Abstract
Background/Aims
Recently, a three-plane symmetric needle with Franseen geometry was developed for endoscopic ultrasound-guided fine needle biopsy (EUS-FNB). In this retrospective study, tissue acquisition per pass was compared between 22-gauge Franseen FNB and standard fine needle aspiration (FNA) needles in patients with solid pancreatic lesions.
Methods
Consecutive patients who underwent EUS-FNA or EUS-FNB for solid pancreatic lesions between October 2014 and March 2018 were retrospectively studied. The tissue acquisition rate and the diagnostic performance per session, per pass, and at first pass were compared.
Results
A total of 663 passes (300 by the FNB needle and 363 by the standard FNA needle) were performed in 154 patients (71 FNB and 83 FNA). The tissue acquisition rate per session and at first pass in the FNB and FNA groups was 100% and 95% (p=0.13) and 87% and 69% (p=0.007), respectively. The multivariate analysis revealed that among the patients, EUS-FNB (odds ratio, 3.07; p=0.01) was associated with a higher first-pass tissue acquisition rate. While the tissue acquisition rate reached a plateau after the 4th pass with FNA, it reached a plateau after the 2nd pass with FNB. Among the 129 malignant cases, the histological tissue acquisition rate per session was similar (100% and 94%), but the sensitivity by histology alone per session was higher for FNB than for FNA (93% and 73%, p<0.01).
Conclusions
The results of our retrospective analysis indicated that compared with a standard FNA needle, a 22-gauge Franseen FNB needle was associated with a higher first-pass tissue acquisition rate.
Keywords: Endoscopic ultrasound, Endoscopic ultrasound-guided fine needle aspiration, Endoscopic ultrasonography-guided fine-needle biopsy, Histology, Pancreatic neoplasms
INTRODUCTION
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is an established procedure for tissue acquisition and pathological diagnosis of pancreatic solid lesions.1-3 However, histological tissue acquisition of EUS-FNA is not necessarily sufficient for additional diagnostic techniques or for the identification of molecular markers often necessary for the final diagnosis and appropriate clinical management. To overcome these disadvantages of EUS-FNA, several novel needles have been introduced for EUS-guided fine needle biopsy (EUS-FNB).4-20
Recently, a three-plane symmetric needle with Franseen geometry has been developed for performing EUS-FNB.15-20 While rapid on-site evaluation (ROSE) reportedly increases the diagnostic performance and decreases the number of passes,21 its role in EUS-FNB is not fully established in clinical practice. In addition, ROSE is not readily available in many institutions including our center. We conducted this retrospective study comparing tissue acquisition rates per pass between 22-gauge Franseen FNB needle and 22-gauge standard FNA needle to evaluate the role of EUS-FNB without ROSE in patients with pancreatic solid lesions.
MATERIALS AND METHODS
1. Patients
This is a single center, retrospective study in the University of Tokyo Hospital. Data on consecutive patients who underwent EUS-FNA between October 2014 and September 2016 and EUS-FNB between October 2016 and March 2018 for pancreatic solid lesions were retrieved from a prospectively maintained database at the University of Tokyo Hospital. Inclusion criteria were patients who underwent EUS-guided tissue acquisition using a standard 22-gauge FNA needle (Expect needle: Boston Scientific Japan, Tokyo, Japan) or a 22-gauge EUS-FNB needle (Acquire needle: Boston Scientific Japan) for pancreatic solid lesions and whose cytological and histological analyses per passes were available. Exclusion criteria were patients with severe coagulopathy and patients with a pancreatic cystic lesion or an extra-pancreatic lesion. This study was approved by the local ethical committee (approval number: 1804) and all patients provided informed consent for EUS-guided tissue acquisition.
2. EUS-FNA or EUS-FNB procedure
All EUS procedures were performed using a curved linear array echoendoscope (GF-UCT260; Olympus Medical Systems, Tokyo, Japan or EG-580-UT; Fujifilm Medical Systems, Tokyo, Japan), which was connected to a processor featuring color Doppler function (EU-ME2; Olympus Medical Systems or SU-1, Fujifilm Medical Systems) under moderate sedation with intravenous midazolam and pethidine hydrochloride. After EUS evaluation including regional vasculature with color Doppler function, EUS-guided tissue acquisition was performed. The FNA or FNB needle with a stylet was advanced into the target lesion under EUS guidance. A stylet was completely removed and 10 to 20 to-and-fro movements within the target lesion were performed while a 10-mL suction was applied. The needle was withdrawn after suction was released, and the obtained material was expressed entirely onto a glass slide by reinsertion of the stylet. The material on the slide was then carefully inspected and visible core, if any, was lifted off the slide and placed into a formalin bottle for histologic examination. The remaining specimen was sent for cytological examination. No ROSE was performed. The number of passes was decided at the discretion of the attending physician but EUS-FNA or FNB was basically repeated until enough visible core tissue was obtained macroscopically (Fig. 1). Cytological and histological reports were retrospectively reviewed. Cellularity and blood contamination for each slide of cytological specimen were evaluated based on the consensus between two cytotechnologists who were blind to FNA or FNB needles, and the semi-quantified scores used in this study were routinely recorded in the pathological reports as follows; 0 (none), 1 (few aggregates), 2 (fair cellularity) and 3 (abundant cellularity) for cellularity and 0 (none), 1 (few), 2 (moderate) and 3 (high) for blood contamination. Cellularity score was based on the amount of all types of cells including tumor cells. Pathological diagnosis was based on the combination of cytological and histological diagnoses as previously reported.22 All procedures were performed by 10 experts (≥5 years of EUS-FNA experiences) or by 12 trainees (<5 years of EUS-FNA experiences) under supervision by experts.
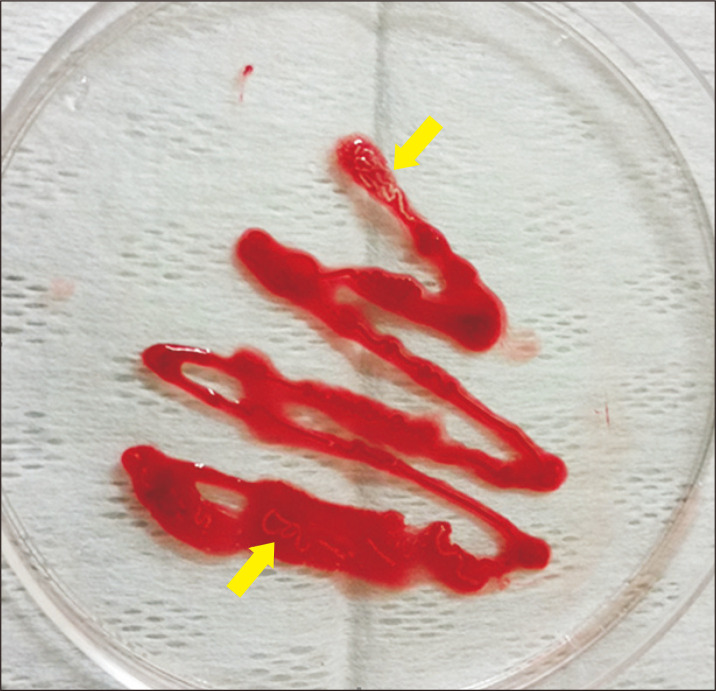
Fig. 1.
Macroscopic findings of endoscopic ultrasound-guided fine needle biopsy specimens. A whitish core tissue was clearly observed upon visual inspection (arrows).
3. Final diagnosis
The final diagnosis was made either by the pathological diagnosis based on surgically resected specimen, by EUS-FNA or EUS-FNB diagnosis positive for malignancy with compatible clinical outcomes, or by EUS-FNA or EUS-FNB diagnosis negative for malignancy with lack of deterioration on follow-up more than 6 months.
4. Outcome measurements
Primary endpoint of this study was the histological tissue acquisition rate in a first pass and per session. The histological tissue acquisition rate was defined as the acquisition rate of the sufficient material for adequate histological interpretation. Secondary outcomes were the diagnostic performance by histology, cytology and combination per pass, per session and in the first pass, cellularity and bloodiness of cytological specimen per pass, adverse events and the prognostic factors for first-pass tissue acquisition and histological sensitivity. Technical failure was defined as failure to obtain macroscopic visible specimen before needle exchange. Adverse events were defined and graded according to the lexicon.23 Post EUS-FNA or FNB pancreatitis was defined as a new onset of abdominal pain or a worsening of an existing one, associated with an increase in amylase or lipase more than 3 times the upper cutoff value. Computed tomography was performed for evaluation of pancreatitis in those cases with abdominal pain associated with elevated pancreatic enzymes.
5. Statistical analysis
Continuous variables were presented as the median and range, and categorical variables as the number and percentage. Statistical comparisons were performed with the chi-square test or the Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Logistic regression models were used to evaluate prognostic factors of first-pass tissue acquisition and histological sensitivity. Potential prognostic factors of first-pass tissue acquisition were age, sex, tumor size, tumor location, puncture route, needle type, and final diagnosis of pancreatic cancer. A p-value of <0.05 in a two-tailed test was considered as a statistically significant difference. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).24
RESULTS
1. Patient characteristics and EUS procedures
A total of 663 passes (300 by the FNB needle and 363 by the FNA needle) were performed in 154 patients (71 FNB and 83 FNA) with pancreatic solid lesions during the study period. Patient characteristics and final diagnoses are shown in Table 1. In both groups, the final diagnosis was pancreatic cancer in 72%. The median tumor size was 25 mm in the FNB group and 24 mm in the FNA group. The median number of passes was four in both groups.
Table 1.
Patient Characteristics and EUS-Guided Tissue Acquisition
| Characteristics | FNB (n=71) | FNA (n=83) | p-value |
|---|---|---|---|
| Age, yr | 68 (26–85) | 70 (32–93) | 0.65 |
| Male sex | 46 (65) | 49 (59) | 0.51 |
| Final diagnosis | |||
| Malignant | 57 (80) | 72 (87) | |
| Pancreatic cancer | 51 (72) | 60 (72) | |
| Neuroendocrine tumor | 3 (4) | 8 (10) | |
| Metastatic pancreatic tumor | 3 (4) | 4 (5) | |
| Benign | 14 (20) | 11 (13) | |
| Autoimmune pancreatitis | 5 (7) | 5 (6) | |
| Chronic pancreatitis | 3 (4) | 2 (2) | |
| Nonspecific inflammation | 3 (4) | 2 (2) | |
| Serous cyst neoplasm | 1 (1) | 1 (1) | |
| Pancreatic abscess | 1 (1) | 1 (1) | |
| Schwannoma | 1 (1) | 0 | |
| Tumor size, mm | 25 (5–66) | 24 (7–67) | 0.65 |
| Tumor location | 0.90 | ||
| Head | 29 (41) | 36 (43) | |
| Body | 31 (44) | 36 (43) | |
| Tail | 11 (15) | 11 (13) | |
| Puncture site | 0.50 | ||
| Stomach | 39 (55) | 51 (61) | |
| Bulb | 25 (35) | 22 (27) | |
| D2 | 7 (10) | 10 (12) | |
| No. of passes | 4 (2–8) | 4 (1–8) | 0.29 |
| Procedures | 0.01 | ||
| Experts | 32 (45) | 56 (67) | |
| Trainee | 39 (55) | 27 (33) |
Data are presented as median (range) or number (%).
EUS, endoscopic ultrasound; FNB, fine needle biopsy; FNA, fine needle aspiration; D2, the second portion of the duodenum.
In the FNA group, there was one technical failure case in which macroscopic visible specimen could not be obtained before needle exchange to a 25-gauge FNA needle. In additional 14 cases (four EUS-FNB and 10 EUS-FNA), though EUS-FNB or EUS-FNA was technically successful, the needle was exchanged to a 25-gague FNA needle at the discretion of endoscopists. The reasons for needle exchange were difficult angle for puncture in 10 (three EUS-FNB and seven EUS-FNA) and needle malfunction in four (one EUS-FNB and three EUS-FNA).
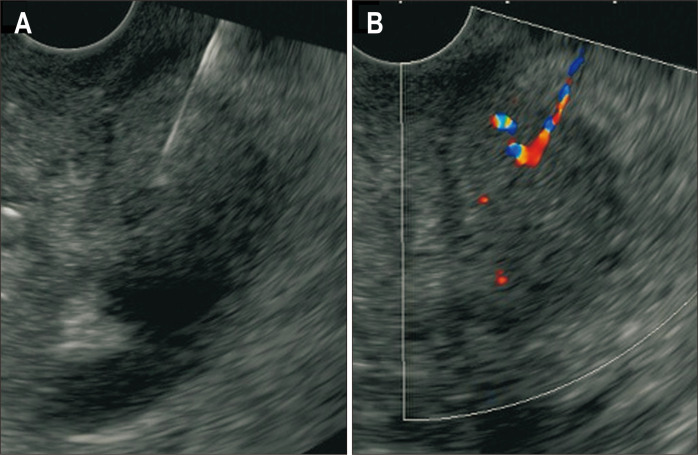
As procedure-related adverse events, two cases (4%) in the FNB group developed mild pancreatitis. Doppler signal along the needle tract was observed in some cases after EUS-FNB (Fig. 2) but clinically overt bleeding was not observed. Doppler signal along the needle tract was rarely observed in the FNA group. There were no adverse events in the FNA group.
Fig. 2.
(A) The EUS image of EUS-FNB puncture. (B) After removal of the FNB needle, a Doppler signal was temporarily observed along the needle tract.
EUS, endoscopic ultrasound; FNB, fine needle biopsy.
2. Histological tissue acquisition in the FNB and FNA groups
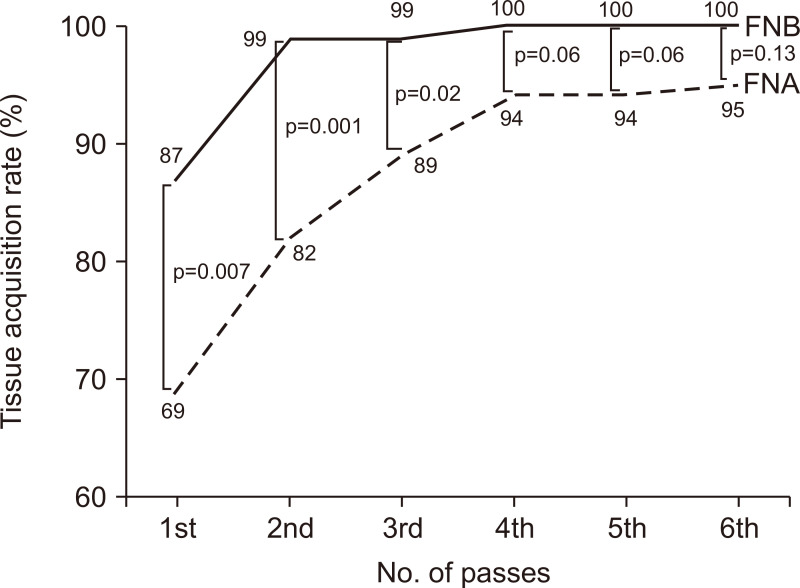
The histological tissue acquisition rate in the first pass was significantly higher in the FNB group: 87% and 69% in the FNB and FNA groups (p=0.007), respectively. After a median of four passes in both groups, the histological tissue acquisition rate was 100% and 95% in the FNB and FNA groups (p=0.13), respectively. While tissue acquisition rate reached a plateau after 4th pass with FNA, it reached a plateau after 2nd pass with FNB (Fig. 3). The histological tissue acquisition was confirmed after the procedure, so the median number of passes was same in both groups.
Fig. 3.
Tissue acquisition rates of EUS-FNB and EUS-FNA. The tissue acquisition rate reached a plateau after the 4th pass with EUS-FNA, whereas it reached a plateau after the 2nd pass with EUS-FNB.
EUS-FNB, endoscopic ultrasound-guided fine needle biopsy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration.
Prognostic factor analyses of first-pass tissue acquisition are shown in Table 2. In the multivariate analysis, EUS-FNB (odds ratio [OR], 3.07; 95% confidence interval, 1.31 to 7.17; p=0.01) and puncture from the stomach (OR, 0.42; 95% confidence interval, 0.18 to 0.98, p=0.04) were the significant factors associated with a higher tissue acquisition rate.
Table 2.
Prognostic Factors of First-Pass Tissue Acquisition
| Factor | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age ≥70 yr | 1.09 (0.51–2.33) | 0.82 | - | - | |
| Male sex | 0.97 (0.45–2.12) | 0.94 | - | - | |
| Pancreatic cancer | 1.75 (0.79–3.90) | 0.17 | - | - | |
| Tumor size ≥20 mm | 1.81 (0.82–3.99) | 0.14 | - | - | |
| Puncture site, stomach | 0.40 (0.17–0.93) | 0.03 | 0.42 (0.18–0.98) | 0.04 | |
| Needle type, FNB needle | 3.14 (1.36–7.27) | 0.01 | 3.07 (1.31–7.17) | 0.01 | |
| Endoscopist, expert | 1.16 (0.54–2.48) | 0.70 | - | - | |
OR, odds ratio; CI, confidence interval; FNB, fine needle biopsy.
3. Cytological evaluation in the FNB and FNA groups
Results of cytological evaluation are shown in Table 3. The accuracy, sensitivity, specificity, positive and negative predictive value of cytology alone per session (n=154) were 76%, 71%, 100%, 100% and 40%, respectively, while those of cytology alone per pass (n=663) were 60%, 52%, 100%, 100% and 29%. When the FNB and FNA groups were compared, accuracy per pass tended to be high in the FNB group: 64% in the FNB group and 56% in the FNA group (p=0.06).
Table 3.
The Diagnostic Performance of the Cytological Specimens
| Diagnostic performance | Per session | Per pass | |||||
|---|---|---|---|---|---|---|---|
| FNB (n=71) |
FNA (n=83) |
p-value | FNB (n=300) |
FNA (n=363) |
p-value | ||
| Accuracy | 79 | 74 | 0.46 | 64 | 56 | 0.06 | |
| Sensitivity | 74 | 69 | 0.70 | 55 | 49 | 0.20 | |
| Specificity | 100 | 100 | 1.00 | 100 | 100 | 1.00 | |
| PPV | 100 | 100 | 1.00 | 100 | 100 | 1.00 | |
| NPV | 48 | 33 | 0.30 | 35 | 24 | 0.02 | |
Data are presented as percentage.
FNB, fine needle biopsy; FNA, fine needle aspiration; PPV, positive predictive value; NPV, negative predictive value.
Blood contamination was more prominent in cytological specimen of the FNB group: blood contamination scores ≥2 in 73% and 55% of the FNB and FNA groups, respectively (p<0.01). However, the cellularity score did not increase in the FNB group. The cellularity scores of ≥2 were 59% and 52% in the FNB and FNA groups (p=0.07).
4. The diagnostic performance of EUS-FNB and EUS-FNA in malignancy
A total of 553 passes were performed in 129 malignant cases and the diagnostic performance is shown in Table 4. Histological tissue acquisition rate per session was not significantly different: 100% and 94% in the FNB and FNA group (p=0.13), respectively. However, sensitivity of histology alone per session was higher in the FNB group: 93% and 73% in the FNB and FNA group (p<0.01), respectively.
Table 4.
The Diagnostic Performance of EUS-FNB and EUS-FNA per Session, per Pass and at First Pass
| Diagnostic performance | Per session | Per pass | First pass | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FNB (n=57) |
FNA (n=72) |
p-value | FNB (n=241) |
FNA (n=312) |
p-value | FNB (n=57) |
FNA (n=72) |
p-value | |||
| Histological tissue acquisition | 100 | 94 | 0.13 | 89 | 73 | <0.01 | 88 | 69 | 0.02 | ||
| Histology positive for malignancy | 93 | 73 | <0.01 | 70 | 46 | <0.01 | 63 | 43 | 0.03 | ||
| Cytology positive for malignancy | 74 | 69 | 0.70 | 55 | 49 | 0.20 | 47 | 46 | 1.00 | ||
| Histology or cytology positive for malignancy | 97 | 85 | 0.04 | 79 | 64 | <0.01 | 72 | 58 | 0.14 | ||
Data are presented as percentage.
EUS, endoscopic ultrasound; FNB, fine needle biopsy; FNA, fine needle aspiration.
In the per pass analysis, EUS-FNB demonstrated a higher histological specimen acquisition rate (89% and 73%, p<0.01) and higher sensitivity in histology (70% vs 46%, p<0.01) per pass. The sensitivity of cytology per pass was similar (55% and 49% in the FNB and FNA groups, p=0.20) and the combined histological and cytological sensitivity per pass was significantly higher (79% and 64% in the FNB and FNA groups, respectively, p<0.01). The incremental increase of the diagnostic performance by adding cytology was 4% and 14% in the FNB and FNA groups (p=0.07).
In the first-pass analysis, both the histological tissue acquisition rate (88% vs 69%, p=0.02) and the histological sensitivity (63% vs 43%, p=0.03) were significantly higher in the EUS-FNB group, too. Prognostic factor analyses for histological sensitivity in a first pass are shown in Table 5. In the multivariate analysis, there were no statistically significant prognostic factors of first-pass histological diagnostic sensitivity. However, EUS-FNB (OR, 1.76; p=0.14) and the tumor size of ≥20 mm (OR, 2.15; p=0.07) had a tendency toward positive malignancy in a first pass, though not statistically significant.
Table 5.
Prognostic Factors of First-Pass Histological Sensitivity
| Factor | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age ≥70 yr | 0.90 (0.44–1.84) | 0.76 | - | - | |
| Male sex | 1.01 (0.49–2.11) | 0.97 | - | - | |
| Tumor size ≥20 mm | 2.23 (0.99–5.05) | 0.05 | 2.15 (0.95–4.91) | 0.07 | |
| Puncture site, stomach | 0.77 (0.37–1.60) | 0.48 | - | - | |
| Needle type, FNB needle | 1.83 (0.87–3.85) | 0.11 | 1.76 (0.83–3.74) | 0.14 | |
| Endoscopist, expert | 0.86 (0.43–1.72) | 0.67 | - | - | |
OR, odds ratio; CI, confidence interval; FNB, fine needle biopsy.
In the first-pass analysis of 111 pancreatic cancer cases (51 EUS-FNB and 60 EUS-FNA), the histological tissue acquisition rate was 96% and 90% (p=0.28) and the diagnosis of definitive cancer was obtained in 84% and 63% by EUS-FNB and by EUS-FNA (p=0.02).
DISCUSSION
In our retrospective comparative analysis, EUS-FNB using a 22-gauge Franseen needle was associated with a higher tissue acquisition rate in the first pass compared with EUS-FNA using a 22-gauge standard needle in EUS-guided tissue acquisition of pancreatic solid lesions. While tissue acquisition rate reached a plateau after four passes of FNA, it reached a plateau after two passes of FNB, suggesting that this FNB needle would provide a higher acquisition rate of core tissue with fewer passes even without ROSE. The diagnostic performance of malignancy was also higher in the EUS-FNB group in per pass and per session analyses. The Franseen needle has three cutting edges to facilitate greater tissue acquisition and improve diagnostic ability.15 In our study, transgastric approach was associated with lower tissue acquisition rate in the first pass. In general, transduodenal puncture is technically difficult, especially when a 19-gauge or FNB needle is used. In a comparative study of 19- and 22-gauge FNA needles,25 while 22-gauge FNA needle demonstrated similar diagnostic accuracy throughout the pancreas, 19-gauge FNA needle was superior to 22-gauge FNA needle in the body and tail of pancreas, but not in the head of pancreas. In another study comparing 19-gauge FNA and reversed bevel FNB needles,9 FNB needle showed better diagnostic accuracy only by transgastric puncture. Given the better diagnostic performance in transduodenal puncture using this 22-Franseen needle, it is suggested that this needle has good technical feasibility even in the duodenum. In addition, the stroke technique might affect the study outcomes. In a comparative study of the stroke methods using 22-gauge FNA needle,26 the major study outcome is that the door-knocking method showed better diagnostic accuracy in the stomach. However, when the conventional stroke technique is used, the tissue acquisition rate was 100% in the duodenum and 85.7% in the stomach. In our study cohort, the conventional stroke was used in most cases, rather than the door-knocking method, which might lead to the better tissue acquisition rate in the duodenum using the 22-gauge Franseen needle.
Although EUS-guided tissue acquisition has been established as a method for the pathological diagnosis of pancreatic lesions, there is some controversy on its techniques such as the needle size, the application of suction or a stylet, the number of passes and so on. EUS-FNB is a recent issue of debate in this field since a few types of FNB needles are now commercially available: Procore,4-9 fork-tip,11-14 and Franseen15-20 needles. There are comparative studies between FNB and FNA needles or between FNB needles with conflicting data but a recent meta-analysis of 11 randomized controlled trials demonstrated superiority of EUS-FNB to EUS-FNA in terms of specimen adequacy, sensitivity and the number of passes. After the promising initial results of EUS-FNB using the Franseen needle,15 Bang et al.16 conducted a small randomized controlled trial comparing the Franseen needle and the conventional FNA needle, showing a larger total tissue, tumor and desmoplastic fibrosis were obtained by two dedicated passes for histological analysis by cell block. In another retrospective comparative study, the Franseen needle provided better tissue acquisition rate with a mean of 2.1 passes compared to 3.2 passes by the conventional FNA needle.19 Our study also confirmed similar findings with a larger cohort; histology acquisition rate of EUS-FNB reached a plateau after two passes with better sensitivity compared to EUS-FNA in per pass analysis.
While ROSE reportedly reduces the number of passes in EUS-FNA,27 it is not globally available in clinical practice. The optimal number of passes in EUS-FNA without ROSE is still unclear.28-30 While seven passes were required to obtain a sensitivity of 83.3% with a single pass sensitivity as low as 16.7% in a prospective study published in 2001,29 a recent prospective study demonstrated that sensitivity of 93% was reached within four passes if the pancreatic tumor was >2 cm.30 In our analysis, the number of passes necessary to reach a plateau of tissue acquisition was four in EUS-FNA and two in EUS-FNB without ROSE. A recent meta-analysis21 also showed that EUS-FNA and EUS-FNB were comparable in the presence of ROSE but that EUS-FNB was associated with a better diagnostic adequacy within fewer passes in pancreatic lesions. Macroscopic on-site quality evaluation31 was reportedly useful in EUS-FNA using a 19-gauge standard needle in the absence of ROSE but conflicting data were reported in EUS-FNA using a 22-gauge needle.32,33 Macroscopic on-site quality evaluation using 22-gauge Franseen needle showed promising results, with >90% sensitivity. Given the relatively large histological core tissue obtained by the Franseen needle, specimen adequacy can be speculated by macroscopic observation.
There are some concerns on adverse events in EUS-FNB using the Franseen needle. While this Franseen needle can procure the tissue in the pancreatic lesion, three cutting edges can potentially injure the vessels in the gastrointestinal wall or the pancreas. In the initial report,15 one arterial bleeding (3%), which required endoscopic hemostasis, was reported. In another study,34 two hematoma (4%) and two post-procedure pain (4%) were reported. In our study, there were no bleeding but two cases of pancreatitis (4%) developed after EUS-FNB. We also noticed that temporary subclinical bleeding along the needle tract was prominent after EUS-FNB compared to EUS-FNA, similar to a previous report.19 These findings did not correlate with significant differences in the rate and severity of adverse events in our study but the adverse event rate appeared relatively high. Finally, it is important to clarify whether this bleeding may lead to the increase in tumor seeding or not in the long-term follow-up of a large study population.
Our study has some limitations. First, this was a single center, retrospective study with a relatively small study population. However, consecutive patients were included in the analysis and the needle selection was solely based on the chronological order, which we believe reduces the risk of selection bias. In addition, the availability of per pass analysis was the strength of our study. Second, our study design of historical cohorts can potentially lead to some bias. The results of EUS-guided tissue acquisition could be affected by the experiences of endoscopists and cytopathologists. In our study, EUS-FNB was more often performed by trainees and there were no significant differences in outcomes between experts and trainees. Therefore, we believe learning curve effects did not exist in our study. In addition, we have performed EUS-guided tissue acquisition for 20 years and the processing of procedure is standardized and it is unlikely that the cohort effect existed in the evaluation by cytopathologists in this study period. Third, the uniform criteria for quality and quantity in the histological specimen obtained by EUS-FNB were lacking. Fourth, we only included pancreatic solid lesions in our study. Although pancreatic solid lesions are the major target of EUS-guided tissue acquisition, the performance of the Franseen needle in evaluating lesions other than pancreatic masses should be further evaluated.
In conclusion, 22-gauge Franseen FNB needle was associated with a higher tissue acquisition rate in the first pass compared with 22-gauge standard FNA needle in EUS-guided tissue acquisition of pancreatic solid lesions.
Footnotes
CONFLICTS OF INTEREST
Y.N. and H.I. received a research grant from Boston Scientific Japan. The other authors declare that they have no conflicting interests.
AUTHOR CONTRIBUTIONS
Study concept and design: K.I., Y.N. Data acquisition: K.I., Y.N., H.O., S.K., T. Suzuki, T.N., T. Sato, R.H., K.S., T. Saito, N.T., T.H., S.M., H.K., M.T., H.I., K.K. Data analysis and interpretation: K.I., Y.N. Drafting of the manuscript; critical revision of the manuscript for important intellectual content: K.I., Y.N., H.I. Statistical analysis: K.I., Y.N. Obtained funding: Y.N., H.I. Administrative, technical, or material support; study supervision: Y.N., H.I.
REFERENCES
- 1.Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–331. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 2.Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas. 2013;42:20–26. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 3.Eltoum IA, Alston EA, Roberson J. Trends in pancreatic pathology practice before and after implementation of endoscopic ultrasound-guided fine-needle aspiration: an example of disruptive innovation effect? Arch Pathol Lab Med. 2012;136:447–453. doi: 10.5858/arpa.2011-0218-OA. [DOI] [PubMed] [Google Scholar]
- 4.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–1196. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 5.Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909–915. doi: 10.1016/j.gie.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Larghi A, Iglesias-Garcia J, Poley JW, et al. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733–3738. doi: 10.1007/s00464-013-2957-9. [DOI] [PubMed] [Google Scholar]
- 7.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–349. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 8.Chong CCN, Teoh AYB, Tang RSY, Chan AWH, Ng EKW, Lai PBS. EUS-FNA using 22G nitinol or ProCore needles without on-site cytopathology. Endosc Ultrasound. 2018;7:56–60. doi: 10.4103/eus.eus_113_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashita T, Nakai Y, Mukai T, et al. A 19-gauge histology needle versus a 19-gauge standard needle in endoscopic ultrasound-guided fine-needle aspiration for solid lesions: a multicenter randomized comparison study (GREATER Study) Dig Dis Sci. 2018;63:1043–1051. doi: 10.1007/s10620-018-4913-y. [DOI] [PubMed] [Google Scholar]
- 10.Fujie S, Ishiwatari H, Sasaki K, et al. Comparison of the diagnostic yield of the standard 22-gauge needle and the new 20-gauge forward-bevel core biopsy needle for endoscopic ultrasound-guided tissue acquisition from pancreatic lesions. Gut Liver. 2019;13:349–355. doi: 10.5009/gnl18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: a case-control study. Gastrointest Endosc. 2016;84:1034–1039. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 12.Jovani M, Abidi WM, Lee LS. Novel fork-tip needles versus standard needles for EUS-guided tissue acquisition from solid masses of the upper GI tract: a matched cohort study. Scand J Gastroenterol. 2017;52:784–787. doi: 10.1080/00365521.2017.1306879. [DOI] [PubMed] [Google Scholar]
- 13.Naveed M, Siddiqui AA, Kowalski TE, et al. A Multicenter comparative trial of a novel EUS-guided core biopsy needle (SharkCore(TM)) with the 22-gauge needle in patients with solid pancreatic mass lesions. Endosc Ultrasound. 2018;7:34–40. doi: 10.4103/eus.eus_27_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attili F, Rimbaş M, Fantin A, et al. Performance of a new histology needle for EUS-guided fine needle biopsy: a retrospective multicenter study. Dig Liver Dis. 2018;50:469–474. doi: 10.1016/j.dld.2018.01.128. [DOI] [PubMed] [Google Scholar]
- 15.Bang JY, Hebert-Magee S, Hasan MK, Navaneethan U, Hawes R, Varadarajulu S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: initial assessment. Dig Endosc. 2017;29:338–346. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 16.Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018;67:2081–2084. doi: 10.1136/gutjnl-2017-315154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitri RD, Rimbaş M, Attili F, et al. Performance of a new needle for endoscopic ultrasound-guided fine-needle biopsy in patients with pancreatic solid lesions: a retrospective multicenter study. Endosc Ultrasound. 2018;7:329–334. doi: 10.4103/eus.eus_33_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432–1438. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Mukai S, Itoi T, Yamaguchi H, et al. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019;8:50–57. doi: 10.4103/eus.eus_11_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa T, Kawashima H, Ohno E, et al. Clinical impact of EUS-guided fine needle biopsy using a novel franseen needle for histological assessment of pancreatic diseases. Can J Gastroenterol Hepatol. 2019;2019:8581743. doi: 10.1155/2019/8581743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–E375. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–1585. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 23.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–1745. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 26.Mukai S, Itoi T, Ashida R, et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos) Gastrointest Endosc. 2016;83:1210–1217. doi: 10.1016/j.gie.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–1439. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 28.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/S0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 29.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/S0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 30.Mohamadnejad M, Mullady D, Early DS, et al. Increasing number of passes beyond 4 does not increase sensitivity of detection of pancreatic malignancy by endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2017;15:1071–1078. doi: 10.1016/j.cgh.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–185. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen YP, Maple JT, Zhang Q, et al. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2009;69:1264–1270. doi: 10.1016/j.gie.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 33.Ishiwatari H, Sato J, Fujie S, et al. Gross visual inspection by endosonographers during endoscopic ultrasound-guided fine needle aspiration. Pancreatology. 2019;19:191–195. doi: 10.1016/j.pan.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 34.El Hajj II, Wu H, Reuss S, et al. Prospective assessment of the performance of a new fine needle biopsy device for EUS-guided sampling of solid lesions. Clin Endosc. 2018;51:576–583. doi: 10.5946/ce.2018.053. [DOI] [PMC free article] [PubMed] [Google Scholar]