Abstract
In 2007, the first associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure was performed in Regensburg, Germany. ALPPS is a variation of two-stage hepatectomy to induce rapid liver hypertrophy allowing the removal of large tumors otherwise considered irresectable due to a too small future liver remnant. In 2012, the international ALPPS registry was created, and it now contains more than 1,000 cases. During the past years, improved patient selection and refinements in operative techniques, in particular, less invasive approaches such as Partial ALPPS, Tourniquet ALPPS, Ablation-assisted ALPPS, Hybrid ALPPS or Laparoscopic or Robotic approaches, have resulted in significant improvements in safety. The most frequent indication for ALPPS is colorectal liver metastases. In the first randomized controlled study, ALPPS provided a higher resectability rate than conventional two-stage hepatectomy, with similar complication rates. Long-term outcome data are still missing. The initial results of ALPPS for hepatocellular carcinoma and for perihilar cholangiocarcinoma were devastating, but with progress in surgical technic and better patient selection, ALPPS could serve as a treatment alternative in carefully selected cases, even for these tumors. ALPPS has enlarged the armamentarium of hepato-pancreato-biliary surgeons, but there is still discussion regarding how to use this novel technique, which may allow resection of tumors that are otherwise deemed irresectable.
Keywords: ALPPS; ALPPS registry; Two-stage hepatectomy; Carcinoma, hepatocellular; Perihilar cholangiocarcinoma
INTRODUCTION
In 2007 Schlitt from Regensburg, Germany, performed somewhat by chance the first “associating liver partition and portal vein ligation for staged hepatectomy” (ALPPS) procedure. The original procedure consisted of a right portal vein ligation combined with simultaneous parenchymal transection during stage 1, followed by resection of the tumor-bearing extended right lobe 9 days later.1 A novel surgical concept–named “in-situ split liver resection”–was born and soon gained tremendous interest nationwide. In 2011, at the 9th congress of the European-African Hepato-Pancreato-Biliary Association (E-AHPBA) in Cape Town, South Africa, a first report of three cases was presented by the group from Mainz, Germany.2 One year later, the initial experience from five German centers, Regensburg, Mainz, Göttingen, Gießen and Tübingen with 25 cases of “in-situ-split liver resection” was published (Fig. 1). In this paper, a hypertrophy of the future liver remnant (FLR) of 74% after a median of 9 days and a 100% staged resectability were reported in tumors otherwise irresectable due to an insufficient FLR volume.3 In an accompanying editorial–as the term “in-situ-split” was already used for the partition of the liver in segmental/pediatric liver transplantation–de Santibañes and Clavien4 suggested the acronym “ALPPS” as a self-explanatory denomination of this new procedure. Soon after, many HPB groups started to work in the field of ALPPS and reports from all over the world confirmed the initial promising results of rapid and excessive induction of hypertrophy.5-7 In addition, it could be shown that ALPPS was effective even after failure of portal vein embolization (PVE).8 In 2012, following the 10th world congress of the International Hepato-Pancreato-Biliary Association in Paris, France, the international ALPPS registry (https://www.ALPPS.net) was created (founding members P.A. Clavien, H. Lang, E. de Santibañes) to collect data on this new procedure. A first major report of the registry appeared in 2014, and in May 2019 the registry accounted for more than 1,100 cases from 142 centers in 42 countries.9 In 2017, at the 12th congress of the E-AHPBA in Mainz, Germany, an expert meeting “10th anniversary of ALPPS” summarized the status quo and future perspectives of this innovative procedure.10
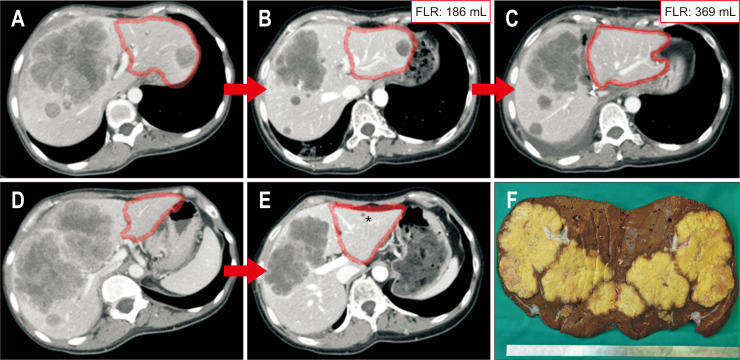
Fig. 1.
Classic ALPPS (complete parenchymal transection and ligation of the right portal vein) for colorectal liver metastases. (A, B) CT scan at the time of diagnosis; FLR encircled (red). (C, D) CT scan after 12 cycles of chemotherapy and targeted therapy (folfiri+bevacizumab) (*cyst); FLR volume=186 mL. (E) CT scan 8 days after step 1 (complete parenchymal transection and ligation of the right portal vein plus wedge resection of Seg II/III); FLR with 98% increase in volume (186 to 369 mL). (F) Resection specimen (R0-resection).
ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; CT, computed tomography; FLR, future liver remnant.
With the introduction of ALPPS a milestone in liver surgery seemed to be reached offering patients with an even extensive hepatic tumor burden the chance of resection. However, the early enthusiasm was hampered as the procedure was associated with a high morbidity and mortality rate which was 68% and 12% in the initial series.3 It was assumed that the high early complication rates would lower with increasing experience. This was confirmed by the first major report of 202 ALPPS procedures from the registry, showing a decrease of Clavien-Dindo grade ≥3 morbidity and mortality to 27% and 9%, respectively.9 It became clear that patient age and tumor type were independent risk factors for a poor outcome.9,11 Morbidity and mortality rates for CRLM were lower than for primary hepatobiliary malignancies. Nevertheless, complication rates after ALPPS for CRLM still were higher than after conventional liver resection, but this was attributed to a selection bias in the ALPPS group. It was obvious that patients undergoing ALPPS, although there was no matched pair analysis, had more advanced disease and a higher tumor burden than those patients usually treated by extended hepatectomy after PVE and PVL (portal vein ligation).
At the same time reports from Belgium, France and Italy demonstrated that post stage 1 complications grade ≥3b were significant predictors of post stage 2 mortality.12,13 Potential risk factors for an unfavorable outcome were in particular post stage 1 biliary fistula or ascites, and infected and/or bilious peritoneal fluid at stage 2.
Due to these data and a better know how and based on a growing experience with the procedure´s pitfalls, continuous efforts were made aiming at a reduction of the surgical morbidity and mortality. Modifications addressed anatomical and diagnostic aspects as well as the surgical technique itself. Anatomically, special attention was paid to the vascularization and biliary drainage of segment 4 in order to avoid ischemia/necrosis or bile leakage. At the end of stage 1 and stage 2 procedure, a bile leak test in order to prevent bile leaks was strongly recommended, and bile duct ligation of the future specimen was considered absolutely contraindicated as it might cause cholestasis, cholangitis or bile leaks.5,6,13 In order to prevent or minimize adhesions and to reduce bleeding and trauma during stage 2, either placement of a silastic bag between the two liver parts or other surface sealing materials not necessarily requiring removal was suggested.13
Further on, the importance of the venous drainage of segment 4 became evident. The concept of preserving the middle hepatic vein during stage 1 operation finally led to the development of “partial ALPPS.” It could be shown that the transection of only 50% to 80% of liver parenchyma resulted in similar effects on the velocity and extent of hepatic hypertrophy but with a significant reduction in perioperative morbidity.14,15 Complete transection of the liver parenchyma during stage 1 seemed to be mandatory only in those cases with a risk of invasion of the tumor into the FLR in between both stages due to close proximity of the tumor to the FLR.
Parallel to anatomical considerations special emphasis was laid on preoperative diagnostics. The two main diagnostic issues in ALPPS are the evaluation of the FLR and the timing of the stage 2 operation. It became clear that there was no good correlation of the CT-scan volumetry with the liver function even when based on three-dimensional reconstruction.16,17 Hepatobiliary scintigraphy (HBS) using 99mTc-labeled iminodiacetic acid derivates could show that volumetry overestimated the liver function in ALPPS while in PVE it was the other way around.18 Although the mechanisms are not fully understood the discrepancy between volume increase and the high rate of liver failure in ALPPS may be attributed to initial edema and enlarged but still at least partially immature and not completely functioning hepatocytes within the first 2 weeks of regeneration.19 This warranted an assessment of both function and volume of the FLR during the interstage course of ALPPS. Combining HBS with SPECT-CT (single photon emission computed tomography-computed tomography) offers quantitative information regarding segmental liver function and therefore provides an accurate measure of the function of the FLR.20 In a preliminary report Kambakamba et al.21 could show that the kinetic growth rate was a predictor for postoperative liver failure after ALPPS with a cutoff-point of 6% per day.
Over the ensuing years manifold other technical variations have been introduced, for example the replacement of parenchymal transection by just applying a tourniquet around the liver in the future transection line or by using radiofrequency or microwave ablation to create a virtual liver partition through a “necrotic groove” (Fig. 2).22-24 Another technical modification aiming to reduce the trauma during stage 1 was to avoid surgical manipulation of the hepatic hilum by replacing the portal vein transection by portal vein embolization (Hybrid ALPPS), either in a simultaneous or a subsequent fashion.25 The combination of only partial parenchymal transection and simultaneous intraoperative portal vein embolization (instead of portal vein ligation) was named Mini ALPPS.26 With the introduction of Mini ALPPS not only a modification of the ALPPS procedure but an entire change of paradigm was accomplished. In contrast to classic ALPPS, the surgical extent and the associated trauma of the stage 1 operation were dramatically reduced while the main surgical steps were performed at stage 2.
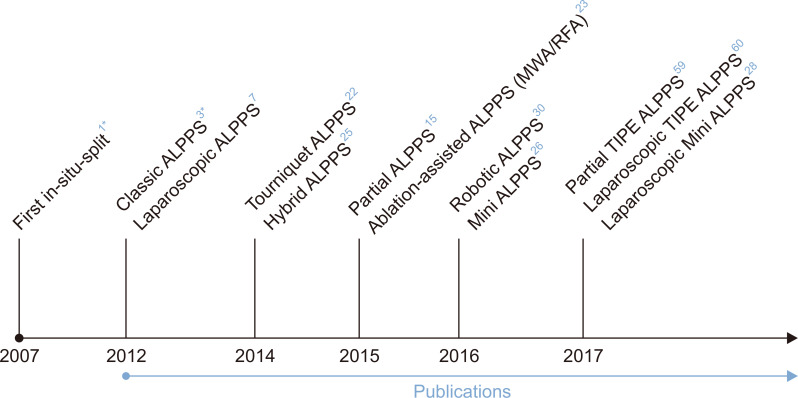
Fig. 2.
Timeline of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) and its most important variations.
Hybrid ALPPS, ALPPS with portal vein embolization instead of portal vein ligation; MWA, microwave ablation; RFA, radiofrequency ablation; Mini ALPPS, partial ALPPS plus simultaneous portal vein embolization; TIPE ALPPS, ALPPS with transileocolic portal vein embolization. *In 2007, the first in-situ split was performed; the first publication (classic ALPPS) was in 2012.
Only shortly after the introduction of ALPPS the first reports on laparoscopic approaches appeared–initially mainly regarding the stage 1 procedure but to a lesser extent also stage 2.7,27 The first larger series on laparoscopic ALPPS from Brazil showed a stage 2 completion rate similar to the open approach with no mortality and no complication Clavien-Dindo grade ≥3a, and a significant shorter hospital stay compared to an open ALPPS group (11 days vs 14 days; p=0.011).27 These encouraging results could be confirmed in further series, and the above mentioned technical variations for the open procedure such as partial or Mini ALPPS were also applicable and successful in the laparoscopic setting.28,29 In the meantime first case reports on robotic ALPPS have appeared.30 Technically, the robotic approach is feasible but needs further evaluation. In order to allow comparability of data, a “consensus” terminology was suggested to harmonize reports.31 All these technical refinements and a better patient selection led to a stepwise reduction in the perioperative complication rate of ALPPS.32 In a subsequent paper from the ALPPS registry in 2017 a continuous drop in early mortality and morbidity was reported.33 These data clearly demonstrated a significant improvement of results of ALPPS. Although a recent meta-analysis by Moris et al.34 still accounted a higher morbidity and mortality in ALPPS but with no difference in liver-related mortality data of the first and so far only randomized controlled trial (RCT; Ligro-Trial) showed no difference between ALPPS and PVE with regard to perioperative complication (Clavien-Dindo grade III-IV) and 90-day mortality.35 These data confirmed that with improved technical experience and patient selection, ALPPS for CRLM should no longer be considered a much riskier operation than a conventional two-stage hepatectomy.
The second major concern in ALPPS was about its oncological benefit. The initial enthusiasm about increased resectability in patients with even extensive tumor load had cooled down with first reports on early and aggressive tumor recurrence in many cases.36 The assumed pathological mechanism was a simultaneous massive stimulation of hepatocellular hypertrophy as well as growth of tumor cells in the systemic circulation and in the liver. However, experimental and also clinical data were contradictory.37 While some authors described an earlier tumor recurrence in admittedly very small series the meta-analysis by Moris et al.34 showed similar tumor recurrence rates for two-stage hepatectomy (TSH) and ALPPS for CRLM. Since there was a great variability in indications and patient selection from center to center comparability of data was limited. The only RCT comparing ALPPS and TSH did not analyze the oncological outcome and therefore cannot help answering this question.35
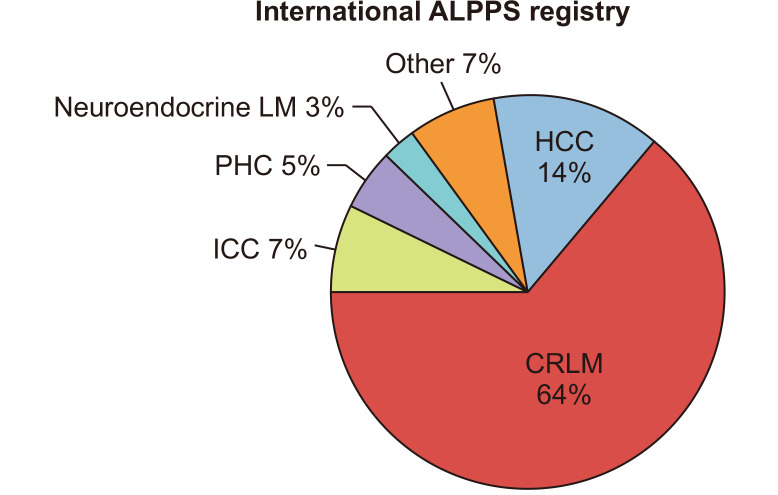
In the initial report indications for ALPPS were CRLM (n=14), hepatocellular carcinoma (HCC; n=3), ICC (n=2), perihilar cholangiocarcinoma (PHC; n=2), gallbladder carcinoma (n=1), malignant epithelioid hemangioendothelioma (n=1), non-colorectal liver metastases (n=2). Looking at the ALPPS registry CRLM are by far the most frequent indication for ALPPS (Fig. 3). Although ALPPS has been performed for almost any primary and secondary liver tumor, there are some larger series only about ALPPS for HCC and, to a much lesser extent, for PHC.
Fig. 3.
Indications for ALPPS, data from the registry (May 2019; https://ALPPS.net).
ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; CRLM, colorectal liver metastases; ICC, intrahepatic cholangiocarcinoma; PHC, perihilar cholangiocarcinoma; LM, liver metastases; HCC, hepatocellular carcinoma.
ALPPS FOR COLORECTAL LIVER METASTASES
ALPPS has expanded the treatment options for patients with CLRM which are currently by far the main indication for this procedure (about 2/3 of patients in the ALPPS registry).38 However, there seems to be a lack of hard criteria when ALPPS is necessary. In a recent analysis by Schnitzbauer et al.39 a tendency for potential overuse of ALPPS was assumed. Although recurrence rates appear higher than those of patients following conventional liver resections, ALPPS is offering the chance for cure in patients who otherwise would never have any surgical option. Robust long-term clinical and oncological outcome studies of ALPPS for CRLM are still lacking. In an initial report about ALPPS the 1- and 2-year overall survival was 76% and 63% for CRLM, respectively.9 These survival data need to be seen in the light of the high initial perioperative mortality of 8% and a probable selection bias considering that ALPPS might have been performed in more extensive hepatic tumor burden. In a series from two high volume centers, a 3-year overall survival of 50% and a disease-free survival of 13% was reported after 58 ALPPS for CRLM, with most of them after chemotherapy.40 In various series it could be shown that neoadjuvant chemotherapy had no negative effect on growth of FLR and on perioperative outcome after ALPPS.41
A recent case-match study from the ALPPS registry compared otherwise irresectable “ALPPS patients” to historic controls receiving palliative chemotherapy and concluded a non-superiority in early oncological outcome in the ALPPS group.42 It has to be pointed out that with a median of seven liver segments affected and a median of four lesions in the FLR the disease in the surgical group was very far advanced. Thus, the poor outcome (data) has to be regarded rather as a failure of patient selection than a failure of the concept of ALPPS.
So far, only a few series were published comparing ALPPS to TSH, showing all in all no survival difference. While Adam et al.43 reported a lower median survival for ALPPS (20 months vs 37 months), Ratti et al.,44 Kambakamba et al.45 and our own series found a similar 1-year survival without a difference in 1-year disease-free survival, all analyses performed in a non-randomized and non-matched-pair fashion.46 Recently, Robles-Campos et al.47 published almost identical data for Tourniquet ALPPS and conventional TSH with 1-, 3- and 5-year overall survival of 81%, 67% and 24% versus 76%, 57% and 23% in a propensity matched pair analysis. Long-term oncological data are almost missing (Table 1).9,13,40,42-49 We have to wait for further data and in particular further RCTs to evaluate whether ALPPS is associated with a higher or faster rate of tumor relapse.
Table 1.
Survival after ALPPS for Colorectal Liver Metastases-Literature Review
| Author | Year | No. of patients | Year, % | Median survival, mo | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | ||||
| Schadde et al.9 | 2014 | 141 | 76 | 63 | - | - | - |
| Oldhafer et al.13 | 2014 | 7 | 57 | - | - | - | - |
| Lang et al.48 | 2015 | 7 | - | - | 64 | - | - |
| Ratti et al.44 | 2015 | 12 | 92 | - | - | - | - |
| Adam et al.43 | 2016 | 17 | - | 42 | - | - | - |
| Björnsson et al.49 | 2016 | 23 | 83 | 59 | - | - | - |
| Kambakamba et al.45 | 2016 | 41 | - | - | - | - | 24.7±2.3 |
| Olthof et al.42 | 2017 | 70 | - | 62 | - | - | - |
| Wanis et al.40 | 2018 | 58 | 93 | 66 | 50 | - | - |
| Robles-Campos et al.47 | 2019 | 21 | 76 | - | 57 | 23 | 36 |
| Baumgart et al.46 | 2019 | 8 | 75 | - | 40 | - | 36.2 |
ALPPS, associating liver partition and portal vein ligation for staged hepatectomy.
ALPPS FOR HCC
Surgery for HCC is often challenging due to an underlying liver cirrhosis with concomitant portal hypertension and/or impaired hepatic function. As such, ALPPS seemed to be an attractive approach to increase resectability. However, a first report from the ALPPS registry with 35 ALPPS for intermediate-stage HCC revealed a 90-day mortality of 31%.50 Such a devastating result certainly called for abandoning the ALPPS procedure for this indication but careful analysis elucidated that ALPPS had been used with wide inclusion criteria and in a somewhat undifferentiated manner. Much better results came from the Hong Kong University and, with smaller numbers, from San Camillo Forlanini in Rome.51,52 In particular the Hong Kong group could outline strict criteria which patients with HCC were good candidates for ALPPS (FLR volume <30% estimated standard liver volume, Child A cirrhosis, indocyanine green clearance rate <20% at 15 minutes, platelet count >100/nL, and no total right portal vein thrombosis). Although the degree of FLR hypertrophy in fibrotic/cirrhotic livers appeared somewhat less than in non-cirrhotic livers–in the initial report there was a volume gain of the FLR of a little less than 50%–the 90-day mortality rate of 7.1% was encouraging.51-53 Of note, in chronic liver disease complete parenchymal transection seems to be associated with a more rapid hypertrophy of the FLR than partial ALPPS.54
A recent single-center study from Fudan University, China, analyzed their outcome of standard ALPPS in 45 HCC patients. The in-detail analysis revealed that the severity of liver disease was inversely correlated with the degree and velocity of hypertrophy. With overall 1- and 3-year survival rates of 64% and 60% the survival of patients undergoing ALPPS was significantly better than of those receiving TACE.55
The experiences from Hong Kong, Rome and Fudan show that in selected patients ALPPS seems to be an attractive approach to increase resectability in HCC patients otherwise left with palliative treatment options.54-56
ALPPS FOR PHC
The first study of the international ALPPS registry reported 11 patients with PHC with a 90-day mortality rate of 27%.9 In 2017, Olthof et al.57 compared the outcome of ALPPS from the international ALPPS registry to PVE and right trisectionectomy for PHC (data from two experienced HPB centers) in a matched case fashion. The postoperative mortality was twice as high in the ALPPS group (48% vs 24%) and the median survival was 6 months only after ALPPS and 29 months in the matched controls (p=0.048). Other reports from the registry also identified biliary tumors to be associated with a significant higher risk for fatal outcome.9,11,13 At that point, the question arose whether PHC should be regarded as a contraindication to ALPPS. However, many of the results of ALPPS in PHC had been obtained by conventional ALPPS procedures, and probably were also inferior as they were from the initial experience, that is, at the lower end of the learning curve with ALPPS. These limitations had been addressed in a subsequent letter to the editor identifying the critical issues of ALPPS in PHC and suggesting possibilities for substantial improvements.58 As already outlined, several modifications had been developed with the aim to reduce morbidity and mortality. The first to provide a modified technique for PHC was Sakamoto59 who refined the Mini ALPPS concept using a transileocolic portal vein embolization (partial TIPE ALPPS) instead of a transmesenteric PVE. In three patients encouraging results could be shown with this technique. Similarly, Balci60 reported on another two successful cases using laparoscopic stage 1 TIPE ALPPS. These promising data in carefully selected cases suggest that PHC should not be regarded as a categorial contraindication for ALPPS.
SUMMARY
Now, more than 10 years ago Schlitt from Regensburg, Germany, performed the first ALPPS procedure. In 2012, the first report on 25 so called “In-Situ Splits” was published as a novel surgical technique to rapidly induce liver hypertrophy. However, early enthusiasm was hampered by an initial high perioperative morbidity and mortality as well as by early and rapid disease-recurrence. Continuous efforts to improve patient selection, to optimize timing of stage 2 and to refine operative technique have led to reduced morbidity and mortality rates. The technical modifications aimed first and foremost to minimize the trauma associated with the stage 1 procedure.
The most frequent indication for ALPPS is CRLM. In a first randomized controlled study ALPPS could be shown to provide a higher resectability rate than conventional TSH but with similar complication rates. Long-term outcome data are still missing. First results of ALPPS for HCC and PHC were devastating but with technical refinements and better patient selection even in these tumors ALPPS could be a treatment alternative allowing resection of otherwise irresectable tumors.
As with every surgery in particular for ALPPS is true that the most essential decision is the indication when to use it. ALPPS certainly does not replace other techniques such as PVE or standard TSH, but may allow tumor resection in selected patients without any other surgical option left. As such, ALPPS is a welcome novel asset in the armamentarium in the hands of experienced hepatobiliary surgeons.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Schlitt HJ, Hackl C, Lang SA. 'In-Situ Split' liver resection/ALPPS-historical development and current practice. Visc Med. 2017;33:408–412. doi: 10.1159/000479850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart J, Lang S, Lang H. A new method for induction of liver hypertrophy prior to right trisectionectomy: a report of three cases. HPB (Oxford) 2011;13 Suppl 2:71–72. [Google Scholar]
- 3.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 4.de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg. 2012;255:415–417. doi: 10.1097/SLA.0b013e318248577d. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814–821. doi: 10.1007/s11605-012-2092-2. [DOI] [PubMed] [Google Scholar]
- 6.Dokmak S, Belghiti J. Which limits to the "ALPPS" approach? Ann Surg. 2012;256:e6. doi: 10.1097/SLA.0b013e318265fd64. [DOI] [PubMed] [Google Scholar]
- 7.Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg. 2012;256:e13. doi: 10.1097/SLA.0b013e318265ff2e. [DOI] [PubMed] [Google Scholar]
- 8.Knoefel WT, Gabor I, Rehders A, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388–394. doi: 10.1002/bjs.8955. [DOI] [PubMed] [Google Scholar]
- 9.Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the international ALPPS registry. Ann Surg. 2014;260:829–838. doi: 10.1097/SLA.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 10.Lang H, de Santibañes E, Schlitt HJ, et al. 10th Anniversary of ALPPS-Lessons Learned and quo Vadis. Ann Surg. 2019;269:114–119. doi: 10.1097/SLA.0000000000002797. [DOI] [PubMed] [Google Scholar]
- 11.Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg. 2015;262:780–786. doi: 10.1097/SLA.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 12.Truant S, Scatton O, Dokmak S, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol. 2015;41:674–682. doi: 10.1016/j.ejso.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Oldhafer KJ, Stavrou GA, van Gulik TM Core Group, author. ALPPS: where do we stand, where do we go? Eight recommendations from the first international expert meeting. Ann Surg. 2016;263:839–841. doi: 10.1097/SLA.0000000000001633. [DOI] [PubMed] [Google Scholar]
- 14.Linecker M, Kambakamba P, Reiner CS, et al. How much liver needs to be transected in ALPPS? A translational study investigating the concept of less invasiveness. Surgery. 2017;161:453–464. doi: 10.1016/j.surg.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 2015;261:e90–e92. doi: 10.1097/SLA.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 16.Cieslak KP, Huisman F, Bais T, et al. Future remnant liver function as predictive factor for the hypertrophy response after portal vein embolization. Surgery. 2017;162:37–47. doi: 10.1016/j.surg.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 17.De Graaf W, van Lienden KP, van den Esschert JW, Bennink RJ, van Gulik TM. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98:825–834. doi: 10.1002/bjs.7456. [DOI] [PubMed] [Google Scholar]
- 18.Olthof PB, Tomassini F, Huespe PE, et al. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: liver volume overestimates liver function. Surgery. 2017;162:775–783. doi: 10.1016/j.surg.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Matsuo K, Murakami T, Kawaguchi D, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery. 2016;159:1289–1298. doi: 10.1016/j.surg.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Cieslak KP, Bennink RJ, de Graaf W, et al. Measurement of liver function using hepatobiliary scintigraphy improves risk assessment in patients undergoing major liver resection. HPB (Oxford) 2016;18:773–780. doi: 10.1016/j.hpb.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambakamba P, Stocker D, Reiner CS, et al. Liver kinetic growth rate predicts postoperative liver failure after ALPPS. HPB (Oxford) 2016;18:800–805. doi: 10.1016/j.hpb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robles R, Parrilla P, López-Conesa A, et al. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br J Surg. 2014;101:1129–1134. doi: 10.1002/bjs.9547. [DOI] [PubMed] [Google Scholar]
- 23.Cillo U, Gringeri E, Feltracco P, et al. Totally laparoscopic microwave ablation and portal vein ligation for staged hepatectomy: a new minimally invasive two-stage hepatectomy. Ann Surg Oncol. 2015;22:2787–2788. doi: 10.1245/s10434-014-4353-7. [DOI] [PubMed] [Google Scholar]
- 24.Edmondson MJ, Sodergren MH, Pucher PH, et al. Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): many routes to the summit. Surgery. 2016;159:1058–1072. doi: 10.1016/j.surg.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Kantas A, Ittrich H, et al. Avoid "all-touch" by hybrid ALPPS to achieve oncological efficacy. Ann Surg. 2016;263:e6–e7. doi: 10.1097/SLA.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 26.De Santibañes E, Alvarez FA, Ardiles V, Pekolj J, de Santibañes M. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbecks Arch Surg. 2016;401:557–563. doi: 10.1007/s00423-016-1424-1. [DOI] [PubMed] [Google Scholar]
- 27.Machado MA, Makdissi FF, Surjan RC, Basseres T, Schadde E. Transition from open to laparoscopic ALPPS for patients with very small FLR: the initial experience. HPB (Oxford) 2017;19:59–66. doi: 10.1016/j.hpb.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Pekolj J, Alvarez FA, Biagiola D, Villegas L, Ardiles V, de Santibañes E. Totally laparoscopic Mini-ALPPS using a novel approach of laparoscopic-assisted transmesenteric portal vein embolization. J Laparoendosc Adv Surg Tech A. 2018;28:1229–1233. doi: 10.1089/lap.2018.0039. [DOI] [PubMed] [Google Scholar]
- 29.Truant S, El Amrani M, Baillet C, et al. Laparoscopic partial ALPPS: much better than ALPPS! Ann Hepatol. 2019;18:269–273. doi: 10.5604/01.3001.0012.7937. [DOI] [PubMed] [Google Scholar]
- 30.Vicente E, Quijano Y, Ielpo B, Fabra I. First ALPPS procedure using a total robotic approach. Surg Oncol. 2016;25:457. doi: 10.1016/j.suronc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Linecker M, Kron P, Lang H, de Santibañes E, Clavien PA. Too many languages in the ALPPS: preventing another tower of babel? Ann Surg. 2016;263:837–838. doi: 10.1097/SLA.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K. Modified ALPPS procedures: more safety through less invasive surgery. Langenbecks Arch Surg. 2017;402:563–574. doi: 10.1007/s00423-017-1588-3. [DOI] [PubMed] [Google Scholar]
- 33.Linecker M, Björnsson B, Stavrou GA, et al. Risk adjustment in ALPPS is associated with a dramatic decrease in early mortality and morbidity. Ann Surg. 2017;266:779–786. doi: 10.1097/SLA.0000000000002446. [DOI] [PubMed] [Google Scholar]
- 34.Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg. 2018;42:806–815. doi: 10.1007/s00268-017-4181-6. [DOI] [PubMed] [Google Scholar]
- 35.Sandström P, Røsok BI, Sparrelid E, et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian multicenter randomized controlled trial (LIGRO Trial) Ann Surg. 2018;267:833–840. doi: 10.1097/SLA.0000000000002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldhafer KJ, Donati M, Jenner RM, Stang A, Stavrou GA. ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J Surg. 2014;38:1504–1509. doi: 10.1007/s00268-013-2401-2. [DOI] [PubMed] [Google Scholar]
- 37.Kambakamba P, Linecker M, Schneider M, et al. Impact of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) on growth of colorectal liver metastases. Surgery. 2018;163:311–317. doi: 10.1016/j.surg.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Lang H, Baumgart J, Mittler J. Associating liver partition and portal vein ligation for staged hepatectomy in the treatment of colorectal liver metastases: current scenario. Dig Surg. 2018;35:294–302. doi: 10.1159/000488097. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzbauer AA, Schadde E, Linecker M, et al. Indicating ALPPS for colorectal liver metastases: a critical analysis of patients in the international ALPPS registry. Surgery. 2018;164:387–394. doi: 10.1016/j.surg.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Wanis KN, Ardiles V, Alvarez FA, et al. Intermediate-term survival and quality of life outcomes in patients with advanced colorectal liver metastases undergoing associating liver partition and portal vein ligation for staged hepatectomy. Surgery. 2018;163:691–697. doi: 10.1016/j.surg.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 41.Hasselgren K, Malagò M, Vyas S, et al. Neoadjuvant chemotherapy does not affect future liver remnant growth and outcomes of associating liver partition and portal vein ligation for staged hepatectomy. Surgery. 2017;161:1255–1265. doi: 10.1016/j.surg.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 42.Olthof PB, Huiskens J, Wicherts DA, et al. Survival after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for advanced colorectal liver metastases: a case-matched comparison with palliative systemic therapy. Surgery. 2017;161:909–919. doi: 10.1016/j.surg.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Adam R, Imai K, Castro Benitez C, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg. 2016;103:1521–1529. doi: 10.1002/bjs.10256. [DOI] [PubMed] [Google Scholar]
- 44.Ratti F, Schadde E, Masetti M, et al. Strategies to increase the resectability of patients with colorectal liver metastases: a multi-center case-match analysis of ALPPS and conventional two-stage hepatectomy. Ann Surg Oncol. 2015;22:1933–1942. doi: 10.1245/s10434-014-4291-4. [DOI] [PubMed] [Google Scholar]
- 45.Kambakamba P, Linecker M, Alvarez FA, et al. Short chemotherapy-free interval improves oncological outcome in patients undergoing two-stage hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2016;23:3915–3923. doi: 10.1245/s10434-016-5419-5. [DOI] [PubMed] [Google Scholar]
- 46.Baumgart J, Jungmann F, Bartsch F, et al. Two-stage hepatectomy and ALPPS for advanced bilateral liver metastases: a tailored approach balancing risk and outcome. J Gastrointest Surg. 2019;23:2391–2400. doi: 10.1007/s11605-019-04145-9. [DOI] [PubMed] [Google Scholar]
- 47.Robles-Campos R, Brusadin R, López-Conesa A, et al. Long-term outcome after conventional two-stage hepatectomy versus tourniquet-ALPPS in colorectal liver metastases: a propensity score matching analysis. World J Surg. 2019;43:2281–2289. doi: 10.1007/s00268-019-05031-w. [DOI] [PubMed] [Google Scholar]
- 48.Lang SA, Loss M, Benseler V, Glockzin G, Schlitt HJ. Long-term results after in-situ split (ISS) liver resection. Langenbecks Arch Surg. 2015;400:361–369. doi: 10.1007/s00423-015-1285-z. [DOI] [PubMed] [Google Scholar]
- 49.Björnsson B, Sparrelid E, Røsok B, et al. Associating liver partition and portal vein ligation for staged hepatectomy in patients with colorectal liver metastases: intermediate oncological results. Eur J Surg Oncol. 2016;42:531–537. doi: 10.1016/j.ejso.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 50.D'Haese JG, Neumann J, Weniger M, et al. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol. 2016;23:1335–1343. doi: 10.1245/s10434-015-5007-0. [DOI] [PubMed] [Google Scholar]
- 51.Chan AC, Poon RT, Chan C, Lo CM. Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann Surg. 2016;263:e14–e16. doi: 10.1097/SLA.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 52.Vennarecci G, Grazi GL, Sperduti I, et al. ALPPS for primary and secondary liver tumors. Int J Surg. 2016;30:38–44. doi: 10.1016/j.ijsu.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 53.Chia DKA, Yeo Z, Loh SEK, Iyer SG, Madhavan K, Kow AWC. ALPPS for hepatocellular carcinoma is associated with decreased liver remnant growth. J Gastrointest Surg. 2018;22:973–980. doi: 10.1007/s11605-018-3697-x. [DOI] [PubMed] [Google Scholar]
- 54.Chan ACY, Chok K, Dai JWC, Lo CM. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepatocellular carcinoma: complete-ALPPS versus partial-ALPPS. Surgery. 2017;161:357–364. doi: 10.1016/j.surg.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Peng Y, Hu J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg. 2020;271:534–541. doi: 10.1097/SLA.0000000000002942. [DOI] [PubMed] [Google Scholar]
- 56.Vennarecci G, Ferraro D, Tudisco A, et al. The ALPPS procedure: hepatocellular carcinoma as a main indication: an Italian single-center experience. Updates Surg. 2019;71:67–75. doi: 10.1007/s13304-018-0596-3. [DOI] [PubMed] [Google Scholar]
- 57.Olthof PB, Coelen RJS, Wiggers JK, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:381–387. doi: 10.1016/j.hpb.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang H, de Santibanes E, Clavien PA. Outcome of ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:379–380. doi: 10.1016/j.hpb.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 59.Sakamoto Y, Matsumura M, Yamashita S, Ohkura N, Hasegawa K, Kokudo N. Partial TIPE ALPPS for perihilar cancer. Ann Surg. 2018;267:e18–e20. doi: 10.1097/SLA.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 60.Balci D. Pushing the envelope in perihiler cholangiocellularcarcinoma surgery: TIPE-ALPPS. Ann Surg. 2018;267:e21–e22. doi: 10.1097/SLA.0000000000002604. [DOI] [PubMed] [Google Scholar]