Abstract
Rationale: Lymphangioleiomyomatosis (LAM) is a metastatic neoplasm of reproductive-age women associated with mutations in tuberous sclerosis complex genes. LAM causes cystic remodeling of the lung and progressive respiratory failure. The sources and cellular characteristics of LAM cells underlying disease pathogenesis remain elusive.
Objectives: Identification and characterization of LAM cells in human lung and uterus using a single-cell approach.
Methods: Single-cell and single-nuclei RNA sequencing on LAM (n = 4) and control (n = 7) lungs, immunofluorescence confocal microscopy, ELISA, and aptamer proteomics were used to identify and validate LAMCORE cells and secreted biomarkers, predict cellular origins, and define molecular and cellular networks in LAM.
Measurements and Main Results: A unique cell type termed LAMCORE was identified, which was distinct from, but closely related to, lung mesenchymal cells. LAMCORE cells expressing signature genes included known LAM markers such as PMEL, FIGF, CTSK, and MLANA and novel biomarkers validated by aptamer screening, ELISA, and immunofluorescence microscopy. LAM cells in lung and uterus are morphologically indistinguishable and share similar gene expression profiles and biallelic TSC2 mutations, supporting a potential uterine origin for the LAMCORE cell. Effects of LAM on resident pulmonary cell types indicated recruitment and activation of lymphatic endothelial cells.
Conclusions: A unique population of LAMCORE cells was identified in lung and uterus of patients with LAM, sharing close transcriptomic identity. LAM cell selective markers, secreted biomarkers, and the predicted cellular molecular features provide new insights into the signaling and transcriptional programs that may serve as diagnostic markers and therapeutic targets to influence the pathogenesis of LAM.
Keywords: lymphangioleiomyomatosis, single-cell RNA, pulmonary remodeling, uterus, tuberous sclerosis complex
At a Glance Commentary
Scientific Knowledge on the Subject
Lymphangioleiomyomatosis (LAM) is a metastatic neoplasm of reproductive-age women associated with mutations in TSC (tuberous sclerosis complex) genes. LAM causes cystic remodeling of the lung and progressive respiratory failure. Current knowledge on LAM cells has been limited to histological and immunohistochemical studies that demonstrate that cells within the LAM lesions are morphologically heterogeneous, including spindled cells with a smooth muscle phenotype, epithelioid cells that variably stain for PMEL, and stromal cells derived from mesenchymal, hematopoietic, and endothelial lineages. The sources and cellular characteristics of LAM cells underlying disease pathogenesis in LAM remain elusive.
What This Study Adds to the Field
The present study identified a unique population of mesenchymal cells in both pulmonary and uterine tissues of patients with LAM that are distinct from normal lung mesenchymal cells. These cells express signature genes including known LAM markers such as PMEL and VEGFD; therefore, they are termed as LAMCORE cells. Evidence presented in this study supports the uterus as a likely source of LAM cells in the lung. In addition, novel LAM cell–selective markers, secreted biomarkers, and transcriptional programs identified from this study may serve as diagnostic markers and therapeutic targets to influence the pathogenesis of LAM. To our knowledge, this is the first comprehensive study of the LAM lung and uterus using the single-cell transcriptomic approach.
Lymphangioleiomyomatosis (LAM) is a rare, neoplastic lung disease primarily affecting women of childbearing age, which causes diffuse cystic tissue remodeling and progressive respiratory failure (1). LAM is caused by deleterious mutations in TSC1 or TSC2, which encode components of a heterooligomer that controls the activity of the mTORC1 (mechanistic target of rapamycin complex 1) (2). A sporadic form of the disease occurs in women who do not have any evidence of heritable illness. LAM lesions have recurred in the allografts of some patients who have undergone lung transplants, supporting a metastatic mechanism for the disease (3). Mitotically quiescent cells within the LAM lesions (4) are morphologically heterogeneous and include spindled cells with a smooth muscle phenotype, epithelioid cells that variably stain for PMEL with HMB45 antibody, and stromal cells derived from mesenchymal, hematopoietic, and endothelial lineages (5). LAM shares many morphological and immunohistochemical features with renal angiomyolipomas (AMLs), and both are members of the perivascular epithelioid cell neoplasm family of rare mesenchymal tumors that can arise in diverse tissues and exhibit pathological behaviors ranging from benign to malignant (6). Lymphangiogenic growth factors are expressed in the LAM lesions, and VEGF-D (vascular endothelial growth factor D) is increased in the serum of patients with LAM (7–9). The mTOR inhibitor sirolimus is a U.S. Food and Drug Administration–approved suppressive therapy that stabilizes lung function in most patients with LAM (10, 11); however, the drug does not eliminate LAM cells, and it can be associated with significant side effects (10). There is therefore an urgent need to develop new molecular targets and treatments for LAM. Intractable uncertainties regarding the origins of the LAM cells, the predilection for reproductive-age women, and the cellular and molecular mechanisms contributing to LAM cell migration, differentiation, and dysfunction have been obstacles to the development of new therapeutic strategies (12).
Because LAM cells are difficult to isolate, grow poorly, and lose differentiated features in culture, an authentic human cell model has not been developed, and the precise molecular and cellular features of LAM cells that drive the pathogenesis of pulmonary LAM have remained elusive. Recent advances in single-cell RNA sequencing (scRNA-seq) enable detailed transcriptomic mapping of individual cells within organs and tissues, providing a unique opportunity to interrogate cellular heterogeneity, cell–cell interactions, and cell–environment interactions in their native state (13–15). In this study, we used scRNA-seq, immunostaining, and confocal microscopy to identify LAMCORE cells, predict LAM cell origins, identify biomarkers, and predict molecular mechanisms underlying the pathogenesis of LAM.
Some of the results of these studies have been previously reported in the form of a preprint (bioRxiv, 8 October 2019 https://doi.org/10.1101/784199) and a conference abstract (16).
Methods
Fresh resected lung tissue was obtained from the National Diseases Research Interchange with the assistance of the LAM Foundation. Female control donor lung tissue was obtained from the LifeCenter Donor Network, Cincinnati. LAM uterine tissue was obtained from St. Vincent Women’s Hospital, Indianapolis, with the help of Dr. Angela Stevens. Explanted tissues were prepared for scRNA-seq and single-nuclei RNA-seq (snRNA-seq) analyses in an analytic pipeline described in the online supplement. Immunostaining, serum aptamer (Somalogic), ELISA analyses, and RT-PCR were used to identify known and potential biomarkers of LAM.
Results
scRNA-seq Analysis of LAM Lung Explants Identifies a Unique Cluster of LAMCORE Cells
scRNA-seq and snRNA-seq were performed on four unique LAM lung explants (LAM1/2/3/4) (Figure 1), normal lung, a renal AML, LAM uterus (from patient LAM5), and normal uterine tissue. Publicly available scRNA-seq data from six additional female donor lungs were downloaded from the Gene Expression Omnibus (17) and included for integrative analyses. A total of 92,444 cells (21,964 cells from LAM lung and AML samples, 47,906 donor lung cells, and 22,574 cells from LAM uterus and normal uterus) were used for further analysis (Methods). The mutation findings of comprehensive TSC1 and/or TSC2 genetic analysis from patients with LAM or AML are summarized in Table 1. Additional clinical data and quality control statistics related to single-cell and single-nuclei RNA-seq data are summarized in Table E1 in the online supplement.
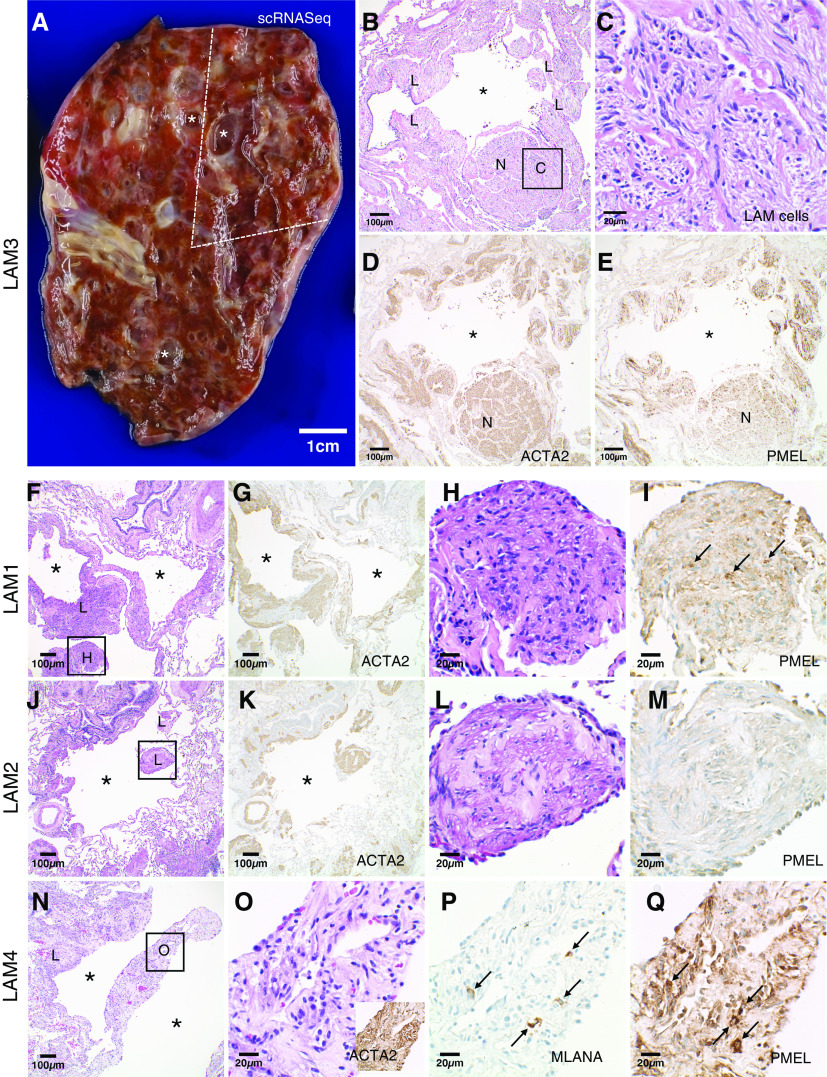
Figure 1.
Patient lungs with lymphangioleiomyomatosis (LAM) for single-cell RNA transcriptomic analysis. (A) Fresh LAM lung (patient LAM3) with multiple variable-sized cysts (*). The portion used for single-cell RNA sequencing (scRNA-seq) analysis is indicated by dashed lines. Scale bar, 1 cm. (B) Histologic image of LAM3 lung adjacent to the portion used for scRNA-seq analysis showing a cyst (*) surrounded by LAM cells (denoted as “L”) arranged as bundles and as a nodule (denoted as “N”). Scale bar, 100 μm. (C) Higher-magnification image of the boxed area in B showing haphazardly arranged bundles of spindled and epithelioid LAM cells with eosinophilic to clear cytoplasm. Scale bar, 20 μm. (D and E) The LAM cells are diffusely positive for ACTA2 with a variable subpopulation staining for PMEL. Scale bars, 100 μm. (F–Q) Diagnostic histologic features of LAM were also present in patients LAM1 (F–I), LAM2 (J–M), and LAM4 (N–Q). Multiple variable-sized cysts (*) with surrounding LAM cell (denoted as “L”) bundles positive for ACTA2 were present in all lungs. Higher-magnification images of the boxed areas in F, J, and N (H, L, and O, respectively) show characteristic bundles of spindled and epithelioid LAM cells with (H, L, and O) eosinophilic to clear cytoplasm. LAM cells staining for PMEL were detected in LAM1 (I, arrows) and LAM4 (Q, arrows) lungs with no PMEL-positive LAM cells detected in the LAM2 lung (M) from a patient being treated with sirolimus. Only very rare LAM cells were MLANA positive (P, arrows), representing a much smaller subpopulation than PMEL-positive cells. Scale bars: F, G, J, K, and N, 100 μm; H, I, L, M, and O–Q, 20 μm.
Table 1.
Identification of TSC1 and TSC2 Mutations in Tissues of Patients with LAM/AML
| Patient ID | Age (yr) | Rx | Tissue | Genomic Location (hg19) | Nucleotide Change | Protein Change | Mutation Type | Mutant Allele Frequency |
|---|---|---|---|---|---|---|---|---|
| LAM1 | 72 | No | Lung | chr16:2112983 | TSC2; c.1372C>T | p.(Arg458*) | Nonsense | 2.0–4.0% |
| LAM2 | 65 | Yes | Lung | chr16:2136360 | TSC2; c.4829G>A | p.(Trp1610*) | Nonsense | 3.0% |
| LAM3 | 50 | No | Lung | chr16:2122880 | TSC2; c.2251C>T | p.(Arg751*) | Nonsense | 5.7% |
| LAM4† | 52 | No | Lung | chr16:2103385 | TSC2; c.268C>T | p.(Gln90*) | Nonsense | 6.1% |
| chr16:2129160 | TSC2; c.3094C>T | p.(Arg1032*) | Nonsense | 4.0% | ||||
| LAM5† | 37 | Yes | Lung | chr16:2135028 | TSC2; c.4569 + 1G>T | N/A | Splice donor | 4.0% |
| chr16:2114333 | TSC2; c.1504delC | p.(His502Thrfs*33) | Frameshift | 4.5% | ||||
| Uterus | chr16:2135028 | TSC2; c.4569 + 1G>T | N/A | Splice donor | 1.7% | |||
| chr16:2114333 | TSC2; c.1504delC | p.(His502Thrfs*33) | Frameshift | 2.0% | ||||
| AML | 47 | No | Kidney | chr9:135781167 | TSC1; c1798C>T | p.(Gln600*) | Nonsense | 79.0% |
Definition of abbreviations: AML = angiomyolipoma; Arg = arginine; chr = chromosome; del = deletion; Gln = glutamine; His = histidine; LAM = lymphangioleiomyomatosis; N/A = no protein changes; Rx = sirolimus treatment; Thr = threonine; Trp = tryptophan.
Two-hit gene mutations were identified.
We identified 18 distinct cell types from merged scRNA-seq data from LAM1 and LAM3 lung samples (Figures 2A and 2B and E1). These included epithelial cells (alveolar type 1 and alveolar type 2 [AT2] and airway), vascular endothelial cells (vascular type 1 and vascular type 2), lymphatic endothelial cells, immune cells (T cell, B cell, macrophage, dendritic cell, monocyte, mast cell, natural killer cell, and plasmacytoid dendritic cells), and mesenchymal cells. A small cluster of unique cells expressed multiple known LAM-associated genes, including PMEL (18, 19), ACTA2 (19), ESR1 (20), and FIGF (VEGFD) (21), hereafter termed LAMCORE cells (Figure 2A, magenta; Figure E1). LAMCORE cells were distinct from, but closely related to, an adjacent cluster of cells expressing typical lung mesenchymal cell signature genes (Figure 2A, dark blue), suggesting the mesenchymal nature of the LAMCORE cells. Predicted cell types were validated using cell-type selective markers (Figures 2B and E1).
Figure 2.


Identification of LAMCORE cells. (A) Single-cell RNA sequencing (scRNA-seq) data of LAM1 and LAM3 lungs. The dashed line encircles LAMCORE cells that express known lymphangioleiomyomatosis (LAM) markers. Cells are visualized in two-dimensional space calculated by uniform manifold approximation and projection (UMAP) and colored by cell types. (B) Expression of known LAM markers (PMEL and ACTA2) in all cells of scRNA-seq
LAMCORE cells were also identified via unbiased clustering of integrated (LAM1–4) as well as in individual LAM1, LAM3, and LAM4 lungs but not detected in scRNA-seq of LAM2 lung (Figures 2C and E2). The absence of LAMCORE cells in LAM2 may have been related to ongoing use of sirolimus in that patient up to the date of transplant. Consistent with the single-cell data, PMEL expression was detected by immunohistochemistry in the LAM cells surrounding the cysts in the LAM1, LAM3, and LAM4 lungs but not in the LAM2 lung (Figure 1). Because no LAMCORE cells were detected in LAM2, data from this lung were not included in further analyses. A total of 125 LAMCORE cells were identified from single-cell transcriptomic profiling of LAM1, 3, and 4 (Figure 2C). A total of 777 LAMCORE signature genes were identified, including well-known LAM markers, such as PMEL, MLANA (22), FIGF, ESR1, and many new and more selective markers, including HOXD11, HOXA11, VGLL2, and SLC35F1 (Figures 2D and 2E and Table E2). As shown in Figure 2F, LAMCORE signature genes were enriched in bioprocesses involving extracellular matrix organization, collagen and muscle fiber formation, cell migration and adhesion, prostaglandin metabolic and biosynthetic processes, and the development and differentiation of vessels, neurons, and the urogenital system. LAMCORE signature genes shared closest identity with disease genes associated with neoplasms of the female genitourinary tract, including neoplasms of ovary, uterine fibroids, and secondary malignant neoplasms of lung and lymph node (Figure 2F). Pathway analysis predicted the activation and interactions among mTOR, WNT (23), YAP-HIPPO (24), VEGF, PI3K-AKT, prostaglandin synthesis (25), Rho GTPases, ILK, and ERK/MAPK (26) signaling pathways (Figure E3). Proliferation markers were not increased in LAMCORE cells (Figure E1C).
AML is commonly present in patients with LAM. We analyzed scRNA-seq from a sporadic renal AML. AML lesions consist of three major cell types: ACTA2+ cells, immune cells, and endothelial cells. Renal AML shared some signature genes with pulmonary LAMCORE cells, including the expression of known and newly predicted LAM markers in ACTA2+ cells (Figure E4). A distinct characteristic of the AML ACTA2+ population is that it also shares transcriptomic similarity with epithelial cells, consistent with epithelial–mesenchymal transition activation.
Distinction of LAMCORE from Normal Lung Mesenchymal Cells
Genes differentially expressed in LAMCORE cells and lung mesenchymal cells from control female lungs (Figure E5) were identified using negative binomial probability-based testing (27) (Figure 2G and Table E3). Representative differentially expressed LAMCORE cell-selective markers are shown in Figure 2H. Bioprocesses and pathways induced in LAMCORE cells compared with control lung mesenchymal cells including glucosamine-containing compound catabolic process, uterus development, MAPK signaling pathway, cytoskeletal regulation by RHO GTPase, and FoxO signaling pathway are shown in Figure E5B.
Validation of Representative Markers Expression at the mRNA and/or Protein Level
Expression of known LAMCORE signature RNAs (CTSK, FIGF, PGR, and MYH11) and novel LAMCORE signatures (KCNAB1 and PALLD) were validated by qPCR and immunofluorescence staining (Figure E6). LAMCORE signature genes RAMP1 and MYH11 were coexpressed with ACTA2 in LAM nodules (Figures 3G–3I and E6G), supporting RAMP1 as a new marker for LAMCORE cells. YAP and PTGER3 staining are used to validate the activated pathways in LAMCORE cells, that is, HIPPO/YAP and prostaglandin signaling as shown in Figure E3. Increased nuclear YAP was present in both PMEL+ cells and PMEL− smooth muscle cells in LAM lung (Figures 3A–3F). PTGER3 (28) staining was detected in ACTA2-positive cells in LAM lesions (Figure E6H).
Figure 3.
Validation of expression of LAMCORE signature genes and activation of YAP. (A) Histology (hematoxylin and eosin [H&E]) of normal human lung bronchiole, surrounding smooth muscle, and alveoli. Scale bars: B, 200 μm; C, 50 μm. (B and C) PMEL and YAP coimmunostaining showing weak nuclear staining of YAP in bronchiolar epithelium and absence in stroma in control donor lung; note nonspecific reaction of red blood cells. Scale bars: B, 200 μm; C, 50 μm. (D) Histology (H&E) of a lymphangioleiomyomatosis (LAM) nodule and cyst. Scale bar, 200 μm. (E and F) PMEL and YAP immunostaining demonstrates nuclear YAP in PMEL-positive cells (white arrow) and PMEL-negative smooth muscle cells (yellow arrow). Scale bars: E, 200 μm; F, 50 μm. (G) RAMP1, CGRP, and ACTA2 coimmunostaining demonstrates RAMP1 expression in normal bronchiolar epithelium; and RAMP1 and rare CGRP expression in neuroendocrine cells (white arrow) in a control donor. Scale bar, 50 μm. (H) Abundant RAMP1 staining in ACTA2+ cells within LAM lesions (white arrow). Scale bar, 50 μm. (I) RAMP1 and CGRP costaining is seen in a subset of cells adjacent to ACTA2+ cells in a LAM lesion (white arrow). Scale bar, 50 μm; inset, 25 μm.
LAMCORE Cell Origins Predicted by scRNA-seq Analysis
To predict the tissue of origin of LAMCORE cells, we compared LAMCORE cell signature genes with multiple organ signatures identified from RNA expression data that were downloaded from the HumanGeneAtlas (29), BodyMap (30), and Molecular Signatures Databases (31). Tissue-selective signature genes were identified for 84 different human tissues (Figure 4A) and compared with the LAMCORE signature (Figure 4B and Table E4). Uterine signatures shared the highest similarity with the LAMCORE signature via Fisher’s exact test (P = 4.1 × 10−28). Smooth muscle, adipocyte, brain regions, and retina also shared signature genes with LAMCORE cells (Figure 4B and Table E4). Representative uterine and neural crest genes were significantly enriched in LAMCORE but not in LAM-associated or donor mesenchymal cells (Figure 4C). Interestingly, LAMCORE cells express a unique panel of signature genes characteristic of normal uterus and known to be important in sex steroid action; these included homeobox transcription factors HOXA9, HOXA11, HOXD11, and EMX2. These uterine selective transcription factors were not detected in normal lung. Comparison of LAMCORE signature genes (n = 777) with BodyMap top tissue selective markers showed that the LAMCORE signature most significantly overlapped with that of uterine myometrium (Figure E7 and Table E5).
Figure 4.
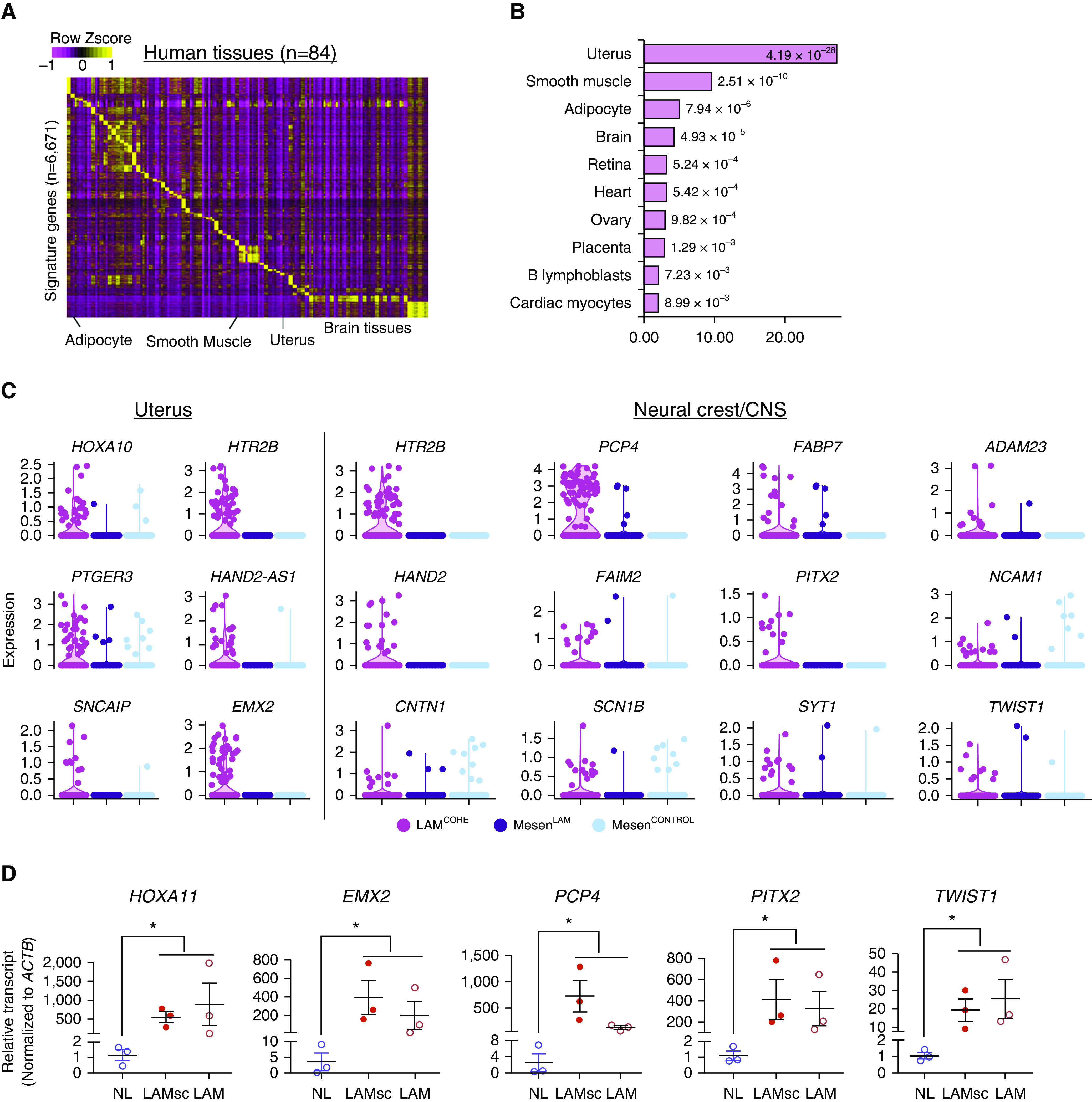

Bioinformatics analysis revealed transcriptomic similarity of LAMCORE cells to uterine cells. (A) Heatmap of expression of predicted signature genes for human tissues. RNA profiles of human tissues were from the HumanGeneAtlas data set. (B) Human tissues whose signatures were most associated with the LAMCORE signature. Significance of association was tested using Fisher’s exact test using whole genome as background. (C) Violin plots of expression of LAMCORE-enriched uterine and neural crest/CNS genes in single-cell RNA sequencing (scRNA-seq) data. MesenLAM represents lymphangioleiomyomatosis (LAM)-associated mesenchymal cells identified by scRNA-seq of LAM lung; MesenCONTROL represents mesenchymal cells identified from scRNA-seq of normal lung. (D) LAMCORE marker genes were measured in normal control and LAM tissues by RT-PCR. LAMsc = samples from LAM lung tissues that were used for single cell sequencing (LAM1, LAM3, and LAM4). LAM = samples from independently archived LAM lung tissues. Data are mean ± SEM. Nonparametric Mann-Whitney U test was used to compare LAM group with control. *P < 0.05. (E–P) Lung tissues from patients with LAM and control donors were immunostained and imaged by confocal immunofluorescence microscopy. Nuclear HOXD11 was detected in MYH11+ cells in LAM lung lesions in LAM lung (E, F, and F’) and was absent in control lung (G and H). PCP4 was present in MYH11+ cells in LAM lesions in LAM lung (I, J, and J’) and was absent in control lung (K and L). PITX2 was present in ACTA2+ cells in LAM lesions in LAM lung (M, N, and N’) and was absent in control lung (O and P). Panels F’, J’, and N’ show staining for HOXD11, MYH11, PCP4, PITX2, or ACTA2 without DAPI. Yellow arrows indicate positive staining, and white arrows indicate negative staining. Scale bars: E, 200 μm; F, F’, J, J’, N, and N’, 10 μm; G, I, K, M, and O, 100 μm; H, L, and P, 20 μm. CNS = central nervous system; NL = normal control.
RNA analysis and immunofluorescence staining were used to validate a number of newly identified LAMCORE cell signature genes that were selectively expressed in uterus and neural crest but not in lung. Uterine- (HOXA11, HOXD11, EMX2, RAMP1, PTGER3, PGR) and brain/neural crest (PCP4, UNC5D, KCNAB1, PITX2 and TWIST1)–associated genes were abundantly expressed in the pulmonary LAM lesions but barely detectable in normal female lungs (Figures 3G–3I, 4D–4P, and E6). Taken together, present RNA and immunofluorescence analyses showed the similarity of LAMCORE and uterine cells, providing support for the concept that uterus is a potential source of LAM cells in the lung.
Identification of LAMCORE Cells in Uterus from a Patient with Pulmonary LAM
Uterine LAM lesions have been reported in rare patients with pulmonary LAM (32). To determine whether uterine LAM cells share the unique LAMCORE cell transcriptome identified by pulmonary LAM single-cell analysis, we performed snRNA-seq on uterine tissue obtained at the time of hysterectomy from a patient with sporadic LAM (LAM5) and a “normal” female donor who was undergoing uterine resection for cervical cancer. Gross inspection and histopathology of the LAM uterus revealed multiple well-circumscribed, firm nodules with a whorled trabecular pattern bulging from the myometrial surface, consistent with leiomyomas. No macroscopic LAM lesions were identified, consistent with previous observations that most uterine LAM lesions in patients with sporadic LAM are microscopic and localized in the subserosal myometrium (32). Unbiased clustering analysis of the snRNA data from LAM uterus identified a distinct cluster of cells sharing RNA expression patterns with pulmonary LAMCORE cells, including known and newly predicted markers (Figures 5A and E8). Uterine myocyte, epithelial, immune, endothelial, and stromal cell subtypes were identified (Figures 5A and E8). Hierarchical clustering of major cell types from LAM lung and LAM uterus demonstrated close correlation of LAMCORE cells from both tissues (Figure 5B). Uterine- and neural crest–associated genes identified in pulmonary LAMCORE cells were assessed in scRNA-seq or snRNA-seq data from the human LAM uterus and normal human/mouse uterus (33). Most neural crest– and uterine-associated genes selectively expressed in pulmonary LAMCORE cells were expressed in the LAMCORE cell cluster from the human LAM uterus (Figures 5A and E8) as well as in myocytes and stromal cells in the normal human and mouse uteri (Figure E9) but not in normal lung. Immunofluorescence staining of uterine marker HOXD11 and neural crest marker PCP4 demonstrated their expression in normal human uterus (Figure E10).
Figure 5.
Expression of LAMCORE signature genes in a lymphangioleiomyomatosis (LAM) uterus. (A) Single-nuclei RNA sequencing (snRNA-seq) analysis of a uterus from a patient with sporadic LAM (LAM5) identified a cluster of cells expressing LAMCORE signature genes. The left panel shows cells in a t-distributed stochastic neighbor embedding (tSNE) plot and colored by major cell types; the dashed line encircles uterine LAMCORE cells (magenta). The right panels show expression of LAMCORE signature genes in tSNE plots of snRNA-seq of a LAM uterus; black arrows indicate the uterine LAMCORE cell cluster. (B) Hierarchical clustering of major cell types of single cells/nuclei from LAM lung and LAM uterus. Endo = endothelial cells; Epi = epithelial cells; Immune = immune cells; LL = sc/snRNA-seq data from LAM lungs; LU = snRNA-seq data from a LAM uterus; Mesen = mesenchymal cells. The text in magenta labels LAMCORE cells. (C–R) LAM lesions in the uterus of a patient with LAM. (C) Fresh uterine tissue from patient LAM5 showing subserosal myometrium adjacent to tissue used for snRNA-seq analysis. No macroscopic LAM lesions were identified. Scale bar, 1 cm. (D–J) LAM lesions in the uterus identified microscopically resembled lung LAM lesions. Uterine LAM lesions were composed of variably dilated spaces (D and E, *) surrounded by bundles and nodules of ACTA2-stained (E) spindled and epithelioid LAM cells with eosinophilic to clear cytoplasm (F). Lesions were lined by podoplanin (PDPN)-stained lymphatic endothelial cells (G, arrows), with a variable subpopulation of LAM cells stained with HMB-45 antibody for PMEL (H, arrows). LAM cell clusters (I, arrow) surrounded by a monolayer of PDPN-stained lymphatic endothelial cells (J, arrow) within lymphatic spaces (I and J, *) were present. (K–R) A second uterine lesion with abundant PMEL immunopositive cells (L, arrows) is shown. Higher-power images demonstrate bundles of spindled LAM cells (M) with characteristic granular cytoplasmic PMEL staining (N). Lesions were again lined by PDPN-stained lymphatic endothelial cells (O, arrows), around the bundles of LAM cells that diffusively stained for ACTA2 (P). Vascular involvement by LAM cells was present with infiltration of the vessel (Q, labeled v) wall by spindled and epithelioid LAM cells (R). Scale bars: D, E, and Q, 100 μm; K, L, O, and P, 50 μm; M and N, 25 μm; F–J and R, 20 μm. LymEndo = lymphatic endothelial cell; Stromal-1 = stromal cell subtype 1; Stromal-2 = stromal cell subtype 2; VasEndo = vascular endothelial cell.
The presence of LAMCORE cells within the uterine LAM tissues used for snRNA-seq was demonstrated by microscopic and immunohistochemical analysis of contiguous histologic sections (Figures 5C–5R). Uterine LAM lesions stained diffusely for ACTA2 and focally for PMEL (Figures 5E–5P). LAM cell clusters composed of LAM cells surrounded by an abundance of lymphatic endothelial cells were observed in the uterine LAM lesions (7) (Figures 5I and 5J) and are consistent with the widely proposed roles for lymphangiogenesis and lymphangitic spread in the pathogenesis of LAM.
Notably, genotyping on LAM lung and uterus biopsy samples from the same patient demonstrated that the uterine LAM cells and pulmonary LAM cells share the same biallelic TSC2 mutations (Table 1). This finding together with their almost identical morphological characteristics and genetic signatures indicates that they arise from a common source or that one of these organs is the source and the other is the target. We submit that the presence of LAMCORE cells in both the lung and uterus, evidence presented here for lymphatic and vascular invasion by LAMCORE cells in the uterus, multiple case reports of LAM lesions in the uterus of patients with sporadic LAM and TSC subjects with pulmonary LAM (32, 34, 35), and the known descending gradient-like involvement of axial lymphatic nodes from pelvis to thorax in patients with LAM support the uterus as the likely source of LAMCORE cells.
The LAMCORE Secretome Predicted by scRNA-seq
Because only a fraction (<20%) of the cells in LAM lesions contain mutations in TSC genes (5), it is likely that most cells that make up LAM nodules are recruited by the LAMCORE cells. To identify genes and processes active in LAMCORE cells that are predicted to influence pulmonary parenchymal and circulating cells, we identified LAMCORE RNAs encoding “secretome” proteins. A total of 162 LAMCORE signature genes encoded secretome proteins (Table E2). Of these, 11 secretome proteins were exclusively found in the lungs of patients with LAM and were not expressed in normal control lungs. Disease and functional enrichment analysis using Ingenuity Pathway Analysis (IPA) suggested that 9 of 11 were associated with tumorigenesis of the female reproductive tract (PMEL, IGSF21, C10orf90, NELL2, GOLM1, CPA6, PRLR, MMP11, and CHI3L1) and 6 of 11 were associated with uterine carcinoma (C10orf90, NELL2, GOLM1, CPA6, PRLR, and MMP11). Aptamer proteomics of serum from female patients with TSC-LAM identified increased levels of proteins encoded by LAMCORE signature genes compared with females with TSC mutations without LAM, including CHRDL1, CNTN1, COL18A1, FGFR1, IGFBP4 (36), IGFBP6, MATN2, MFGE8, TGFBR3, and THBS2 (Figure 6A). CHRDL1, IGFBP4, and the COL18A1 cleavage product, endostatin, were increased in LAM tissues or serum as validated via qRT-PCR or ELISA assay (Figures 6B and E6).
Figure 6.

Prediction and validation of the LAMCORE cell “secretome.” (A) LAMCORE signature genes encoding predicted secreted proteins and their enriched functional annotations are shown. Human Protein Atlas and VerSeDa databases were used to annotate secretome proteins. ToppGene suite was used to perform functional enrichment analysis. Network diagram was generated using Cytoscape (v3.7.1). Hexagon-shaped nodes represent predicted secreted proteins; link colors represent different bioprocesses/pathways. Pink nodes represent proteins associated with lymphangioleiomyomatosis (LAM) in the literature. Proteins in yellow were validated by aptamer proteomics of the serum of patients with LAM. Proteins in cyan nodes were identified in the serum of patients with LAM by ELISA. Proteins in nodes with both yellow and cyan colors were validated by both aptamer proteomics and ELISA. (B) ELISA analysis of serum levels of IGFBP-4 and endostatin (derived from proteolysis of COL18A1) in female TSC (tuberous sclerosis complex) subjects with and without pulmonary LAM (TSC-LAM and TSC, respectively), and serum samples from healthy control subjects. *P < 0.05; one-way ANOVA with Dunnett’s multiple comparison test in ELISA. ECM = extracellular matrix associated; GSG/PG = glycosaminoglycan/proteoglycans; HC = healthy control.
The Pleiotropic Changes in Gene Expression in Other Cell Types in the LAM Lung
Complex changes in transcriptomic programs occur in multiple cell types in the LAM lung, including lymphatic endothelial, airway and alveolar epithelial, and inflammatory cells (37, 38). Expression of genes associated with metastatic invasion of lymphatic vessels (UNC5B, NECTIN2, CD200, ESAM, and ENG) (39) was increased in LAM-associated lymphatic endothelial cells (Figures E11A and E11B). Factors controlling lymphatic vascular development, including PROX1, NR2F2, NRP2, FLT4 (VEGFR3), and TBX1, were abundantly expressed in lymphatic endothelial cells of LAM samples (Figure 7). Epithelial cell gene expression in LAM lung was distinct from that of controls. For example, expression of genes involved in host defense and innate immunity (DMBT1, LCN2, and SFTPD) and cytokine/IL signaling (IFI27 and IL20RA) was increased in LAM-associated AT2 cells. Bioprocesses characteristic of normal AT2 cells, including biosynthesis and metabolism of lipids and lipoproteins, SREBP-mediated cholesterol biosynthesis, and activity of the ERK1 and ERK2 cascade, were suppressed in LAM-associated AT2 cells (Figure E11C). Effects of LAM on AT2 cells included activation of innate immune functions and suppression of surfactant biosynthesis. The expression of multiple cytokines and chemokines involved in the recruitment of monocytes (IL1B, CSF1R, and TLR2), T cells (CD8A and CD3D), B cells (CD79A and CD19), mast cells (KIT and FCER1A), natural killer cells (NKG7 and KLRF1), and other inflammatory cells was noted. The pleiotropic changes in gene expression in multiple cell types in the LAM lesions are consistent with the complexity of histopathological changes associated with pulmonary LAM lesions.
Figure 7.

Predicted schematic model summarizing interactions between LAMCORE and lymphatic endothelial cells in lymphangioleiomyomatosis (LAM). Left panel: interactions between LAMCORE and lymphatic endothelial cells in LAM; right panel: gene expression in single-cell RNA sequencing of LAM lungs. In LAMCORE cells, TSC2 mutations lead to mTOR activation, enhancing secretion of VEGFD, CCBE1, ECM, and collagens, which activate VEGFR3 signaling in lymphatic endothelial cells via multiple ligand–receptor interactions, including VEGFR3, KDR, NRP2, and ITGB1. Within the activated lymphatic endothelial cells, transcription factors PROX1, NR2F2, TBX1 and SOX18 regulate lymphatic endothelial cell specification and morphogenesis by inducing VEGFC/VEGFD/VEGFR3-dependent lymphangiogenic programs in LAM. AirwayEpi = airway epithelial cell; AT1 = alveolar type 1 cell; AT2 = alveolar type 2 cell; B-cell-1 = B cell subtype 1; B-cell-2 = B cell subtype 2; DC = dendritic cell; ECM = extracellular matrix; Mast = mast cell; NK = natural killer cell; pDC = plasmacytoid dendritic cell; pMacrophage = proliferative macrophage; VasEndo-1 = vascular endothelial cell subtype 1; VasEndo-2 = vascular endothelial cell subtype 2.
Discussion
Recurrence of pulmonary LAM following lung transplantation supports the concept that LAM is a metastatic neoplasm rather than an interstitial lung disease arising from expansion of tissue-resident mesenchymal cells (3, 4). Sources of cells that make up the LAM lesion and the mechanisms that control tumorigenesis, metastasis, invasion, and cystic remodeling have remained elusive (1, 4, 40). In the present study, single-cell transcriptomic analysis of lung and uterine LAM lesions identified a unique population of cells, which we termed LAMCORE cells, that shared close transcriptomic similarity with myometrial and stromal cells from normal human and mouse uteri. A prominent neural crest signature was also identified in pulmonary and uterine LAMCORE cells and in normal uterus. Single-cell transcriptomic profiling, together with aptamer proteomics of patient serum, quantitative PCR, immunofluorescence confocal microscopy, and serum ELISA, identified novel secreted proteins, cell-specific signature genes, and altered bioprocesses and signaling pathways characteristic of LAMCORE cells.
LAMCORE cells expressed a unique gene signature likely driven both by cell autonomous effects of mTOR activation and by interactions with neighboring and recruited cells in their pulmonary niche. The transcriptomic profile of the LAMCORE cells was not consistent with that of any known pulmonary cell and shared closest transcriptomic similarity with normal uterine myocytes and neural crest. The similarity of gene expression in uterine and pulmonary LAMCORE cells is consistent with a uterine source for pulmonary LAM cells and is supported by the close relationship between myometrial tumors and spontaneous pulmonary metastasis in the mouse model of myometrium-specific Tsc2 deletion (34, 35). The clinical features of female sex restriction, pelvic predominant distribution of lymph node involvement, hormonal responsiveness, and prior case reports of uterine LAM provide further support for the concept that LAM is a metastatic uterine tumor. The occurrence of LAM in men indicates that other sources for pulmonary LAM cells must exist, and the occurrence of primary perivascular epithelioid cell neoplasms in other locations within the male and female genitourinary tract (e.g., kidney, bladder, ovary, and prostate), gastrointestinal tract (e.g., stomach, small intestine, colon, and liver), soft tissue, and bone support additional sources of the tumor in men and women with LAM (6). The possibility that mTOR activation may drive aberrant gene expression that closely mimics uterine programs cannot be completely excluded by the data presented.
A model for LAM has been proposed in which somatic mutations in TSC2 lead to mTOR activation, cellular proliferation, and VEGF-D–dependent lymphangiogenic programs that drive recruitment of lymphatic endothelial cells, lymphatogenous spread to the lung, and cystic lung remodeling (1, 7, 8). Evidence for this concept has been limited to histological and immunohistochemical studies that demonstrate LAM cell clusters lined by VEGFR-3–expressing lymphatic endothelial cells lining dysmorphic lymphatic channels in lung and uterus of patients with LAM (7, 8). The present study provides new evidence at single-cell resolution that supports the concept that LAMCORE cells interact with lymphatic endothelial cells and activate VEGFC/VEGFD–VEGFR3 signaling pathways that enhance lymphangiogenesis (Figure 7). VEGF signaling pathways play a critical role in lymphatic endothelial cell proliferation and migration (41, 42). The findings that both lung and uterine LAMCORE cells express VEGFD, that FLT4 (VEGFR3) RNA is highly expressed in lymphatic endothelial cells, and that VEGFC is selectively expressed in vascular endothelial cells support an important role for this signaling network in the pathogenesis of LAM. Key regulators driving VEGF signaling and lymphangiogenesis are selectively expressed in LAMCORE and lymphatic endothelial cells (Figure 7). Specifically, LAMCORE cells express VEGFD, CCBE1, ECM, and collagens, which are known to activate VEGF signaling in lymphatic endothelial cells via multiple ligand–receptor interactions, including VEGFR3, KDR, NRP2, and ITGB1 (43, 44). PROX1 (45), NR2F2 (46), TBX1 (47), and SOX18 (48), known regulators of lymphatic morphogenesis, are expressed in the LAM-associated lymphatic endothelial cells. NR2F2, a nuclear receptor expressed in LAMCORE cells and lymphatic endothelial cells, was recently identified by a genome-wide association study as a susceptibility locus in LAM (49). EP3 (PTGER3) (28), a regulator of tumor-associated lymphangiogenesis that enhances expression of VEGFC/VEGFD and VEGFR-3 in tumor stromal tissues (50), is selectively expressed in LAMCORE cells. CCBE1, a secreted extracellular matrix protein selectively expressed in LAMCORE cells, is required for embryonic lymphangiogenesis (51) and is mutated in Hennekam syndrome (52). Taken together, the data presented support a central role for the VEGFC/VEGFD–VEGFR3 axis in the molecular pathogenesis of LAM in which LAMCORE cells interact with lymphatic endothelial cells to stimulate lymphangiogenesis (Figure 7), disseminate via blood and lymphatics, and drive cystic remodeling in the lung (53, 54).
LAMCORE cells expressed multiple growth factors, including VEGFD, PDFGRA/B, FGF7, IGFBP2/4/6, and TGFB1I1, that likely mediate active crosstalk among pulmonary LAMCORE and surrounding cells (Figure 6A and Table E2). RNAs encoding the LAMCORE cell “secretome” provide insights into potential mechanisms by which LAM cells orchestrate tissue destruction and shape the cellular microenvironment. For example, genes encoding extracellular matrix, including glycosaminoglycans, proteoglycans, diverse collagens, proteolytic enzymes (MMPs and ADAMs), and a number of matricellular proteins, including thrombospondins, fibulins, SPARC, CYR61 and CTGF, were the most enriched functional classes of “secretome” proteins in LAMCORE cells (Figure 6A). The finding that LAMCORE cells selectively expressed VEGF-D (FIGF) in the lung supports its use as a biomarker of mTOR pathway activation in LAM (9). New potential serum biomarkers were identified from the LAMCORE cell secretome analysis (Figure 6A and Table E2), including Col18A1 and IGFBP-4, which were increased in serum of patients with TSC-LAM (Figure 6B). Endostatin, an antiangiogenic cleavage product of matrix protein Col18A1 (55), may bias vasculogenesis toward lymphatic lineages, and IGFBP-4, a protein that binds and enhances activation of IGF-1 and IGF-2 (36), may provide a growth advantage to LAM cells.
LAMCORE cells expressed a unique panel of signature genes shared with uterine LAM cells, and with normal human and mouse uterus cells, but not with normal human lung. LAMCORE cells and normal myometrial cells shared close transcriptomic similarities, many of which imply regulation by female sex hormones, including estrogen, progesterone, and prolactin receptors (35, 56). LAMCORE cells expressed a unique panel of uterine-selective transcription factors, including HOXD11, HOXA11, and EMX2, that are regulated by the menstrual cycle and play important roles in female reproductive tract development and oncogenesis (57–63).
There are several limitations in the present study, most related to the rarity of LAM disease and limited availability of fresh tissue samples. The yield of mesenchymal cells, including LAM cells, isolated from the tissues for scRNA-seq analysis of all lung specimens was relatively low, perhaps related to incomplete proteolytic liberation of the cells from matrix or effects of the digestion protocol on cell viability. Another point worth noting is that there is no perfect control of human samples. The control lung tissue used in our studies included explants declined for organ donation (17); thus, cells and RNA expression may have been altered by prior medical and physiologic conditions. Nevertheless, present observations regarding distinct uterine-related gene signatures and transcriptional programs in LAM lung were not likely influenced by comparisons made between LAM and the explanted control samples.
In summary, we used scRNA and snRNA sequencing to identify a unique population of LAMCORE cells in both LAM lung and uterus. Evidence presented supporting the uterus as a likely source of LAM cells in the lung includes the following: 1) LAM cells in the lung and uterus are morphologically indistinguishable, 2) LAM cells in the lung and uterus share the same biallelic TSC2 mutations, 3) LAMCORE cells in the lung and uterus of a patient with sporadic LAM show close transcriptomic identity, and 4) LAMCORE cells are readily distinguished from endogenous lung cells and shared closest transcriptomic similarity to normal uterus. Novel LAMCORE cell–specific secretome proteins were identified, which may serve as biomarkers for diagnosis, prognosis, and therapeutic responses in LAM. The present findings provide new insights into the source and cellular characteristics of LAM cells; predict signaling networks by which LAMCORE cells infiltrate, recruit, and interact with other cells in the lung; and inform the development of molecular pathogenesis–driven biomarkers and therapeutic targets.
Supplementary Material
Acknowledgments
Acknowledgment
The LAM Foundation and National Diseases Research Interchange assisted with tissue collection. The LAM Foundation funded the single-cell analyses. This study was also supported by services from the Pathology Research Core shared facility in the Cincinnati Children’s Research Foundation with the assistance of Betsy DiPasquale; by UC Pathology for providing normal uterine tissue; by St. Vincent Women’s Hospital for providing LAM uterine tissue; and by Cincinnati Life Center for providing normal lung. The authors thank Andrew Wagner for data mining assistance.
Footnotes
Supported by the LAM Foundation (LAM0138E01-19 to A.K.P. and Y.X.; LAM0131PB07-18 to F.X.M. and A.K.P.) and the NHLBI (U01HL122642 and U01HL148856 to J.A.W., S.S.P., and Y.X.; U01HL134745 to J.A.W. and Y.X.; R01 HL131661 to A.K.P. and Y.X.; R01HL138481 to J.J.Y.; T32 HL007752 to J.A.W. and M.R.; U01 U54HL127672 and U01HL131022 to F.X.M.).
Author Contributions: J.A.W., F.X.M., and Y.X. conceived and designed the experiments. M.G., P.S., and Y.X. designed and performed lymphangioleiomyomatosis (LAM) single-cell analysis and associated integrative bioinformatics analyses and interpreted data. J.J.Y. and E.Y.Z. designed the validation experiments using qRT-PCR and immunofluorescent staining. A.K.P. and M.R. designed and analyzed LAMCORE cells and subtypes spatial location and signature genes validation using immunofluorescent confocal imaging. K.G. and D.J.K. conducted mutation analysis. F.X.M. designed and validated LAM secretome findings in the serum of patients with LAM using aptamer screen and ELISA. M.A., A.P., and S.S.P. conducted single-cell RNA-seq experiments and contributed to single-cell data analysis. E.J.K. coordinated tissue collection and regulatory issues. K.A.W.-B. examined, processed, and pathologically evaluated human tissues, selected human lung and uterus samples for single-cell and single-nuclei RNA-seq experiments, confirmed lung and established uterine pathologic diagnoses, and determined immunophenotype of LAM cells. S.R.H. provided input regarding uterine origins of LAM cells. E.J.K. and S.S. coordinated acquisition of LAM tissues from transplant centers and hospitals. M.G., J.A.W., F.X.M., and Y.X. wrote the manuscript. All authors contributed to the data interpretation, manuscript writing, and editing. All the authors read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201912-2445OC on June 30, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Henske EP, McCormack FX. Lymphangioleiomyomatosis: a wolf in sheep’s clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack FX, Travis WD, Colby TV, Henske EP, Moss J. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med. 2012;186:1210–1212. doi: 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badri KR, Gao L, Hyjek E, Schuger N, Schuger L, Qin W, et al. Exonic mutations of TSC2/TSC1 are common but not seen in all sporadic pulmonary lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2013;187:663–665. doi: 10.1164/ajrccm.187.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119–132. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumasaka T, Seyama K, Mitani K, Souma S, Kashiwagi S, Hebisawa A, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;29:1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 8.Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28:1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 9.Young L, Lee HS, Inoue Y, Moss J, Singer LG, Strange C, et al. MILES Trial Group. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1:445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta N, Lee HS, Young LR, Strange C, Moss J, Singer LG, et al. NIH Rare Lung Disease Consortium. Analysis of the MILES cohort reveals determinants of disease progression and treatment response in lymphangioleiomyomatosis. Eur Respir J. 2019;53:1802066. doi: 10.1183/13993003.02066-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay G. The LAM cell: what is it, where does it come from, and why does it grow? Am J Physiol Lung Cell Mol Physiol. 2004;286:L690–L693. doi: 10.1152/ajplung.00311.2003. [DOI] [PubMed] [Google Scholar]
- 13.Zhu W, Zhang X-Y, Marjani SL, Zhang J, Zhang W, Wu S, et al. Next-generation molecular diagnosis: single-cell sequencing from bench to bedside. Cell Mol Life Sci. 2017;74:869–880. doi: 10.1007/s00018-016-2368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellsworth DL, Blackburn HL, Shriver CD, Rabizadeh S, Soon-Shiong P, Ellsworth RE. Single-cell sequencing and tumorigenesis: improved understanding of tumor evolution and metastasis. Clin Transl Med. 2017;6:15. doi: 10.1186/s40169-017-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perl A-KT, Adam M, Green J, Sherman S, Yu JJ, Kopras EJ, et al. Single cell RNAseq of lymphangioleiomyomatosis (LAM) lungs reveals unique populations consistent with LAM cells and lymphatic endothelial cells [abstract] Am J Respir Crit Care Med. 2018;197:A7699. [Google Scholar]
- 17.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoon V, Thung SN, Kaneko M, Unger PD. HMB-45 reactivity in renal angiomyolipoma and lymphangioleiomyomatosis. Arch Pathol Lab Med. 1994;118:732–734. [PubMed] [Google Scholar]
- 19.Zhe X, Schuger L. Combined smooth muscle and melanocytic differentiation in lymphangioleiomyomatosis. J Histochem Cytochem. 2004;52:1537–1542. doi: 10.1369/jhc.4A6438.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gao L, Yue MM, Davis J, Hyjek E, Schuger L. In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor. Virchows Arch. 2014;464:495–503. doi: 10.1007/s00428-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 21.Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;4:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 22.Busam KJ, Jungbluth AA. Melan-A, a new melanocytic differentiation marker. Adv Anat Pathol. 1999;6:12–18. doi: 10.1097/00125480-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Mak BC, Kenerson HL, Aicher LD, Barnes EA, Yeung RS. Aberrant beta-catenin signaling in tuberous sclerosis. Am J Pathol. 2005;167:107–116. doi: 10.1016/s0002-9440(10)62958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang N, Pende M. YAP enters the mTOR pathway to promote tuberous sclerosis complex. Mol Cell Oncol. 2015;2:e998100. doi: 10.1080/23723556.2014.998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Lee PS, Sun Y, Gu X, Zhang E, Guo Y, et al. Estradiol and mTORC2 cooperate to enhance prostaglandin biosynthesis and tumorigenesis in TSC2-deficient LAM cells. J Exp Med. 2014;211:15–28. doi: 10.1084/jem.20131080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu X, Yu JJ, Ilter D, Blenis N, Henske EP, Blenis J. Integration of mTOR and estrogen-ERK2 signaling in lymphangioleiomyomatosis pathogenesis. Proc Natl Acad Sci USA. 2013;110:14960–14965. doi: 10.1073/pnas.1309110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, et al. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell. 2016;166:1308–1323, e30. doi: 10.1016/j.cell.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Liu X, Liu Y, Zhang E, Medepalli K, Masuda K, et al. Tuberin regulates prostaglandin receptor-mediated viability, via Rheb, in mTORC1-hyperactive cells. Mol Cancer Res. 2017;15:1318–1330. doi: 10.1158/1541-7786.MCR-17-0077. [DOI] [PubMed] [Google Scholar]
- 29.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hishiki T, Kawamoto S, Morishita S, Okubo K. BodyMap: a human and mouse gene expression database. Nucleic Acids Res. 2000;28:136–138. doi: 10.1093/nar/28.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi T, Kumasaka T, Mitani K, Terao Y, Watanabe M, Oide T, et al. Prevalence of uterine and adnexal involvement in pulmonary lymphangioleiomyomatosis: a clinicopathologic study of 10 patients. Am J Surg Pathol. 2011;35:1776–1785. doi: 10.1097/PAS.0b013e318235edbd. [DOI] [PubMed] [Google Scholar]
- 33.Mucenski ML, Mahoney R, Adam M, Potter AS, Potter SS. Single cell RNA-seq study of wild type and Hox9,10,11 mutant developing uterus. Sci Rep. 2019;9:4557. doi: 10.1038/s41598-019-40923-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prizant H, Sen A, Light A, Cho SN, DeMayo FJ, Lydon JP, et al. Uterine-specific loss of Tsc2 leads to myometrial tumors in both the uterus and lungs. Mol Endocrinol. 2013;27:1403–1414. doi: 10.1210/me.2013-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prizant H, Taya M, Lerman I, Light A, Sen A, Mitra S, et al. Estrogen maintains myometrial tumors in a lymphangioleiomyomatosis model. Endocr Relat Cancer. 2016;23:265–280. doi: 10.1530/ERC-15-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valencia JC, Matsui K, Bondy C, Zhou J, Rasmussen A, Cullen K, et al. Distribution and mRNA expression of insulin-like growth factor system in pulmonary lymphangioleiomyomatosis. J Investig Med. 2001;49:421–433. doi: 10.2310/6650.2001.33787. [DOI] [PubMed] [Google Scholar]
- 37.Osterburg AR, Nelson RL, Yaniv BZ, Foot R, Donica WR, Nashu MA, et al. NK cell activating receptor ligand expression in lymphangioleiomyomatosis is associated with lung function decline. JCI Insight. 2016;1:e87270. doi: 10.1172/jci.insight.87270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis JM, Hyjek E, Husain AN, Shen L, Jones J, Schuger LA. Lymphatic endothelial differentiation in pulmonary lymphangioleiomyomatosis cells. J Histochem Cytochem. 2013;61:580–590. doi: 10.1369/0022155413489311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, et al. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 2008;68:7293–7303. doi: 10.1158/0008-5472.CAN-07-6506. [DOI] [PubMed] [Google Scholar]
- 40.Krymskaya VP, McCormack FX. Lymphangioleiomyomatosis: a monogenic model of malignancy. Annu Rev Med. 2017;68:69–83. doi: 10.1146/annurev-med-050715-104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet. 2000;67:295–301. doi: 10.1086/303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Planas-Paz L, Strilić B, Goedecke A, Breier G, Fässler R, Lammert E. Mechanoinduction of lymph vessel expansion. EMBO J. 2012;31:788–804. doi: 10.1038/emboj.2011.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galvagni F, Pennacchini S, Salameh A, Rocchigiani M, Neri F, Orlandini M, et al. Endothelial cell adhesion to the extracellular matrix induces c-Src-dependent VEGFR-3 phosphorylation without the activation of the receptor intrinsic kinase activity. Circ Res. 2010;106:1839–1848. doi: 10.1161/CIRCRESAHA.109.206326. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, et al. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 2014;28:2175–2187. doi: 10.1101/gad.216226.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011;25:2187–2197. doi: 10.1101/gad.16974811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Mupo A, Huynh T, Cioffi S, Woods M, Jin C, et al. Tbx1 regulates Vegfr3 and is required for lymphatic vessel development. J Cell Biol. 2010;189:417–424. doi: 10.1083/jcb.200912037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 49.Kim W, Giannikou K, Dreier JR, Lee S, Tyburczy ME, Silverman EK, et al. A genome-wide association study implicates NR2F2 in lymphangioleiomyomatosis pathogenesis. Eur Respir J. 2019;53:1900329. doi: 10.1183/13993003.00329-2019. [DOI] [PubMed] [Google Scholar]
- 50.Kubo H, Hosono K, Suzuki T, Ogawa Y, Kato H, Kamata H, et al. Host prostaglandin EP3 receptor signaling relevant to tumor-associated lymphangiogenesis. Biomed Pharmacother. 2010;64:101–106. doi: 10.1016/j.biopha.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 51.Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41:396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- 52.Alders M, Hogan BM, Gjini E, Salehi F, Al-Gazali L, Hennekam EA, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet. 2009;41:1272–1274. doi: 10.1038/ng.484. [DOI] [PubMed] [Google Scholar]
- 53.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP, Jr, Wang JA, Kumaki F, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112:138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wickström SA, Alitalo K, Keski-Oja J. Endostatin signaling and regulation of endothelial cell-matrix interactions. Adv Cancer Res. 2005;94:197–229. doi: 10.1016/S0065-230X(05)94005-0. [DOI] [PubMed] [Google Scholar]
- 56.Terasaki Y, Yahiro K, Pacheco-Rodriguez G, Steagall WK, Stylianou MP, Evans JF, et al. Effects of prolactin on TSC2-null Eker rat cells and in pulmonary lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2010;182:531–539. doi: 10.1164/rccm.200911-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27:331–355. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- 58.Du H, Taylor HS. The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med. 2015;6:a023002. doi: 10.1101/cshperspect.a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgan R, El-Tanani M, Hunter KD, Harrington KJ, Pandha HS. Targeting HOX/PBX dimers in cancer. Oncotarget. 2017;8:32322–32331. doi: 10.18632/oncotarget.15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly ZL, Michael A, Butler-Manuel S, Pandha HS, Morgan RG. HOX genes in ovarian cancer. J Ovarian Res. 2011;4:16. doi: 10.1186/1757-2215-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 62.Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol Reprod. 1997;56:1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.