Efficient gas exchange is the result of an intimate matching of regional ventilation and perfusion across alveoli. Pulmonary embolism (PE) produces partial or complete blockage of perfusion to lung regions, resulting in very high / mismatch and/or dead-space ventilation. In addition, the interruption of blood flow to some regions diverts perfusion to areas without embolism. Normal ventilation may not be enough to oxygenate the surplus of blood flow within these units, and their / relation decreases, producing low / units (1) and consequent hypoxemia. Imaging of / matching can therefore become an invaluable diagnostic tool for PE.
Cyclic variations in pulmonary air and blood content are the major determinants for the changes in thoracic impedance. Besides features like being a bedside imaging tool and providing the possibility of around-the-clock monitoring, the high temporal resolution is a crucial aspect of electrical impedance tomography (EIT) imaging that allows for the study not only of ventilation but also of faster physiological phenomena, such as pulmonary perfusion (2–5) and the pulsatility of the lung during the cardiac cycle (6, 7).
The measurement of lung pulsatility correlates well with stroke volume measured by thermodilution (6, 7) and can be used to track relative changes in stroke volume, helping optimize hemodynamics (6). Lung pulsatility, however, cannot reliably estimate pulmonary perfusion (8) because the cyclic perturbations in local impedance caused by the passage of the stroke volume through the lung can be influenced by changes in parenchyma architecture (8), distensibility of the small pulmonary vessels (9), and size and patency of the pulmonary microvascular bed (10). We favor the estimation of lung perfusion based on first-pass kinetics of an EIT-indicator dilution curve using a hypertonic saline bolus, which is similar to X-ray computed tomography estimation of perfusion using iodinated contrast. A study that compared the pulsatility and the hypertonic-based approach using single-photon emission computed tomography demonstrated that the approach using a hypertonic saline bolus was superior (8). A fundamental reason for the poorer performance of the pulsatility method is probably related to the fact that it measures the pulsatile changes in pulmonary blood volume instead of measuring real forward blood flow. Changes in vascular compliance can confound the relationship between the cyclic pulsatile changes in regional impedance and the perfusion to those regions. The largest difference between local blood flow measured by single-photon emission computerized tomographic and EIT pulsatility (8) was found during unilateral lung collapse. Stronger pulsatile changes were found within the collapsed lung, whereas the amount of blood flowing to this region was significantly reduced. These data agree with findings (11) of a retrograde flow from atelectatic zones to healthy ones during the diastolic phase.
In this issue of the Journal, He and colleagues (pp. 1464–1467) described EIT regional ventilation and perfusion measures that were able to distinguish patients with acute PE from other patients with other types of acute respiratory failure (12). After demonstrating the clinical use of this method in a recent case (13), the authors have now confirmed the feasibility of bedside detection of acute PE by EIT imaging. The group with PE had significantly higher dead space % than patients without PE. Dead space % (an EIT imaging–derived parameter) from all measured parameters had the best performance in diagnosing PE. A cutoff value of 30.37 for dead space % resulted in remarkable sensitivity (90.9%) and specificity (98.6%). Of course, these results require external validation.
This is the first prospective clinical study confirming PE with EIT imaging. In this proof-of-concept investigation, quantitative assessments of regional lung perfusion were made with injection of a bolus of hypertonic saline. The lung pulsatility signal was also computed. Of note, no correlation was found between the saline bolus method and the pulsatility method for the investigated parameters of the study in patients with PE, consistent with previous findings in the literature discussed above. Whether the pulsatility method has a role on describing regional pulmonary vascular mechanics in multiple conditions including PE deserves further studies.
Some limitations of the study encourage further investigations. A full computation of / depends on the cardiac output and that was not measured. The threshold values used in the study to identify ventilated and perfused regions were not optimized and may have decreased the sensitivity to small perfusion changes. The bolus was injected during expiratory pauses, and perfusion distribution may be dissimilar during an expiratory pause and during ongoing ventilation. For instance, compression of capillaries in the open areas during inspiration may decrease the perfusion to those areas, redistributing blood flow to nonventilated areas. The PE group presented more hypoxemia together with less intrapulmonary shunt %. We hypothesize that such hypoxemia is due to the occurrence of low / areas, but the methods proposed by the authors did not include a specific parameter to compute low / areas. Interestingly, ongoing research suggests that cardiac output can be calculated from the same saline bolus curve measured by EIT imaging, using a modified Stewart-Hamilton approach (Figure 1). Thus, computation of low-/ areas will be soon a reality.
Figure 1.
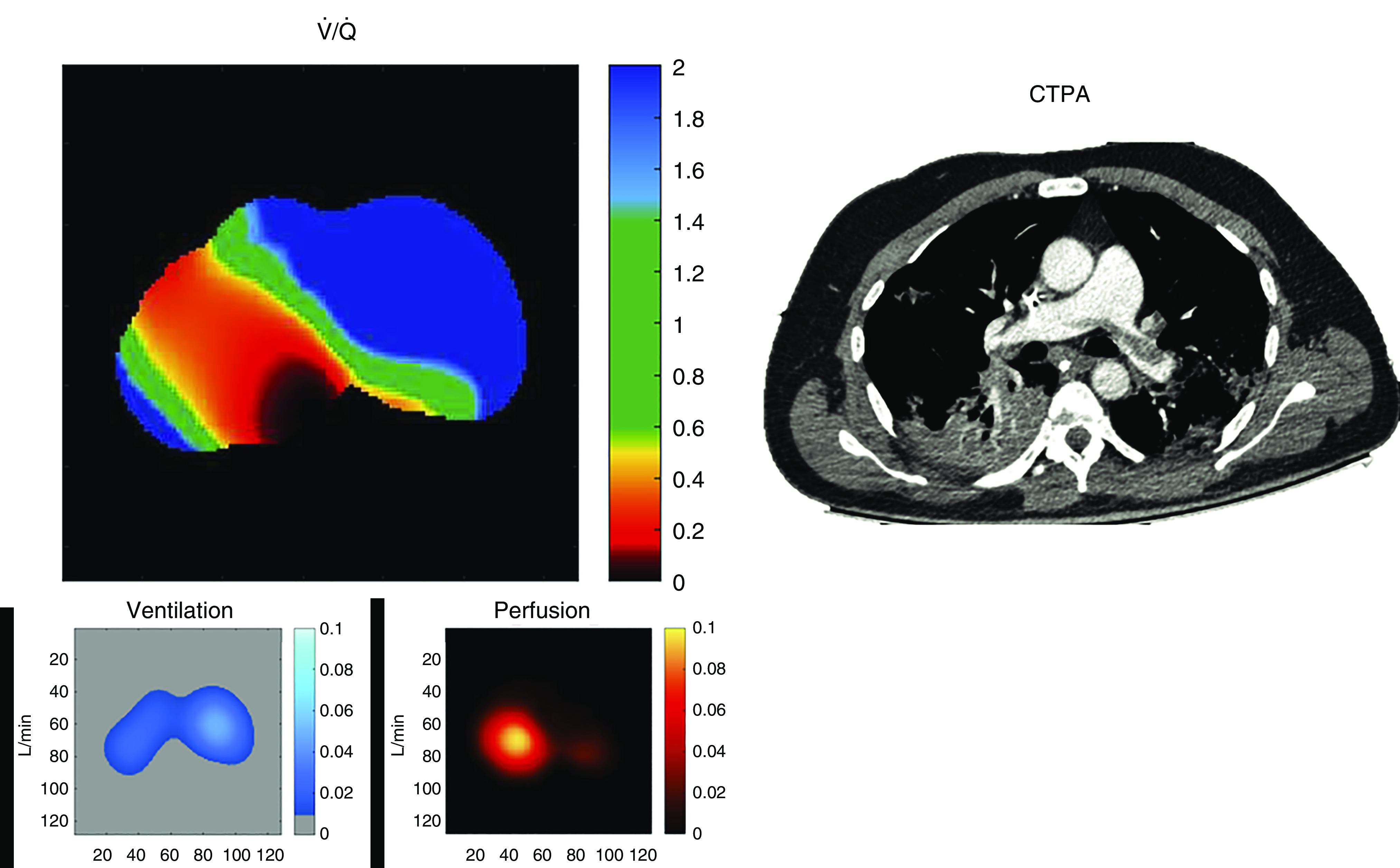
Electrical impedance tomography map of regional / in a patient with acute respiratory distress syndrome and coronavirus disease (COVID-19) lung disease under invasive mechanical ventilation (PaO2/FiO2 ratio of 71) who developed acute pulmonary embolism (confirmed by CTPA). We estimated regional / for each pixel in the electrical impedance tomography functional image. The ventilatory component region of interest (ROI) was calculated as the alveolar e multiplied by the fraction of Vt directed to each pixel (%ROIV; see Reference 15), after discounting the anatomical dead space (assumed to be 30% of Vt directed to each ROI, when Vt is around 6–8 ml/kg). The perfusion component was estimated as the fraction of lung perfusion directed to each ROI (%ROIQ), multiplied by the estimated cardiac output (CO). Fractional perfusion was estimated as the sum of maximum slopes (negative slopes of impedance decay after the bolus of hypertonic solution) for pixels composing the ROI, divided by the sum of maximum slopes for all pixels within the lung (see Reference 8). Thus, / = ([respiratory rate × Vt × (1 − 0.3)] × %ROIV) / (CO × %ROIQ). CO was estimated by thermodilution at each clinical condition or by the inverse relationship between the area under the impedance curve (after the bolus of hypertonic contrast) versus cardiac output, analogous to the principle of Stewart-Hamilton. This inverse relationship was previously validated in a clinical study. Note that hypoxemia is explained by the low-/ areas (<0.5; red/yellow) happening in the contralateral (right) lung, which is receiving extra blood flow coming from the diversion of perfusion away from occluded vessels in the left lung. The areas most affected by the emboli present a dead-space effect (blue pixels in the ventral-left lung showing / ratios above 2). CTPA = computed tomography pulmonary angiography.
For critically ill patients, bedside monitoring of key aspects of lung function must be performed in real time, to detect the very moment when the lung tissue behavior changes and/or react in response to therapeutic interventions. A very illustrative example is the process of pulmonary collapse and recruitment. These phenomena are very dynamic because of the unstable nature of lung tissue regarding its three-dimensional morphology. Lung units change from an open to a closed state and from a closed to an open state very quickly. And when reaeration occurs in previously collapsed units, capillaries are also opened, mainly by the immediate release of the hypoxic pulmonary vasoconstriction reflex as oxygen enters the recruited areas. As the pulmonary opening and closing pressures vary among different zones of the lungs and among patients, around-the-clock monitoring of these pressures and their / effects becomes a prerequisite in tailoring ventilator treatment. Real-time and timely elucidation and monitoring of regional / matching during ongoing ventilation will be very welcome, and this is not only limited to use in patients with PE.
In an editorial (14) responding to an article published in the Journal in 2004 (15), Hedenstierna concluded that “a bedside tool . . . with the capacity of measuring regional ventilation and perfusion would be worth waiting for.” This study brings us one step closer to what he envisioned.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202007-2896ED on August 24, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Manier G, Castaing Y, Guenard H. Determinants of hypoxemia during the acute phase of pulmonary embolism in humans. Am Rev Respir Dis. 1985;132:332–338. doi: 10.1164/arrd.1985.132.2.332. [DOI] [PubMed] [Google Scholar]
- 2. Reinius H, Borges JB, Engström J, Ahlgren O, Lennmyr F, Larsson A, et al. Optimal PEEP during one-lung ventilation with capnothorax: an experimental study. Acta Anaesthesiol Scand. 2019;63:222–231. doi: 10.1111/aas.13247. [DOI] [PubMed] [Google Scholar]
- 3. Reinius H, Borges JB, Fredén F, Jideus L, Camargo ED, Amato MB, et al. Real-time ventilation and perfusion distributions by electrical impedance tomography during one-lung ventilation with capnothorax. Acta Anaesthesiol Scand. 2015;59:354–368. doi: 10.1111/aas.12455. [DOI] [PubMed] [Google Scholar]
- 4. Borges JB, Cronin JN, Crockett DC, Hedenstierna G, Larsson A, Formenti F. Real-time effects of PEEP and tidal volume on regional ventilation and perfusion in experimental lung injury. Intensive Care Med Exp. 2020;8:10. doi: 10.1186/s40635-020-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frerichs I, Hinz J, Herrmann P, Weisser G, Hahn G, Quintel M, et al. Regional lung perfusion as determined by electrical impedance tomography in comparison with electron beam CT imaging. IEEE Trans Med Imaging. 2002;21:646–652. doi: 10.1109/TMI.2002.800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. da Silva Ramos FJ, Hovnanian A, Souza R, Azevedo LCP, Amato MBP, Costa ELV. Estimation of stroke volume and stroke volume changes by electrical impedance tomography. Anesth Analg. 2018;126:102–110. doi: 10.1213/ANE.0000000000002271. [DOI] [PubMed] [Google Scholar]
- 7. Vonk-Noordegraaf A, II, Janse A, Marcus JT, Bronzwaer JG, Postmust PE, Faes TJ, et al. Determination of stroke volume by means of electrical impedance tomography. Physiol Meas. 2000;21:285–293. doi: 10.1088/0967-3334/21/2/308. [DOI] [PubMed] [Google Scholar]
- 8. Borges JB, Suarez-Sipmann F, Bohm SH, Tusman G, Melo A, Maripuu E, et al. Regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse. J Appl Physiol (1985) 2012;112:225–236. doi: 10.1152/japplphysiol.01090.2010. [DOI] [PubMed] [Google Scholar]
- 9. Schuster DP, Haller J. Regional pulmonary blood flow during acute pulmonary edema: a PET study. J Appl Physiol (1985) 1990;69:353–361. doi: 10.1152/jappl.1990.69.1.353. [DOI] [PubMed] [Google Scholar]
- 10. Smit HJ, Vonk Noordegraaf A, Marcus JT, Boonstra A, de Vries PM, Postmus PE. Determinants of pulmonary perfusion measured by electrical impedance tomography. Eur J Appl Physiol. 2004;92:45–49. doi: 10.1007/s00421-004-1043-3. [DOI] [PubMed] [Google Scholar]
- 11. Newell JC, Levitzky MG, Krasney JA, Dutton RE. Phasic reflux of pulmonary blood flow in atelectasis: influence of systemic PO2. J Appl Physiol. 1976;40:883–888. doi: 10.1152/jappl.1976.40.6.883. [DOI] [PubMed] [Google Scholar]
- 12. He H, Chi Y, Long Y, Yuan S, Zhang R, Frerichs I, et al. Bedside evaluation of pulmonary embolism by saline contrast electrical impedance tomography method: a prospective observational study [letter] Am J Respir Crit Care Med. 2020;202:1464–1467. doi: 10.1164/rccm.202005-1780LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He H, Long Y, Frerichs I, Zhao Z. Detection of acute pulmonary embolism by electrical impedance tomography and saline bolus injection. Am J Respir Crit Care Med. 2020;202:881–882. doi: 10.1164/rccm.202003-0554IM. [DOI] [PubMed] [Google Scholar]
- 14. Hedenstierna G. Using electric impedance tomography to assess regional ventilation at the bedside. Am J Respir Crit Care Med. 2004;169:777–778. doi: 10.1164/rccm.2401010. [DOI] [PubMed] [Google Scholar]
- 15. Victorino JA, Borges JB, Okamoto VN, Matos GF, Tucci MR, Caramez MP, et al. Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med. 2004;169:791–800. doi: 10.1164/rccm.200301-133OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


