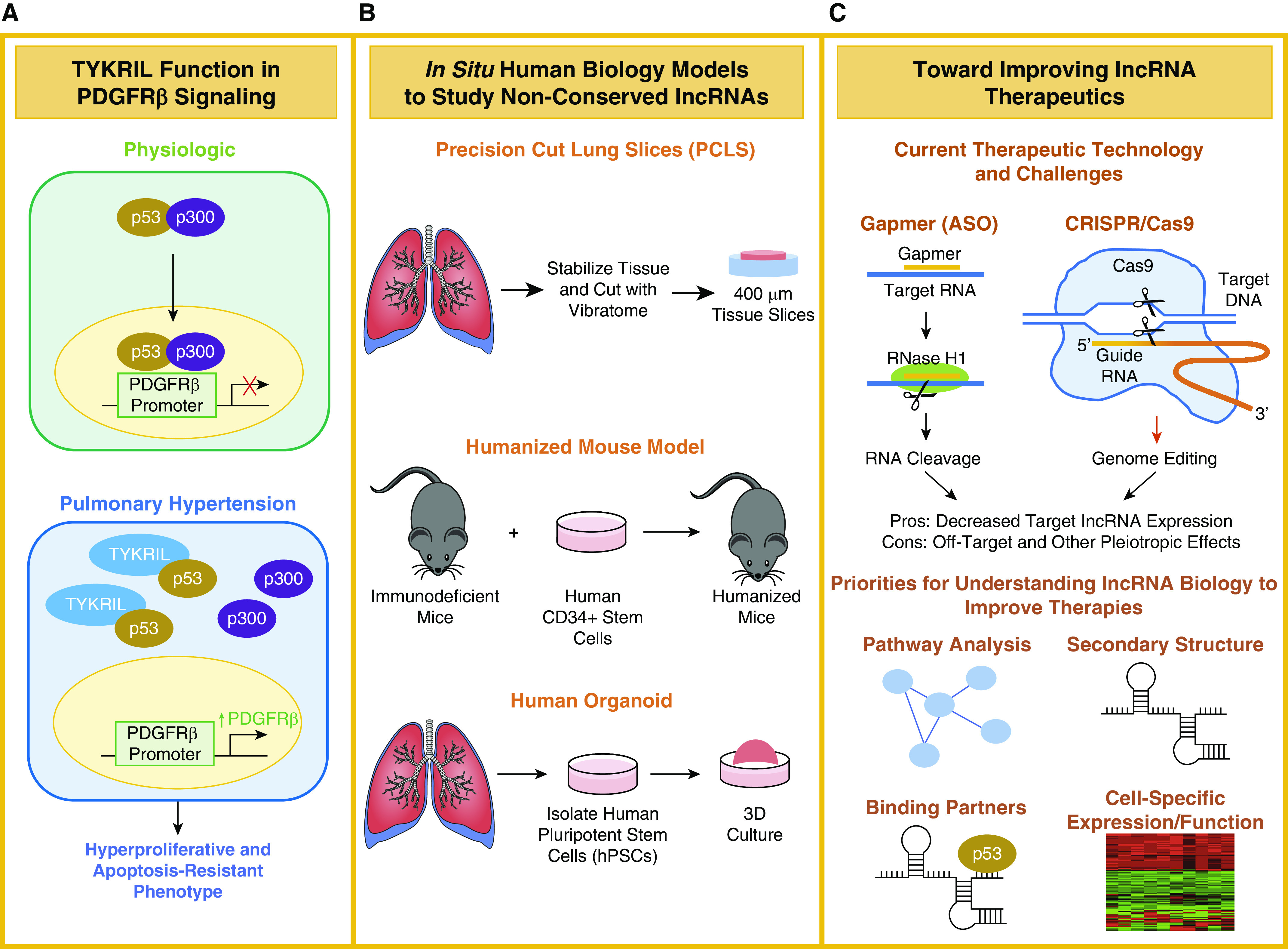
A new era for studies in long noncoding RNA (lncRNA) biology for pulmonary arterial hypertension. (A) Representation of TYKRIL (tyrosine kinase receptor–inducing lncRNA) function in the p53/PDGFRβ signaling axis under physiologic and pulmonary hypertension conditions. (B) Advancing methodologies for creating three in situ models to study human pathophysiology driven by nonconserved lncRNAs: precision-cut lung slices, humanized mouse models, and human organoid models. (C) Currently proposed therapeutic technologies and their drawbacks for inhibiting lncRNAs (Gapmers/antisense oligonucleotides [ASO] and CRISPR/Cas9 genome editing). Priorities for understanding lncRNA biology to develop more effective RNA therapies include systems biology analyses for defining RNA secondary structure, binding partners, and cell type–specific expression and function. 3D = three-dimensional.
Overall, this study offers a glimpse into the next generation of studies that are fast approaching to characterize lncRNA biology in PAH. As the first known lncRNA to regulate the central p53/PDGFRβ axis, TYKRIL may indeed serve as a key mediator across multiple cell types of PAH. Yet, because this lncRNA is not conserved in rodents, traditional approaches to study its mechanisms of action were not possible in live animals. Instead, the team used an ex vivo precise cut lung slice model (7), whereby explanted human lung slices containing all lung cell types could be cultured and manipulated at the molecular level. As such, the use of precision lung slices here served as a clever method to gain insight into this lncRNA’s role in controlling vascular remodeling. Such a discovery platform may open up key avenues to study other nonconserved lncRNAs in human lung diseases. Furthermore, given the well-documented limitations of current animal models of PAH (8), such work highlights the potential for future development of complex in situ or synthetic human biology models of PAH that otherwise have not been possible to date. For example, such approaches could be envisioned using three-dimensional human organoid modeling (9) or humanized mice engrafted with human biological tissues, often useful in modeling human immune cell interactions with the vasculature (10) (Figure 1B).
lncRNA Therapeutics
These novel discovery platforms may also open a door for development of specific lncRNAs as therapeutic targets in PAH. However, stemming from the number of unknowns that still exist in noncoding RNA biology, current technology for lncRNA inhibition may not yet be advanced enough for true therapeutic performance. Here, Zehendner and colleagues inhibit TYKRIL using gapmers, chimeric antisense oligonucleotides that engage target lncRNA and induce RNase H-based degradation (11). These can be particularly useful in targeting nuclear RNAs as compared with siRNAs that target cytoplasmic messenger transcripts. Nonetheless, gapmers and other existing RNA interference methods are difficult to implement therapeutically because of low bioavailability and off-target effects (12). Those off-target effects are further compounded by the innate biology of lncRNAs that often employs extreme and varied pleiotropic cellular reprogramming, as this group also found by RNA sequencing of cells after TYKRIL knockdown. Finally, the cell-type specificity of actions of lncRNAs such as TYKRIL can further complicate the biology. For instance, in this study, beyond pericytes and PASMCs, TYKRIL was also found to be upregulated in PAECs. However, because TYKRIL’s target p53 displays divergent expression patterns and activity in PAECs in PAH (13), TYKRIL’s ultimate actions may be more nuanced and distinct, depending on cell type.
Ultimately, for more reliable therapeutic development in this space, a better system would be necessary to catalog and discern the key regulatory targets and pathways of an individual lncRNA across its multilayered pleiotropy. Improvements in our ability to predict the secondary structure of lncRNAs and their binding potential to other RNAs and proteins should be prioritized. Furthermore, the development of therapeutic delivery systems in vivo to target or genomically edit lncRNAs in specific cells or cell types may also be warranted (Figure 1C). Despite these challenges, this study exemplifies the progress being made toward a more complete understanding and druggable landscape for lncRNAs in PAH.
Supplementary Material
Footnotes
Supported by NIH grants R01 HL124021, HL 122596, HL 138437, and UH2/UH3 TR002073, and American Heart Association Established Investigator Award 18EIA33900027 (S.Y.C.).
Originally Published in Press as DOI: 10.1164/rccm.202007-2632ED on July 29, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lammers S, Scott D, Hunter K, Tan W, Shandas R, Stenmark KR. Mechanics and function of the pulmonary vasculature: implications for pulmonary vascular disease and right ventricular function. Compr Physiol. 2012;2:295–319. doi: 10.1002/cphy.c100070. [DOI] [PubMed] [Google Scholar]
- 2.Jin Q, Zhao Z, Zhao Q, Yu X, Yan L, Zhang Y, et al. Long noncoding RNAs: emerging roles in pulmonary hypertension. Heart Fail Rev. 2020;25:795–815. doi: 10.1007/s10741-019-09866-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun M, Kraus WL. From discovery to function: the expanding roles of long noncoding RNAs in physiology and disease. Endocr Rev. 2015;36:25–64. doi: 10.1210/er.2014-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao R-W, Wang Y, Chen L-L. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Hong R, Chen W, Xu M, Wang L. The role of long noncoding RNA in major human disease. Bioorg Chem. 2019;92:103214. doi: 10.1016/j.bioorg.2019.103214. [DOI] [PubMed] [Google Scholar]
- 6.Zehendner CM, Valasarajan C, Werner A, Boeckel J-N, Bischoff FC, John D, et al. Long noncoding RNA TYKRIL plays a role in pulmonary hypertension via the p53-mediated regulation of PDGFRβ. Am J Respir Crit Care Med. 2020;202:1445–1457. doi: 10.1164/rccm.201910-2041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, Betts C, Cunoosamy DM, Åberg PM, Hornberg JJ, Sivars KB, et al. Use of precision cut lung slices as a translational model for the study of lung biology. Respir Res. 2019;20:162. doi: 10.1186/s12931-019-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das M, Fessel J, Tang H, West J. A process-based review of mouse models of pulmonary hypertension. Pulm Circ. 2012;2:415–433. doi: 10.4103/2045-8932.105030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wörsdörfer P, I T, Asahina I, Sumita Y, Ergün S. Do not keep it simple: recent advances in the generation of complex organoids. J Neural Transm (Vienna) doi: 10.1007/s00702-020-02198-8. [online ahead of print] 8 May 2020; DOI: 10.1007/s00702-020-02198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong KSM, Her Z, Chen Q. Humanized mice as unique tools for human-specific studies. Arch Immunol Ther Exp (Warsz) 2018;66:245–266. doi: 10.1007/s00005-018-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J-S, Mendell JT. Antisense-mediated transcript knockdown triggers premature transcription termination. Mol Cell. 2020;77:1044–1054, e3. doi: 10.1016/j.molcel.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Yang K, Zheng Q, Zhang C, Tang H, Babicheva A, et al. Divergent changes of p53 in pulmonary arterial endothelial and smooth muscle cells involved in the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2019;316:L216–L228. doi: 10.1152/ajplung.00538.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


