To the Editor:
Improvements in perinatal care have increased survival of infants born preterm. Preterm birth has been associated with alveolar simplification and perturbed pulmonary microvascular development (e.g., vascular simplification), which is a known risk factor for the development of pulmonary vascular disease (PVD) in neonates and children (1). Recent work from our group demonstrated that young adults born preterm exhibited early PVD (2) and impaired right ventricular (RV)–PV coupling (3). However, it is unknown what contribution the pulmonary microvasculature plays in these previous findings. Dynamic contrast–enhanced (DCE) magnetic resonance imaging (MRI) is a minimally invasive technique that can be used to probe the pulmonary microvasculature on a regional scale (4). DCE MRI has been used to evaluate PVD in patients with pulmonary hypertension, with prolonged pulmonary mean transit time (MTT) associated with increased pulmonary vascular resistance and reduced cardiac index (CI) values (5, 6). Here, we report that male young adults born prematurely exhibit reduced lung perfusion metrics from DCE MRI and that these metrics correlate with gold-standard metrics of cardiopulmonary function as well as perinatal outcomes. Some of the results of this study have been previously reported in the form of an abstract (7).
Our substudy analyzed prospectively acquired data from the Newborn Lung Project (2, 3) and included adults born prematurely (n = 8; 3 male; 26.8 ± 0.4 yr of age at the time of the study; gestational age, 28.9 ± 0.9 wk; birth weight, 1174 ± 102 g). The Newborn Lung Project is a cohort of infants born in Wisconsin and Iowa between 1988 and 1991. Control subjects born at term (n = 9; 7 male; 25.8 ± 0.3 yr of age at the time of the study; gestational age, 40.2 ± 0.2 wk) were recruited from the general population. The institutional review board at the University of Wisconsin–Madison, School of Medicine and Public Health, approved all procedures. Informed consent was obtained from all subjects.
Cardiac and DCE MR images were acquired on a 3-T scanner (Discovery MR750; GE Healthcare), including ECG-gated balanced steady-state free precession images throughout the heart. DCE MRI using gadobenate dimeglumine 0.025 mmol/kg (Gd-BOPTA, Multihance; Bracco Imaging SpA) during end-expiratory breath-hold was used to determine quantitative pulmonary blood flow (PBF) metrics on the basis of indicator dilution theory (8). Voxel-wise perfusion metrics were obtained via deconvolution using standard-form Tikhonov regularization and L-curve criterion optimization. The RV end-diastolic volume, end-systolic volume, stroke volume, and Q̣̇ were calculated using cardiac MRI with a short-axis cine balanced steady-state free precession series and ventricular volumes were indexed to body surface area (e.g., CI) (cvi42, version 5.6.6; Circle Cardiovascular Imaging, Inc.).
Pulmonary function test results using the Global Lung Initiative tools were reported previously, in the study from which the subjects for this substudy were recruited (2). Spirometric values of FEV1, FVC, FEV1/FVC, and midexpiratory-phase forced expiratory flow (FEF25–75) were similar between term and preterm subjects, whereas DlCO was significantly lower (P < 0.05) in preterm subjects as compared with term subjects (2). Given the unbalanced groups by biological sex, we stratified the measures of pulmonary function by biological sex, and these data revealed that males were driving the reductions in DlCO, with significantly reduced % predicted values (P < 0.05), whereas females were not different (P = 0.21).
RV volumes calculated from cardiac MR revealed no significant differences in body surface area–indexed volumes between preterm and term-born subjects. The CI was slightly elevated in the preterm group, but not significantly so (4.07 ± 0.28 vs. 3.36 ± 0.22 L/min/m2; P = 0.07).
Lung-perfusion values of MTT of blood flow through the lungs, PBF, and pulmonary blood volume (PBV) were assessed after segmenting the left and right lungs into apex, middle, and base subregions. Preterm subjects exhibited similar PBF but exhibited reduced MTT (P = 0.038) (Figure 1). There were strong relationships between MTT with DlCO (r = 0.70) and CI (r = −0.73) (Figure 1). These correlations revealed that MTT related to canonical measures of pulmonary and cardiac function, respectively. The groups were unbalanced by biological sex. Specifically, females made up most of the preterm group (n = 5 out of a total n = 8), and males made up most of the term group (n = 7 out of a total n = 9). Therefore, we further analyzed our data by sex. Notably, females showed no differences in PBV but exhibited lower MTT (P = 0.009), which was likely due to increased CI values (P = 0.0035) as compared with males (Figure 1). Sex-dependent differences in MTT were not related to lung volumes calculated from the segmented MR images (r = 0.30; P = 0.24) (data not shown).
Figure 1.
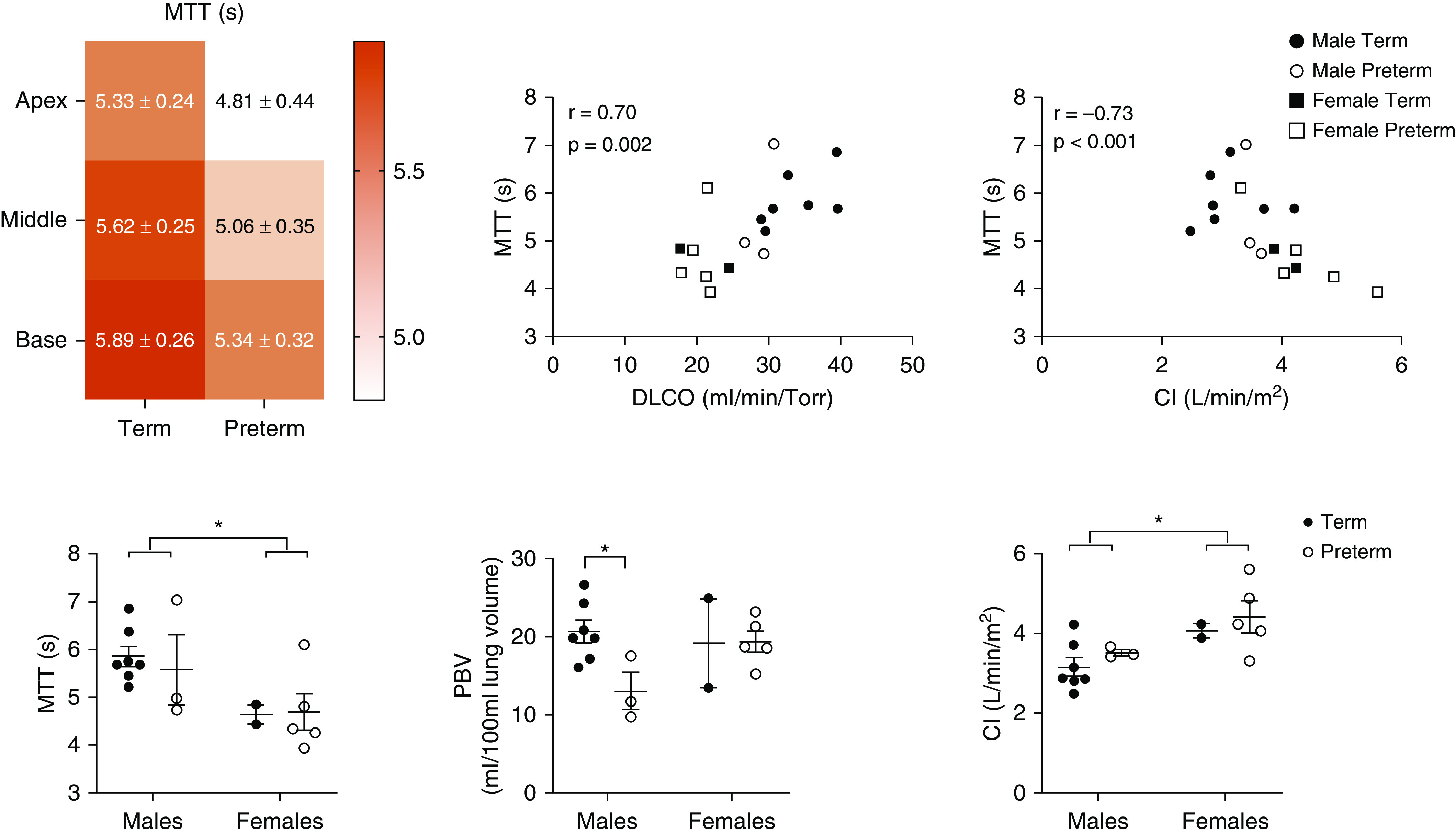
Two-way ANOVA for lung regions in the apex, middle, and base (top left image) shows differences in mean transit time (MTT) (P = 0.038) and pulmonary blood volume (PBV) (P = 0.034; data not shown) between term and preterm groups and by lung region for PBV (P = 0.036). Although qualitatively lower in individuals born preterm, pulmonary blood flow shows no significant differences (data not shown) by group or lung region. Furthermore, Spearman correlation analysis revealed that MTT was moderately to significantly related to DlCO and cardiac index (CI), respectively. However, the data highlight a sex-dependent reduction in MTT (P = 0.009) (bottom left image) that is independent of birth status, likely owing to increased CI (P = 0.0035) (bottom right image), given that there were no sex differences in PBV (bottom middle image). Notably, we see males born prematurely exhibit lower PBV as compared with term-born males (P = 0.02). *P < 0.05.
Interestingly, males born preterm exhibited reduced perfusion metrics compared with term-born males. Preterm males exhibited reduced PBF and PBV compared with term-born males (P = 0.039 and P = 0.02, respectively) (Figures 1 and 2). However, there were no differences in lung-perfusion metrics between females by birth status.
Figure 2.
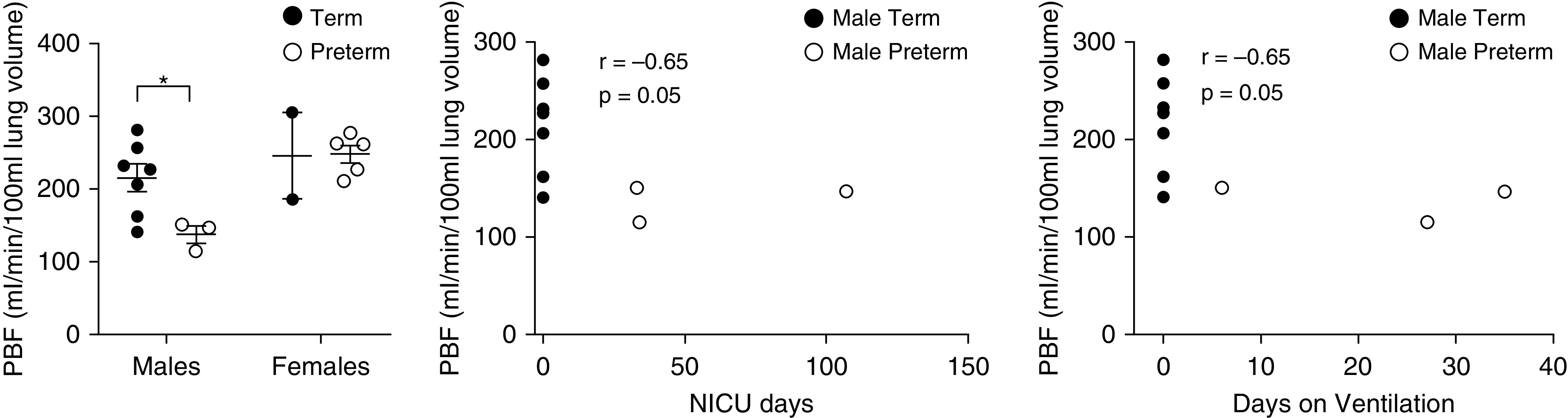
Dynamic contrast–enhanced measures of pulmonary blood flow (PBF) stratified by biological sex. A Student’s t test was used to determine differences between term and preterm subjects by biological sex. Males born preterm exhibit significant reductions in PBF (P = 0.035) compared with term males. Females born prematurely do not exhibit any differences in any of the dynamic contrast–enhanced measures of lung perfusion. PBF (r = −0.65; P = 0.05) is moderately correlated with days in the neonatal ICU (NICU) and with total days on ventilation in males, but not in females, when correlating PBF with days in the NICU (r = 0.04; P = 0.95) and total days on ventilation (r = 0.04; P = 0.95), respectively (data not shown). *P < 0.05.
DCE MRI revealed a preferential reduction of PBF and PBV in the lungs of male adults born prematurely (Figures 1 and 2) compared with male term-born control subjects, despite similar CI values. Reduced PBF and PBV, and decreased DlCO combined with previous work showing increased pulmonary vascular impedance (3) suggests a potential biological sex dependence; specifically, it suggests that males born preterm may have unresolved vascular simplification into adulthood. Moreover, these findings are consistent with those of a recent report that males born prematurely may be more susceptible to cardiac growth arrest than females born prematurely (9).
The decreased PBF, PBV, and DlCO observed in the male population of this small substudy is necessarily preliminary. However, larger studies to elucidate the impact of biological sex on perturbed regional / relationships in individuals born prematurely is clearly warranted.
In summary, young male adults born preterm with no overt cardiopulmonary disease exhibit altered pulmonary microvascular hemodynamics as measured by DCE MRI. These data add to a growing body of knowledge that prematurity may have persistent, lifelong consequences in pulmonary vascular health that may be more pronounced in males.
Supplementary Material
Footnotes
Supported by NIH grants T32AI007635 (G.P.B.); 1R01 HL086897 and R01 HL38149 (M.W.E.); and R01 HL126771 and R01 EB021314 (S.B.F.). K.N.G. is supported by the University of Wisconsin Clinical and Translational Science Award program, through the NIH National Center for Advancing Translational Sciences, grant NIH UL1TR000427 (PI Drezner; 4KL2TR000428-10).
Originally Published in Press as DOI: 10.1164/rccm.202002-0344LE on July 8, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goss KN, Beshish AG, Barton GP, Haraldsdottir K, Levin TS, Tetri LH, et al. Early pulmonary vascular disease in young adults born preterm. Am J Respir Crit Care Med. 2018;198:1549–1558. doi: 10.1164/rccm.201710-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulchrone A, Bellofiore A, Douwes JM, Duong N, Beshish AG, Barton GP, et al. Impaired right ventricular-vascular coupling in young adults born preterm. Am J Respir Crit Care Med. 2020;201:615–618. doi: 10.1164/rccm.201904-0767LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno Y, Hatabu H, Murase K, Higashino T, Kawamitsu H, Watanabe H, et al. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: preliminary experience in 40 subjects. J Magn Reson Imaging. 2004;20:353–365. doi: 10.1002/jmri.20137. [DOI] [PubMed] [Google Scholar]
- 5.Swift AJ, Telfer A, Rajaram S, Condliffe R, Marshall H, Capener D, et al. Dynamic contrast-enhanced magnetic resonance imaging in patients with pulmonary arterial hypertension. Pulm Circ. 2014;4:61–70. doi: 10.1086/674882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley S, Mereles D, Risse F, Grünig E, Ley-Zaporozhan J, Tecer Z, et al. Quantitative 3D pulmonary MR-perfusion in patients with pulmonary arterial hypertension: correlation with invasive pressure measurements. Eur J Radiol. 2007;61:251–255. doi: 10.1016/j.ejrad.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Barton GP, Torres LA, Goss KN, Eldridge MW, Fain SB. Lung perfusion indices in adult survivors of prematurity. Presented at the International Workshop on Pulmonary Functional Imaging. October 18–20, 2019, New Orleans, LA. [Google Scholar]
- 8.Bell LC, Wang K, Munoz Del Rio A, Grist TM, Fain SB, Nagle SK. Comparison of models and contrast agents for improved signal and signal linearity in dynamic contrast-enhanced pulmonary magnetic resonance imaging. Invest Radiol. 2015;50:174–178. doi: 10.1097/RLI.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss KN, Haraldsdottir K, Beshish AG, Barton GP, Watson AM, Palta M, et al. Association between preterm birth and arrested cardiac growth in adolescents and young adults. JAMA Cardiol. doi: 10.1001/jamacardio.2020.1511. 10.1001/jamacardio.2020.1511 [online ahead of print] 20 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


