Abstract
Background/Aims
Esophagogastric junction adenocarcinoma (EJA) is a malignant tumor associated with high morbidity and has attracted increasing attention due to a rising incidence and low survival rate. Pathological biopsy is the gold standard for diagnosis, but noninvasive and effective tests are lacking, resulting in diagnoses at advanced stages. This study explored the diagnostic value of insulin-like growth factor binding protein 7 (IGFBP7) in EJA.
Methods
A total of 120 EJA patients and 88 normal controls were recruited, and their serum levels of IGFBP7 were measured by enzyme-linked immunosorbent assay. Receiver operating characteristic (ROC) curve analysis was used to assess the diagnostic value, and Pearson chi-square analysis was used to evaluate the correlation between IGFBP7 and clinical parameters. Kaplan-Meier survival analysis was carried out to assess the effect of IGFBP7 on overall survival (OS).
Results
The levels of IGFBP7 were higher in both early- and late-stage EJA patients than in normal controls (p<0.001). The area under the ROC curve for EJA patients was 0.794 (95% confidence interval [CI], 0.733 to 0.854), with a cutoff value of 2.716 ng/mL, a sensitivity of 63.3% (95% CI, 54.0% to 71.8%) and a specificity of 90.9% (95% CI, 82.4% to 95.7%). For the diagnosis of early-stage EJA, the same cutoff value and specificity were obtained, but the sensitivity of IGFBP7 was 54.3% (95% CI, 36.9% to 70.8%). Patients with low IGFBP7 protein expression had lower OS than those with high expression (p=0.034). The multivariate analysis showed that IGFBP7 is an independent prognostic factor for EJA (p=0.011).
Conclusions
Serum IGFBP7 acts as a potential diagnostic and prognostic marker for EJA.
Keywords: Insulin-like growth factor binding protein-7, Esophagogastric junction, Adenocarcinoma, Diagnosis, Prognosis
INTRODUCTION
In recent decades, the incidence of esophagogastric junction adenocarcinoma (EJA) has rapidly increased in both western countries and eastern Asia.1 EJA is usually divided into three different types according to the 1998 Siewert classification.2 Tumors with centers from 5 cm to 1 cm above the esophagogastric junction are defined as Siewert type I, while tumors with the centers located 1 cm above to 2 cm below the junction are type II, and tumors with centers located 2 cm to 5 cm below the junction are type III. However, in accordance to the 8th edition of the American Joint Committee on Cancer Cancer Staging Manual,3 esophagogastric junction cancers with centers no more than 2 cm from the inferior esophageal sphincter are staged as esophageal adenocarcinoma (Siewert type I or II), while those more than 2 cm are staged as stomach cancers (Siewert type III).3 Overall survival (OS) for EJA is poor, with a 5-year survival of less than 25% in China.4,5 In clinical practice, the diagnosis of EJA is mostly dependent on pathological biopsy after endoscopy or surgery, both of which are invasive methods. A valuable and noninvasive method is urgently needed for early diagnosis.
The insulin-like growth factor (IGF) family is composed of IGFs, IGF receptors and IGF-binding proteins (IGFBPs),6-8 which play a vital role in regulating cell proliferation, differentiation, and apoptosis.9 Among them, IGFBP7 binds IGF-I and IGF-II with low affinity.10 However, IGFBP7 has been found to be related to several diseases, such as acute kidney injury11 and chronic obstructive pulmonary disease.12 Similarly, both high and low expression of IGFBP7 has been observed in various cancer tissues, such as gastric13 and colorectal cancer.14 In gastroesophageal cancer, IGFBP7 expression also correlates with pathophysiology and tumorigenesis.15 Here, we explored the potential of serum IGFBP7 levels as a diagnostic and prognostic tool for EJA.
MATERIALS AND METHODS
1. Study subjects
In total, we collected 208 serum samples from the Cancer Hospital of Shantou University Medical College: 88 healthy control samples and 120 EJA samples. All participants were diagnosed to be without acute kidney injury or chronic obstructive pulmonary disease. EJA patient serum samples used in this experiment were collected between July 2013 and November 2017 before surgery, and follow-up was made starting from the day of surgery. The exit date was defined as the day of death, withdrawal or completion of follow-up before December 2018. Both patients and normal controls signed informed consent to participate in this study, which was approved by the Institutional Review Board of the Cancer Hospital of Shantou University Medical College and conformed to the requirements of the Declaration of Helsinki. After venous sampling before surgery, serum was obtained by centrifuging blood at 2500 g for 10 minutes, then stored at –80°C until analysis.
EJA was diagnosed by computed tomography or gastroscopy, followed by pathological examination after surgery, endoscopic mucosal resection or biopsy. All patients were accepted for surgery. We defined tumor stage according to the 8th edition of the American Joint Committee on Cancer Cancer Staging Manual.3 In accordance with our previous study, American Joint Committee on Cancer stage I+II was defined as early-stage EJA.16
2. Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay kits for serum IGFBP-7 were purchased from Cusabio® (Catalog number: CSB-E17249h; Houston, TX, USA). The enzyme-linked immunosorbent assay protocol was carried out according to the user manual. The serum samples were diluted to 1:3, while the standard was diluted to a concentration gradient of 10, 5, 2.5, 1.25, 0.625, 0.312, and 0.156 ng/mL, respectively. The 100 μL serum sample, standard or sample diluent (acted as negative control) was added to each well and incubated for 2 hours at 37°C. After removed the liquid, 100 μL biotin-antibody (1X) and 100 μL horseradish peroxidase-avidin (1X) were added in chronological order. 3,3’,5,5’-tetramethylbenzidine substrate was used for color development and stop solution for the termination of color development. The measurement of optical density value of each well was executed on a plate microplate reader (BioTek® Instruments, Winooski, VT, USA) within 5 minutes at 450 nm with 570 nm reference.
3. Statistical analysis
We first changed the optical density values to concentration according to the standard curve plotted by SigmaPlot 10.0. Then the Mann-Whitney U-test was used to evaluate the difference of IGFBP7 levels between two groups. Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic boundaries including the area under the ROC curve (AUC) with the 95% confidence interval (CI), sensitivity and specificity. The cutoff value was calculated by achieving the maximum sensitivity when the specificity was greater than 90%, and by minimizing the distance of the cutoff value to the top-left corner of the ROC curve. We selected a specificity of greater than 90% in order to produce a test that could be beneficial to early cancer detection.17 Correlation between clinical characteristics and the level of IGFBP7 was evaluated by the chi-square test. For optimal cut-point definition in prognostic analysis, we used the X-tile 3.6.1 software to category high and low expression of IGFBP7.18 The Kaplan-Meier survival analysis was used to assess the effect of IGFBP7 on OS. Univariable and multivariable analyses were used to judge the hazard ratio. In all analyses, a p<0.05 (two-tailed) was defined as significance. Statistical analyses were carried out with SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA).
RESULTS
1. Patient characteristics and serum IGFBP7 levels
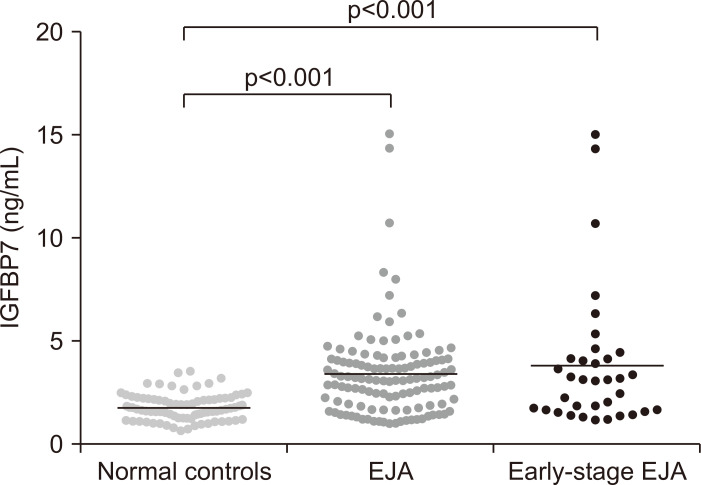
A total of 208 patients and normal controls were analyzed (Table 1). The mean concentration±standard error of the mean (SEM) of IGFBP7 for EJA patients was 3.436±0.1998 ng/mL versus 1.821±0.0663 ng/mL and 3.863±0.5666 ng/mL in the normal control group and early-stage patient group, respectively (Table 2). Thus, early- and late-stage patients with EJA had a significant increase in level of serum IGFBP7, compared with normal controls (p<0.001) (Fig. 1). As illustrated in Fig. 1, similar result can be found in early-stage EJA patients.
Table 1.
Characteristics of the Study Population
| Group | EJA (n=120) | Normal (n=88) | |||
|---|---|---|---|---|---|
| No. | 3-yr survival rate (%) | p-value | No. (%) | ||
| Age, yr | |||||
| Mean (range) | 60 (31–82) | 56 (40–80) | |||
| Sex | 0.469 | ||||
| Male | 99 | 76.4 | 61 (69.3) | ||
| Female | 21 | 49.5 | 27 (30.7) | ||
| Smoke | 0.557 | ||||
| Yes | 79 | 63.6 | - | ||
| No | 41 | 69.0 | - | ||
| Alcohol | 0.038 | ||||
| Yes | 28 | 46.0 | - | ||
| No | 92 | 71.7 | - | ||
| Size of tumor, cm | 0.109 | ||||
| ≥5 | 72 | 60.8 | - | ||
| <5 | 48 | 72.9 | - | ||
| Depth of tumor invasion | 0.012 | ||||
| T1+T2+T3 | 36 (7+4+25) | 79.8 | - | ||
| T4 | 84 | 59.5 | - | ||
| Regional lymph nodes | <0.001 | ||||
| N0 | 36 | 73.3 | - | ||
| N1 | 31 | 80.6 | - | ||
| N2 | 30 | 66.5 | - | ||
| N3 | 23 | 33.5 | - | ||
| Metastasis | <0.001 | ||||
| M0 | 116 | 66.9 | - | ||
| M1 | 4 | 25.0 | - | ||
| Histological grade | 0.019 | ||||
| G1 (High) | 15 | 84.0 | - | ||
| G2 (Middle) | 44 | 68.0 | - | ||
| G3 (Low) | 47 | 53.2 | - | ||
| Unknown | 14 | - | - | ||
| TNM stage | 0.055 | ||||
| I+II | 35 (9+26) | 76.8 | - | ||
| III+IV | 85 (74+11) | 60.7 | - | ||
EJA, esophagogastric junction adenocarcinoma.
Table 2.
Frequency of Circulating IGFBP7
| Group | Mean±SEM | p-value | No. | Positive (%, 95% CI) |
|---|---|---|---|---|
| Normal controls | 1.821±0.0663 | - | 88 | 8 (9.1, 4.7–16.9) |
| All EJA | 3.436±0.1998 | <0.001 | 120 | 76 (63.3, 54.4–71.4) |
| Early-stage EJA | 3.863±0.5666 | <0.001 | 35 | 19 (54.3, 36.9–70.8) |
IGFBP7, insulin-like growth factor binding protein 7; SEM, standard error of the mean; CI, exact confidence interval; EJA, esophagogastric junction adenocarcinoma.
Fig. 1.
Scatter plots of serum IGFBP7 values in the normal control group, EJA group and early-stage EJA group.
IGFBP7, insulin-like growth factor binding protein 7; EJA, esophagogastric junction adenocarcinoma. p<0.001 indicates that the difference between the compared groups is statistically significant.
2. Diagnostic value of IGFBP7
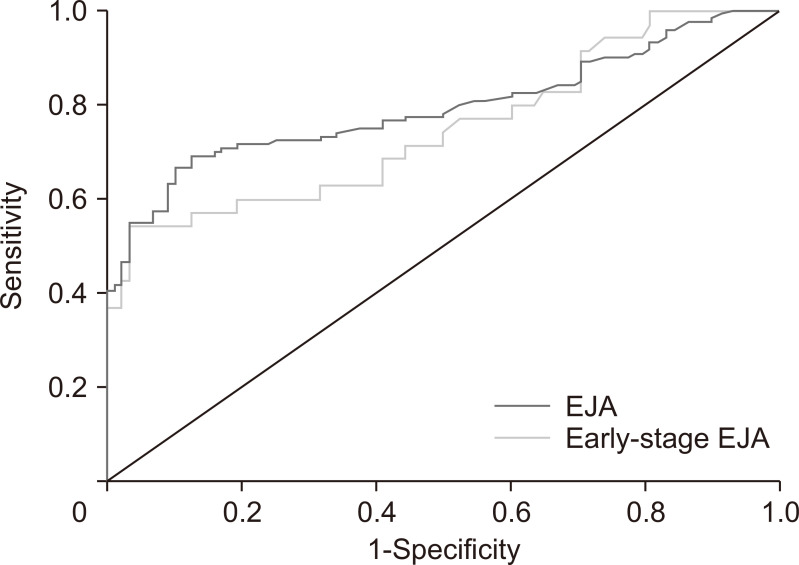
We identified a cutoff value of 2.716 ng/mL for IGFBP7 to diagnose EJA by using the ROC curve to compare the EJA and normal control groups (Fig. 2). We acquired an AUC of 0.794 (95% CI, 0.733 to 0.854) with a sensitivity of 63.3% (95% CI, 54.0% to 71.8%) and a specificity of 90.9% (95% CI, 82.4% to 95.7%) (Table 3). Also, with the same cutoff value, IGFBP7 could identify early-stage EJA with a slightly lower AUC value of 0.749 (95% CI, 0.644 to 0.854), a sensitivity of 54.3% (95% CI, 36.9% to 70.8%) and a specificity of 90.9% (95% CI, 82.4% to 95.7%).
Fig. 2.
Receiver operating characteristic curve analysis for the diagnosis of esophagogastric junction adenocarcinoma (EJA) and early-stage EJA.
Table 3.
Result of the Measurement of IGFBP7 for the Diagnosis of EJA
| IGFBP7 | AUC | SEN, % | SPE, % | FPS, % | FNS, % | PPV, % | NPV, % | PLR | NLR |
|---|---|---|---|---|---|---|---|---|---|
| EJA vs NC | 0.794 (0.733–0.854) |
63.3 (54.0–71.8) |
90.9 (82.4–95.7) |
9.5 (4.5–18.4) |
35.4 (27.2–44.6) |
90.5 (81.6–95.5) |
64.5 (55.4–72.8) |
6.97 (3.55–13.7) |
0.40 (0.32–0.51) |
| Early-stage EJA vs NC | 0.749 (0.644–0.854) |
54.3 (36.9–70.8) |
90.9 (82.4–95.7) |
29.6 (14.5–50.3) |
16.7 (10.1–26.0) |
70.4 (49.7–85.5) |
83.3 (74.0–89.9) |
5.97 (2.89–12.4) |
0.50 (0.35–0.72) |
Numbers in brackets represent the 95% confidence interval.
IGFBP7, insulin-like growth factor binding protein 7; EJA, esophagogastric junction adenocarcinoma; NC, normal controls; AUC, area under the receiver operating characteristic curve; SEN, sensitivity; SPE, specificity; FPS, false-positive rate; FNS, false-negative rate; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
3. Correlation with clinical data
With the cutoff value of 2.716 ng/mL, we defined the positivity of the serum IGFBP7 levels for EJA patients to be over 2.716 ng/mL. Table 4 shows the relationship of serum IGFBP7 levels with clinicopathological features in EJA. IGFBP7 showed no association with any clinical data, including patient age, gender, smoking, drinking, size, depth of invasion, histological grade, lymph node status, metastasis, and early-stage versus advanced-stage groups (all p>0.05).
Table 4.
Relationship between the Positive Rate of IGFBP7 and the Clinical Data in EJA Patients
| Variable | Positive | Negative | p-value |
|---|---|---|---|
| Age, yr | 0.113 | ||
| ≥60 | 54 | 25 | |
| <60 | 22 | 19 | |
| Sex | 0.881 | ||
| Male | 63 | 36 | |
| Female | 13 | 8 | |
| Smoke | 0.699 | ||
| Yes | 51 | 28 | |
| No | 25 | 16 | |
| Alcohol | 0.310 | ||
| Yes | 20 | 8 | |
| No | 56 | 36 | |
| Size of tumor, cm | 0.315 | ||
| ≥5 | 43 | 29 | |
| <5 | 33 | 15 | |
| Depth of tumor invasion | 0.620 | ||
| T1+T2+T3 | 24 | 12 | |
| T4 | 52 | 32 | |
| Regional lymph nodes | 0.741 | ||
| N0 | 22 | 14 | |
| N1+N2+N3 | 54 | 30 | |
| Metastasis | 1.000 | ||
| M0 | 73 | 43 | |
| M1 | 3 | 1 | |
| Histological grade | 0.562 | ||
| G1 | 9 | 6 | |
| G2 | 30 | 14 | |
| G3 | 27 | 20 | |
| TNM grade | 0.187 | ||
| Early stage (I-II) | 19 | 16 | |
| Late stage (III-IV) | 57 | 28 |
Statistical significance was determined using the Pearson chi-square test. Data were from patients with tumor resection.
IGFBP7, insulin-like growth factor binding protein 7; EJA, esophagogastric junction adenocarcinoma.
4. Correlation between IGFBP7 expression and OS
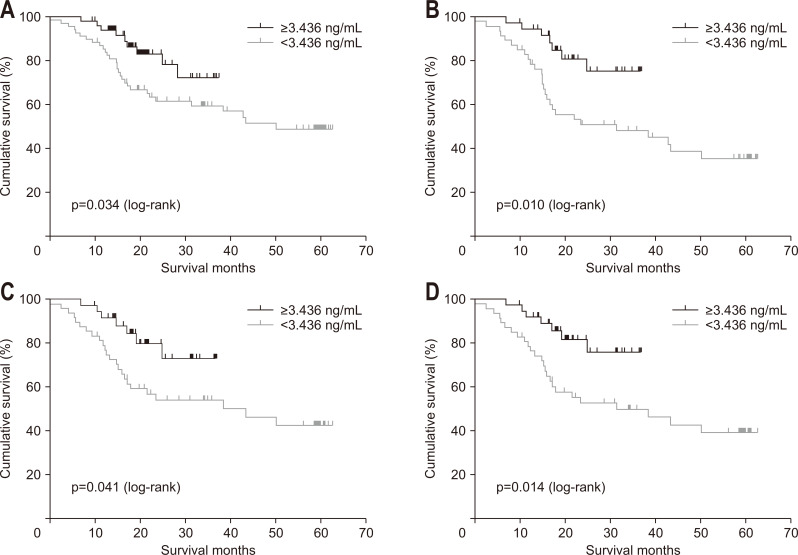
According to X-tile software, we set 3.436 ng/mL as the cutoff value to classify high and low expression of IGFBP7. The difference in 3-year OS for EJA patients with high IGFBP7 levels had 72.4% 3-year OS versus 59.7% for patients with low expression (Fig. 3A), and the difference was statistically significant (p=0.034). When we stratified according to staging, the same results were found in subgroups categorized with T4 (p=0.010), N1+N2+N3 (p=0.041), and III+IV (p=0.014) (Fig. 3B-D). Upon univariable analysis, IGFBP7 was a significant prognostic factor for 3-year OS of EJA patients (p=0.040) (Table 5). Upon multivariable analysis, IGFBP7 was an independent significant prognostic marker for 3-year OS (hazard ratio, 0.351; 95% CI, 0.157 to 0.786; p=0.011). Other significant variables included further metastasis (p<0.001) and depth of tumor invasion (p=0.012).
Fig. 3.
Kaplan-Meier analysis of overall survival in patients with esophagogastric junction adenocarcinoma (EJA) in relation to IGFBP7 expression. (A) All EJA patients, (B) EJA patients categorized as having T4 disease, (C) EJA patients categorized as having N1+N2+N3 disease, and (D) late-stage EJA patients (III+IV).
IGFBP7, insulin-like growth factor binding protein 7.
Table 5.
Univariate and Multivariate Analyses of Factors Associated with Overall Survival in Patients with EJA
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (≥60 yr vs <60 yr) | 0.995 (0.955–1.037) | 0.819 | |||
| Sex (female vs male) | 0.750 (0.343–1.640) | 0.471 | |||
| Smoke (yes vs no) | 1.226 (0.620–2.426) | 0.558 | |||
| Alcohol (yes vs no) | 2.007 (1.025–3.931) | 0.042 | |||
| Tumor size (≥5 cm vs <5 cm) | 1.759 (0.874–3.538) | 0.113 | |||
| T (T4 vs T1+T2+T3) | 3.114 (1.217–7.966) | 0.018 | 3.362 (1.310–8.625) | 0.012 | |
| N (N1+N2+N3 vs N0) | 1.996 (0.917–4.346) | 0.082 | |||
| M (M1 vs M0) | 7.445 (2.225–24.904) | 0.001 | 11.303 (3.101–41.202) | <0.001 | |
| G (G2+G3 vs G1) | 3.734 (0.897–15.553) | 0.070 | |||
| pTNM-stage (III+IV vs I+II) | 2.183 (0.963–4.949) | 0.062 | |||
| IGFBP7 (≥3.436 vs <3.436) | 0.433 (0.195–0.962) | 0.040 | 0.351 (0.157–0.786) | 0.011 | |
Multivariate analysis, Cox proportional hazards regression model. Variables were adopted for their prognostic significance according to the univariate analysis.
EJA, esophagogastric junction adenocarcinoma; HR, hazard ratio; CI, confidence interval; IGFBP7, insulin-like growth factor binding protein 7.
DISCUSSION
In our study, IGFBP7 was significantly higher in both early- and late-stage EJA compared to normal controls (p<0.001). For diagnosis, we achieved an AUC of 0.794 (95% CI, 0.733 to 0.854) and cutoff value of 2.716 ng/mL with sensitivity of 63.3% (95% CI, 54.0% to 71.8%) and specificity of 90.9% (95% CI, 82.4% to 95.7%) in EJA. Furthermore, we found that the level of serum IGFBP7 is not associated with any clinical parameters. However, if diagnosed with EJA, lower IGFBP7 expression may lead to the worse survival.
IGF family members regulate cell proliferation, differentiation and apoptosis through the MAPK and PI3K/Akt signaling pathways.19 They have also been demonstrated to correlate with many cancers.20-22 IGFBP7, also named IGFBP-rP1, is one of the members in IGF family. Although it shares 30% structural homology with IGFBP1 to IGFBP6 at its N-terminal domain, as it binds to IGF with low affinity10,23 and its individual characteristics are different from IGFBP1 to IGFBP6. Methylation of IGFBP7 may reduce the expression of IGFBP7 in gastric cancer and prostate cancer and improve the tumor progression,13,24 and administration of IGFBP7 may be therapeutic for reducing breast cancer growth.25 However, prior studies investigated expression in mostly cells and tissues. Serum expression of IGFBP7 has only been studied in a few diseases, such as chronic obstructive pulmonary disease12 and metabolic syndrome.26 Therefore, we evaluated the potential diagnostic and prognostic value of circulating IGFBP7 in EJA.
Early detection is one of the best methods to reduce cancer mortality and cancer burden.27 In clinical practice, endoscopy contributes to the early diagnosis of EJA.28 However, it is invasive and may produce adverse effects, such as infection, perforation, and bleeding.29 A recent study on serum autoantibody panels to detect EJA aroused our interest to identify noninvasive techniques to detect IGFBP7 in EJA patient serum.16 When accounting for the early detection of IGFBP7 in EJA, through ROC curve analysis, we found an AUC of 0.749 (95% CI, 0.644 to 0.854), which led to a sensitivity of 54.3% (95% CI, 36.9% to 70.8%) and specificity of 90.9% (95% CI, 82.4% to 95.7%). Although there was no statistical correlation between late-stage and early-stage EJA (p=0.187) (Table 4), IGFBP7 might be used as a potential biomarker for the early detection of EJA. Actually, there have been several studies on serum IGFBP7 in other cancers. Overexpression of serum IGFBP7 has been observed in high-grade soft tissue sarcoma,30 and high levels of serum IGFBP7 have been associated with positive nodal status in non-small cell lung cancer.31 However, both of them have not researched on the early-stage cancer. Additionally, our recent study has showed serum IGFBP7 might serve as a diagnostic biomarker for esophageal cancer,32 and it also made sense in early-stage esophageal cancer. Herein, although IGFBP7 is not a unique biomarker for EJA, it still may be a valuable candidate for EJA, in particular for the early detection of EJA.
Although Smith et al.33 suggested that the expression of IGFBP7 is related to poor prognosis in esophageal squamous cell carcinoma, as described in many cancers, such as thyroid,34 lung,35 liver,36 and ovarian,37 a high level of IGFBP7 gene expression could be a potential protective factor to suppress the growth of tumor cells. However, all of these studies were based on IGFBP7 expression at the level of tissues or cells. Therefore, we extended these studies to serum expression. As our study shows, low serum IGFBP7 concentration is associated with poor outcome (p=0.034), and multivariate analysis suggests that the level of serum IGFBP7 protein expression is an independent prognostic factor for EJA (p=0.011), suggesting that serum IGFBP7 might be also a potential prognostic marker for EJA.
In summary, our study offers useful information regarding the diagnostic value of serum IGFBP7 in EJA and suggests that IGFBP7 might be a potential biomarker for the detection and diagnosis of EJA. Additionally, low expression of IGFBP7, acting as an independent factor, might predict the poor prognosis of EJA. However, this was a single-institution study with a small sample size, which may lead to bias. A study with large sample size in multiple institutions should be performed to further verify the diagnostic value of IGFBP7 in EJA, especially early-stage EJA.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (81972801 and 31600632); the Natural Science Foundation of Guangdong Province (2018A030307079); the Innovative and Strong School Project of Guangdong (2018KTSCX068) and the Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Experiments: C.T.L., Y.W.X., X.Y.H. Data analysis: C.T.L., L.Y.C., S.H.Y. Technical or material support: C.Q.H. Serum and clinical data collection: H.G., Y.H.L. Writing - original draft and review and editing: C.T.L., Y.W.X. Study design and obtained funding: Y.W.X., E.M.L., Y.H.P. All authors read and approved the final manuscript.
REFERENCES
- 1.Oda I, Abe S, Kusano C, et al. Correlation between endoscopic macroscopic type and invasion depth for early esophagogastric junction adenocarcinomas. Gastric Cancer. 2011;14:22–27. doi: 10.1007/s10120-011-0001-0. [DOI] [PubMed] [Google Scholar]
- 2.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 3.Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304–317. doi: 10.3322/caac.21399. [DOI] [PubMed] [Google Scholar]
- 4.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thrift AP. The epidemic of oesophageal carcinoma: where are we now? Cancer Epidemiol. 2016;41:88–95. doi: 10.1016/j.canep.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Nagalla SR, Oh Y, Wilson E. False Rosenfeld RG. Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc Natl Acad Sci U S A. 1997;94:12981–12986. doi: 10.1073/pnas.94.24.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–244. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 8.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 9.Fürstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/S1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 10.Oh Y, Nagalla SR, Yamanaka Y, Kim HS, Wilson E, Rosenfeld RG. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7: recombinant human mac25 protein specifically binds IGF-I and -II. J Biol Chem. 1996;271:30322–30325. doi: 10.1074/jbc.271.48.30322. [DOI] [PubMed] [Google Scholar]
- 11.Liu KD, Vijayan A, Rosner MH, Shi J, Chawla LS, Kellum JA. Clinical adjudication in acute kidney injury studies: findings from the pivotal TIMP-2*IGFBP7 biomarker study. Nephrol Dial Transplant. 2016;31:1641–1646. doi: 10.1093/ndt/gfw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan W, Wu M, Shi L, et al. Serum levels of IGFBP7 are elevated during acute exacerbation in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:1775–1780. doi: 10.2147/COPD.S132652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kim WH, Byeon SJ, Lee BL, Kim MA. Epigenetic downregulation and growth inhibition of IGFBP7 in gastric cancer. Asian Pac J Cancer Prev. 2018;19:667–675. doi: 10.1158/1538-7445.AM2017-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao C, Lin SL, Ruan WJ, Wen H, Wu DJ, Deng H. High expression of IGFBP7 in fibroblasts induced by colorectal cancer cells is co-regulated by TGF-beta and Wnt signaling in a Smad2/3-Dvl2/3-dependent manner. PLoS One. 2014;9:e85340. doi: 10.1371/journal.pone.0085340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashyap MK. Role of insulin-like growth factor-binding proteins in the pathophysiology and tumorigenesis of gastroesophageal cancers. Tumour Biol. 2015;36:8247–8257. doi: 10.1007/s13277-015-3972-3. [DOI] [PubMed] [Google Scholar]
- 16.Xu YW, Chen H, Guo HP, et al. Combined detection of serum autoantibodies as diagnostic biomarkers in esophagogastric junction adenocarcinoma. Gastric Cancer. 2019;22:546–557. doi: 10.1007/s10120-018-0894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle P, Chapman CJ, Holdenrieder S, et al. Clinical validation of an autoantibody test for lung cancer. Ann Oncol. 2011;22:383–389. doi: 10.1093/annonc/mdq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 19.Kasprzak A, Kwasniewski W, Adamek A, Gozdzicka-Jozefiak A. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat Res Rev Mutat Res. 2017;772:78–104. doi: 10.1016/j.mrrev.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Wang G, Meng L, et al. Association between circulating levels of IGF-1 and IGFBP-3 and lung cancer risk: a meta-analysis. PLoS One. 2012;7:e49884. doi: 10.1371/journal.pone.0049884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 22.Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257–2263. doi: 10.1016/S0959-8049(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 23.Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer. 2005;41:1515–1527. doi: 10.1016/j.ejca.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan L, Murphy TM, Barrett C, et al. IGFBP7 promoter methylation and gene expression analysis in prostate cancer. J Urol. 2012;188:1354–1360. doi: 10.1016/j.juro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Benatar T, Yang W, Amemiya Y, et al. IGFBP7 reduces breast tumor growth by induction of senescence and apoptosis pathways. Breast Cancer Res Treat. 2012;133:563–573. doi: 10.1007/s10549-011-1816-4. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Wu M, Ling J, et al. Serum IGFBP7 levels associate with insulin resistance and the risk of metabolic syndrome in a Chinese population. Sci Rep. 2015;5:10227. doi: 10.1038/srep10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer. 2003;3:243–252. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 28.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Menachem T, Decker GA, et al. ASGE Standards of Practice Committee, author. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707–718. doi: 10.1016/j.gie.2012.03.252. [DOI] [PubMed] [Google Scholar]
- 30.Benassi MS, Pazzaglia L, Novello C, et al. Tissue and serum IGFBP7 protein as biomarker in high-grade soft tissue sarcoma. Am J Cancer Res. 2015;5:3446–3454. [PMC free article] [PubMed] [Google Scholar]
- 31.Shersher DD, Vercillo MS, Fhied C, et al. Biomarkers of the insulin-like growth factor pathway predict progression and outcome in lung cancer. Ann Thorac Surg. 2011;92:1805–1811. doi: 10.1016/j.athoracsur.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Hong C, Peng Y, et al. The diagnostic value of serum IGFBP7 in patients with esophageal squamous cell carcinoma. J Cancer. 2019;10:2687–2693. doi: 10.7150/jca.32393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith E, Ruszkiewicz AR, Jamieson GG, Drew PA. IGFBP7 is associated with poor prognosis in oesophageal adenocarcinoma and is regulated by promoter DNA methylation. Br J Cancer. 2014;110:775–782. doi: 10.1038/bjc.2013.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vizioli MG, Sensi M, Miranda C, et al. IGFBP7: an oncosuppressor gene in thyroid carcinogenesis. Oncogene. 2010;29:3835–3844. doi: 10.1038/onc.2010.136. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Pacyna-Gengelbach M, Ye F, et al. Insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) has potential tumour-suppressive activity in human lung cancer. J Pathol. 2007;211:431–438. doi: 10.1002/path.2132. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Yoo BK, Santhekadur PK, et al. Insulin-like growth factor-binding protein-7 functions as a potential tumor suppressor in hepatocellular carcinoma. Clin Cancer Res. 2011;17:6693–6701. doi: 10.1158/1078-0432.CCR-10-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gambaro K, Quinn MC, Cáceres-Gorriti KY, et al. Low levels of IGFBP7 expression in high-grade serous ovarian carcinoma is associated with patient outcome. BMC Cancer. 2015;15:135. doi: 10.1186/s12885-015-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]