Abstract
Background
In light of recent Food and Drug Administration (FDA) approval of immune checkpoint inhibitors for mismatch repair deficient (dMMR) malignancies, identifying patients with dMMR malignancies has become increasingly important. Although screening for dMMR in colorectal cancer (CRC) is recommended, it is less common for extracolonic gastrointestinal (GI) malignancies. At Stanford Comprehensive Cancer Institute (SCCI), all GI malignancies have been screened for dMMR via immunohistochemistry since January 2016.
Methods
In this study, we conducted a retrospective review of all patients with GI malignancies screened for dMMR between January 2016 and December 2017. Tumor sequencing was performed on cases negative for germline pathogenic variants where tumor material was available.
Results
A total of 1425 consecutive GI malignancies were screened for dMMR at SCCI during the study period, and 1374 were included for analysis. dMMR was detected in 7.2% of all GI malignancies. We detected the highest prevalence of dMMR in gastric (15 of 150, 10.0%) followed by colorectal (63 of 694, 9.1%), pancreatic (13 of 244, 5.3%), and gastroesophageal malignancy (6 of 132, 4.5%) patients. Lynch syndrome was the most common etiology for dMMR in colorectal cancer (41.5%), double somatic (confirmed or possible) pathogenic variants the most common etiology in pancreatic cancer (44.4%), and somatic MLH1 hypermethylation the most common etiology in gastric (73.3%) and gastroesophageal cancer (83.3%).
Conclusions
Given the relatively high incidence of dMMR in GI malignancies, we recommend screening all GI malignancies. Our results suggest that although a rare occurrence, double somatic pathogenic variants may be a biologically significant pathway causing dMMR in pancreatic cancer.
Mismatch repair deficiency (dMMR) is caused by the inactivation of mismatch repair (MMR) genes, MLH1, MSH2, MSH6, and PMS2, and results in the accumulation of DNA sequence errors in microsatellite regions resulting in microsatellite instability (MSI). dMMR by immunohistochemistry and MSI are the phenotypic hallmarks of molecularly heterogeneous tumors with MMR pathogenic variants that can arise from a germline inheritance, Lynch syndrome, or a sporadic epigenetic or genetic alteration, including promoter hypermethylation of MLH1 (MLH1-hm) and double somatic pathogenic variants in the MMR genes (1–4).
Variations in clinical features and outcomes have been noted between dMMR and mismatch repair proficient (pMMR) or microsatellite stable (MSS) cancers, as well as between the different dMMR molecular etiologies, ie, Lynch syndrome, MLH1-hm, and double somatic pathogenic variants (5,6). Generally, patients with dMMR colorectal cancer (CRC) have poorly differentiated, proximal or right-sided tumors and an early stage at diagnosis whereas patients with dMMR CRC and MLH1-hm are associated with an older age and female gender (6–8). Additionally, patients with dMMR CRC tend to have an improved prognosis compared with those with pMMR CRC, particularly in early stage disease, whereas no difference in prognosis has been found between Lynch syndrome CRC and MLH1-hm CRC (9–12). More recently, patients with dMMR cancers have had a favorable response to PD-1 and PD-L1 immune checkpoint inhibitor therapies in the metastatic setting (13).
The prevalence of dMMR in gastrointestinal (GI) cancers is the highest in CRC, accounting for 10%-15% of CRC cases (1). Two to 3% of all CRC patients have Lynch syndrome (1). The prevalence and clinical features of dMMR is well described in CRC whereas dMMR in non-CRC gastrointestinal (GI) cancers has not been well studied. Gastric cancer (GC) is largely considered the second most common GI cancer with dMMR after CRC. According to large molecular landscape studies including The Cancer Genome Atlas, the prevalence of dMMR in GC cases is reported to be between 17% and 22% (14). Meanwhile, a systematic review of MSI GC studies reports the prevalence of MSI in GC to be in the range of 8.2% to 37% with a favorable prognosis associated with MSI GC (15). The prevalence of dMMR in pancreatic cancer (PC) ranges from 0.3% to 1.6% and is a low 1.6% in gastroesophageal cancer (GEJ) (16–19). To facilitate the ability to detect Lynch syndrome, universal screening for dMMR in CRC patients was recommended by the Evaluation of Genomic Applications in Practice and Prevention workgroup of the Centers for Disease Control and Prevention in 2009, by the National Comprehensive Cancer Network in March 2014, and by the US Multi-Society Task Force in August 2014 (20–22).
Universal screening for dMMR in colorectal as well as endometrial cancer has been performed routinely at many large institutions since 2009. However, universal screening for dMMR across all GI cancers is both uncommon and not yet well studied. At Stanford Comprehensive Cancer Institute (SCCI), all CRC since 2009 and all non-CRC GI cancers since 2016 have been universally screened for dMMR via immunohistochemistry (IHC) testing. We conducted a retrospective review of patients with unselected universally screened GI cancers with dMMR at SCCI from 2016 to 2017. We describe the prevalence of dMMR across all GI cancers and have outlined the molecular etiology and clinical characteristics of patients with dMMR GI cancers.
Methods
Patient Selection and Data Collection
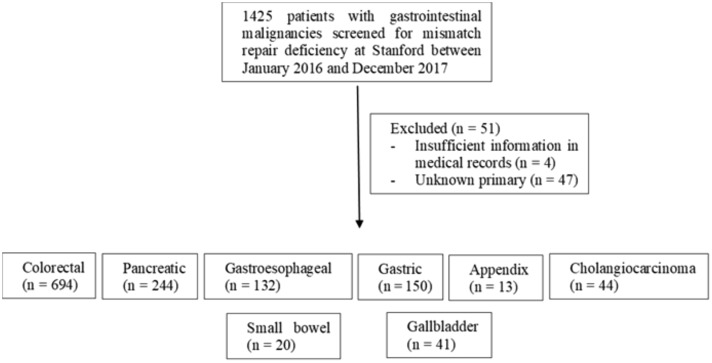
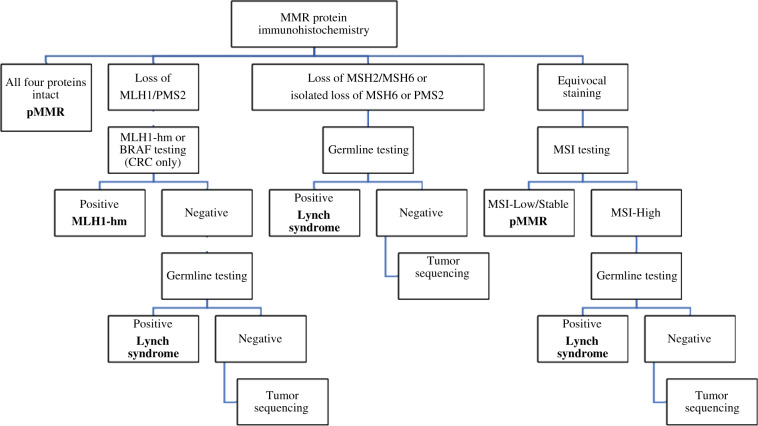
A retrospective review of all patients with GI cancers screened for dMMR from January 2016 to December 2017 at SCCI was conducted. Stanford began universal screening for dMMR on all GI malignancies (of non-squamous etiology, excluding hepatocellular carcinoma and neuroendocrine tumors) either diagnosed or seen for consultation (all pathology was reviewed and confirmed at Stanford) in January 2016. This is automatically performed through pathology on every GI cancer case reviewed at SCCI. MMR status was determined by immunohistochemical staining of the biopsied or resected specimen for MLH1, MSH2, MSH6, and PMS2. A total of 1425 GI malignancies were screened at SCCI for dMMR using IHC from 2016 to 2017 (see Figure 1). Cases with insufficient medical information or unknown primary location were excluded from further analyses. A total of 1374 patients were included. Patients were referred for germline testing for Lynch syndrome if their tumors were deficient for MSH2/MSH6, MSH6 alone or PMS2 alone or if they met the following criteria: MLH1/PMS2 deficient, BRAF mutation negative, and/or MLH1-hm negative. If Lynch syndrome testing was negative, the tumor sample (if available) was sent for somatic tumor sequencing to look for double somatic pathogenic MMR variants. If tumor sequencing was not performed in the clinical setting, tumor DNA was isolated and sent to University of Washington for ColoSeq as part of a research protocol (Figure 2) (14).
Figure 1.
Breakdown of samples sizes during screening process and after excluding cases with insufficient medical records or unknown primary.
Figure 2.
Flowchart of testing procedures to determine dMMR and Lynch syndrome status. CRC = colorectal cancer; MLH1-hm = MLH1 hypermethylation; MSI = microsatellite instability; pMMR = proficient mismatch repair protein.
Histologic and clinical diagnoses were obtained from submitted pathology reports. Ampullary carcinomas were classified with PC for the purposes of this analysis. All mismatch repair stains performed outside of Stanford were confirmed by pathologists at Stanford. Data on patient and tumor characteristics were collected from medical charts. The institutional review board at Stanford University approved this study.
Germline Analysis
Germline analysis and variant classification was performed by one of several commercial clinical laboratories determined by the ordering clinician. Testing utilized massively parallel sequencing technology with germline pathogenic or likely pathogenic variants confirmed with an orthogonal technology, such as Sanger sequencing or array comparative genomic hybridization. All testing included at least sequencing and duplication/deletion analysis for MLH1 NM_000249.3, MSH2 NM_000251.2, MSH6 NM_000179.2, PMS2 NM_000535.5, and duplication/deletion analysis for EPCAM NM_002354.2.
Immunohistochemistry, MLH1 Hypermethylation Testing and Tumor Sequencing
Immunohistochemistry for the four mismatch repair proteins was performed on paraffin-embedded tumor tissue using antibodies for MLH1 (BD Biosciences, San Jose, CA), MSH2 (Calbiochem, San Diego, CA), MSH6 (Cell Marque, Rocklin, CA), and PMS2 (Cell Marque, Rocklin, CA). MLH1-hm testing was done assessing the promoter region using sodium bisulfite modified DNA and sequencing was performed by GoPath Laboratories (Buffalo Grove, IL). Three micrograms of tumor DNA was isolated at University of Washington and analyzed with ColoSeq, a clinical diagnostic assay that uses paired-end sequencing on an Illumina HiSeq 2500 instrument to sequence all exons, introns, and flanking sequences at greater than 300× average coverage as previously described (23).
Statistical Analysis
Descriptive statistics (median with quartiles for age and frequency for categorical variables) were used to describe the patient population. Molecular etiology of dMMR status was stratified by type of cancer and type of pathogenic variant. Prevalence of dMMR, MLH1-hm, double somatic pathogenic variants, and Lynch syndrome across CRC, GC, GEJ cancer, and PC was analyzed. Age at diagnosis was defined as the age when cancer diagnosis was confirmed by pathology. SPSS (IBM Corp. Released 2017. IBM SPSS Statistics, Version 25.0. Armonk, NY: IBM Corp) was utilized for analysis.
Results
dMMR Frequency Across All GI Malignancies
Colorectal cancer (n = 694), PC (n = 244), GC (n = 150), and GEJ (n = 132) were amongst the most frequently screened tumors. dMMR was detected in 7.2% (n = 99) of all GI malignancies. The highest prevalence of dMMR was in GC (15 of 150, 10.0%), followed by CRC (63 of 694, 9.1%), PC (13 of 244, 5.3%—3 of these 13 cases were ampullary carcinomas), and GEJ (6 of 132, 4.5%). A single case of dMMR gallbladder cancer (1 of 41, 2.5%) and a single case of dMMR small bowel cancer (1 of 20, 5.0%) were identified. Of note, 3 of 14 (21.4%) of ampullary carcinomas were dMMR with 2 MLH1-hm cases and 1 Lynch syndrome case. No definitive cases of dMMR were identified in patients with cholangiocarcinoma (n = 44) and cancer of the appendix (n = 13).
Molecular Etiology of dMMR Across All GI Malignancies
We evaluated the underlying molecular etiology of dMMR GI malignancies by reviewing subsequent germline and somatic pathogenic variant testing that was performed as part of standard clinical practice (see Table 1) or as part of a research protocol when tumor testing was not performed in the clinical setting. A total of 41.5% (27 of 65) of patients with dMMR CRC and 16.7% (3 of 18) of patients with dMMR PC had confirmed Lynch syndrome with germline pathogenic or likely pathogenic variants. Hypermethylation of MLH1 was detected in 28.8% (19 of 66) of dMMR CRC, 16.7% (6 of 18) of dMMR PC, 73.3% (11 of 15) of dMMR GC, and 83.3% (5 of 6) of dMMR GEJ. We were unable to identify the molecular etiology of 9 of 65 (13.8%) dMMR CRC, 2 of 18 (11.1%) dMMR PC, 1 of 15 (6.7%) dMMR GC patients, and 1 of 6 (16.7%) dMMR GEJ because of absence of germline testing for Lynch syndrome. Two dMMR CRC patients underwent tumor sequencing but did not have an explained dMMR etiology: the first patient had loss of PMS2 on IHC with no detectable germline pathogenic variant and tumor was found to have only 1 somatic MLH1 (p.K751Sfs*2) pathogenic variant with no loss of heterozygosity detected on ColoSeq testing. The second patient had an equivocal staining of MSH6 with lack of internal control with no germline pathogenic variant and was found to have a somatic MLH1 (p.Gly422Glu) variant of uncertain significance on STAMP (a Stanford next-generation sequencing (NGS) tumor sequencing panel that includes MLH1 and MSH2, but not MSH6 and PMS2). Tumor ColoSeq was performed on 7 additional tumor samples, and 3 of 7 patients were found to have double somatic pathogenic variants. Notably, we found 2 CRC and 5 PC patients with a false positive IHC result (dMMR on IHC not supported on re-review or molecular testing), which were confirmed on re-review of pathology (1 case after no MMR pathogenic variants were detected following tumor sequencing test). We have outlined the dMMR molecular etiology with respect to the IHC stain result for the dMMR CRC (Table 2), PC (Table 3), and GC (Table 4) cases.
Table 4.
Molecular etiology by mismatch repair deficiency in gastric cancer patients
| Mutation type | Lynch syndrome (n = 0) | MLH1-hm (n = 11) | Double somatic (n = 1) | Possible double somatica (n = 2) | Unknownb (n = 1) |
|---|---|---|---|---|---|
| MLH1/PMS2 | – | 11 | 1 | 2 | – |
| Equivocalc | – | – | – | 1 |
Germline testing negative, no tumor testing performed due to lack of tumor DNA for testing.
No germline or tumor sequencing performed.
All 4 mismatch repair stains were equivocal.
Table 5.
Patient and tumor characteristics in mismatch repair deficient gastrointestinal malignancies
| Variable | Cancer |
|||
|---|---|---|---|---|
| Colorectal (n = 63) | Pancreatic (n = 13) | Gastric (n = 15) | Gastroesophageal (n = 6) | |
| Age (median [Q1, Q3]) | 60 (45, 75) | 66 (57, 72) | 77 (71, 86) | 72 (57, 82) |
| Sex, No. (%) | ||||
| Male | 29 (46.0) | 6 (46.2) | 10 (66.7) | 4 (66.7) |
| Female | 34 (54.0) | 7 (53.8) | 5 (33.3) | 2 (33.3) |
| Clinical stage, No. (%) | ||||
| I | 14 (22.2) | 2 (15.4) | 4 (26.7) | 1 (16.7) |
| II | 20 (31.7) | 2 (15.4) | 4 (26.7) | 4 (66.7) |
| III | 20 (31.7) | 3 (23.1) | 3 (20.0) | 1 (16.7) |
| IV | 7 (11.1) | 6 (46.2) | 3 (20.0) | – |
| Missing | 2 (3.2) | – | 1 (6.7) | – |
| T stage, No. (%) | ||||
| T1 | 7 (11.1) | 2 (15.4) | 1 (6.7) | – |
| T2 | 9 (14.3) | – | 5 (33.3) | 1 (16.7) |
| T3 | 32 (50) | 3 (23.1) | 1 (6.7) | 5 (83.3) |
| T4 | 9 (14.3) | 3 (23.1) | 4 (26.7) | – |
| Tx | 1 (1.6) | 5 (38.5) | 3 (20) | – |
| Missing | 5 (7.9) | – | – | – |
| N stage, No. (%) | ||||
| N0 | 33 (52.4) | 5 (38.5) | 5 (33.3) | 2 (33.3) |
| N1 | 12 (20.3) | 2 (15.4) | 4 (26.7) | 2 (33.3) |
| N2 | 11 (19.0) | – | 3 (20.0) | – |
| N3 | – | – | 1 (6.7) | 1 (16.7) |
| Nx | 2 (3.2) | 6 (46.2) | – | 1 (16.7) |
| Missing | 5 (7.9) | – | 2 (13.3) | – |
| Tumor grade, No. (%) | ||||
| G1 | 12 (19.0) | 1 (7.7) | 1 (6.7) | 1 (16.7) |
| G2 | 31 (49.2) | 2 (15.4) | 9 (60.0) | 1 (16.7) |
| G3 | 13 (20.6) | 1 (7.7) | 3 (20.0) | 3 (50.0) |
| Missing | 7 (11.1) | 9 (69.2) | 2 (13.3) | 2 (33.3) |
| Tumor histology, No. (%) | ||||
| Adenocarcinoma | 61a (96.8) | 12 (92.3) | 15 (100.0) | 6 (100.0) |
| Mucinous | 2 (3.2) | – | – | – |
| Medullary | – | 1 (7.7) | – | – |
1 case with squamous features.
Table 1.
Molecular etiology of mismatch repair deficiency by type of gastrointestinal malignancy
| Cancer | Lynch syndrome (n = 30) | MLH1-hm (n = 38) | Double somatic (n = 7) | Possibly double somatica (n = 9) | Unknownb (n = 13) | False positive (n = 7) |
|---|---|---|---|---|---|---|
| Colorectal, No. (%) | 27 (41.5) | 19 (29.2) | 4 (6.2) | 4 (6.2) | 9c (3.1) | 2 (3.0) |
| Pancreatic, No. (%) | 3 (16.7) | 3 (16.7) | 2 (11.1) | 3 (16.7) | 2 (11.1) | 5 (27.8) |
| Gastric, No. (%) | – | 11 (73.3) | 1 (6.7) | 2 (13.3) | 1 (6.7) | – |
| Gastroesophageal, No. (%) | – | 5 (83.3) | – | – | 1 | – |
Germline testing negative, no tumor testing performed due to lack of tumor DNA for testing.
No germline or tumor sequencing performed.
Two patients had all testing done and no etiology was found for dMMR.
Table 2.
Molecular etiology by mismatch repair deficiency in colorectal cancer patients
| Mutation type | Lynch syndrome (n = 27) | MLH1-hm (n = 19) | Double somatic (n = 4) | Possible double somatica(n = 4) | Unknownb(n = 7) | False positive(n = 2) | Mismatch repair deficient, unexplained(n = 2) | |
|---|---|---|---|---|---|---|---|---|
| MLH1/PMS2 | 10 | 19 | 2 | 4 | – | |||
| MSH2/MSH6 | 13 | – | 4 | 1 | 3 | – | ||
| MSH6 | – | – | 1 | – | – | |||
| PMS2 | 2 | – | – | 1 | 1d | |||
| Equivocalc | 2 | – | - | – | 1 | 1e | ||
Germline testing negative, no tumor testing performed due to lack of tumor DNA for testing.
No germline testing performed.
Equivocal staining patterns include: PMS2 equivocal (n = 1), MSH6 equivocal (n = 2), MSH2/MSH6 equivocal (n = 1).
No germline pathogenic variants (somatic MLH1 and loss of heterozygosity not detected).
No germline pathogenic variant, somatic MLH1 variant of uncertain significance on tumor testing (panel does not include MSH6 and PMS2 sequencing).
Patient and Tumor Characteristics
We have outlined the clinical and pathological characteristics of dMMR GI cancer patients (see Table 5—false positive IHC cases are not included in this analysis). The median age at diagnosis was 60 years: (Q1 = 45, Q3 = 75) for CRC, 66 years (Q1 = 57, Q3 = 72) for PC, 72 years (Q1 = 57, Q3 = 82) for GEJ, and 77 years (Q1 = 71, Q3 = 86) for GC. Sex was evenly distributed in dMMR CRC (46% male vs 54% female) and PC (46% male vs 54% female) patients, although there were more males with dMMR GC (67% male vs 33% female) and GEJ (67% male vs 33% female). Seven (11.1%) out of 63 CRC, 6 (46.2%) out of 13 PC, and 3 (20%) out of 15 GC patients with dMMR were diagnosed with metastatic disease. High-grade tumors (Grade 3) were identified in 13 (20.6%) out of 63 CRC, 1 (7.7%) out of 13 PC, 3 (20%) out of 15 GC, and 3 (50%) out of 6 GEJ patients with dMMR although more than half of dMMR PC were missing tumor grade information. Two cases of mucinous carcinoma and 1 case of medullary carcinoma were identified in dMMR CRC and dMMR PC patients, respectively.
Table 3.
Molecular etiology by mismatch repair deficiency in pancreatic cancer patients
| Mutation type | Lynch syndrome (n = 3) | MLH1-hm (n = 3) | Double somatic(n = 2) | Possible double somatica (n = 3) | Unknownb (n = 2) | False positive (n = 5) |
|---|---|---|---|---|---|---|
| MLH1/PMS2 | – | 3 | – | – | – | |
| MSH2/MSH6 | – | – | 1 | – | 1 | 2 |
| MSH6 | 2 | – | 1 | – | – | 1 |
| Equivocalc | 1 | – | 3 | 1 | 2 |
Germline testing negative, no tumor testing performed due to lack of tumor DNA for testing.
No germline testing performed.
Equivocal staining patterns include: PMS2 equivocal (n = 1), MSH6 equivocal (n = 2), MSH2/MSH6 equivocal (n = 3), MLH1/PMS2/MSH2 equivocal (n = 1).
Discussion
Universal screening for dMMR across all GI malignancies is a novel approach instituted by Stanford in 2016. To our knowledge no studies have yet analyzed dMMR prevalence and molecular etiology across an unselected pool of pan-GI malignancies. In this single-institution universal screening study of mismatch repair deficiency we found a 7.2% incidence of dMMR across all stages of GI cancers with dMMR etiology established in most cases. While Lynch syndrome was the most common etiology in CRC, sporadic MLH1-hm and double somatic variants were more frequent etiologies in GC and PC. Identification of the etiology of dMMR in cancer patients has clinical significance as patients with a sporadic etiology would not require intensive surveillance strategies and family members would not require Lynch syndrome testing. Currently, IHC-based and PCR-based approaches are the most common approach to universal screening for dMMR and they have high sensitivity (>90%) for Lynch syndrome detection (24, 25). These approaches, although highly sensitive for dMMR, are unable to diagnostically separate Lynch syndrome patients from those with double somatic pathogenic variants. Next-generation sequencing, in this regard, has been shown to have slightly better sensitivity as compared to IHC + BRAF testing or MSI PCR-based + BRAF testing to detect Lynch syndrome (26). When NGS also includes MSI testing, it may perform as well as multiple sequential tests to diagnose Lynch syndrome and may replace current testing protocols in the future.
The clinicopathologic characteristics of Lynch syndrome cancer patients, particularly in CRC, has been described compared to patients with double somatic MMR pathogenic variants. Pearlman et al. reported that isolated loss of MSH6 and PMS2 is more predictive of Lynch syndrome than double somatic dMMR as double somatic hits to MSH6 or PMS2 are rarely seen (27). In our study, 6 of 7 confirmed double somatic cases had either MLH1 or MSH2 mutations, suggesting that double somatic pathogenic variants affecting MSH6 or PMS2 are more rare. Studies have found double somatic events in more than 50% of cases without germline pathogenic variants and MLH1-hm (34). We found double somatic MMR pathogenic variants in approximately 42% of CRC cases without germline mutations or MLH1-hm but 7 cases had no germline analysis available. Of our sample of 694 patients with CRC, 9.1% or 63 patients showed evidence of dMMR, less than the reported 15% prevalence of MSI in CRC (1, 10). Of note, in population-based studies, the majority (70%-80%) of dMMR or MSI-high CRC is driven by MLH1-hm, which is more common in older patients, but in our cohort, hypermethylation explained only 29.2% of dMMR within our sample of CRC patients (1, 6, 8). This discrepancy is likely because of tertiary referral bias with higher rates of referrals of younger patients and cases with suspected Lynch syndrome and is not generalizable to the national population of CRC patients.
With respect to GC, a high degree of concordance was noted between our results and previously published studies. Of the 150 tumors screened at Stanford, we found a 10% prevalence of dMMR, similar to previously reported estimates of dMMR in 10%-20% of all GC (14). Eleven (73.3%) of 15 GC patients in this study had confirmed MLH1-hm whereas 1 case was explained by double somatic pathogenic variants to MLH1. Although there are a few molecular pathways that can lead to MLH1/PMS2 loss, MLH1-hm is thought to be the most common driver of dMMR in GC with previous studies reporting rates of MLH1-hm of up to 77.8% in dMMR GCs (28). Germline or double somatic pathogenic MMR variants are considered to be less prevalent. Interestingly, we found 6 GEJ adenocarcinoma cases that were dMMR and all but one were caused by somatic MLH1-hm. This tumor location should therefore prompt screening for dMMR, particularly as it could affect treatment choice in the metastatic setting.
In contrast to gastric and colorectal carcinoma, the frequency of dMMR in an unselected cohort of universally screened PC patients has not been previously described. In recent clinic-based studies aimed to evaluate the prevalence of dMMR in PC patients, authors have described dMMR incidence from 0.3% to 1% (18, 19, 29). One study found all 7 dMMR PC cases to have Lynch syndrome (18). In another study with 4 dMMR cases, 3 had double somatic pathogenic variants in MSH2 and 1 had MLH1-hm (30). On the contrary, we report a higher frequency of dMMR in PC (5.3%) arising from somatic and germline etiologies compared with previously reported clinic-based studies. Out of the 16 potential dMMR PC patients who received germline testing, only 3 patients were found to have Lynch syndrome, while 5 patients had a sporadic or likely sporadic dMMR etiology and 5 were excluded as having a false positive IHC result. In 2 dMMR cases we were unable to perform germline or tumor sequencing and can therefore not exclude the possibility of false positive IHC. In the fully tested PC patients with sporadic dMMR, we identified 3 patients with MLH1-hm and 2 patients with a confirmed double somatic pathogenic variant in MSH2 and MSH6, respectively. Notably, 7 out of the 18 dMMR PC patients had equivocal IHC staining due to technical limitations such as a lack of internal positive control, poor nuclear staining, high background stain, or insufficient tissue. High IHC concordance between preoperative biopsies and surgically resected specimens has been described in CRC, but IHC testing may not be as reliable in small biopsy specimens because of technical limitations (29, 31). Moreover, IHC testing may be difficult to assess in extracolonic specimens that are less proliferative and often lack internal control compared with CRC specimens (29). A combination of MSI and NGS testing would be indicated to clarify equivocal IHC findings, particularly for PC patients with a biopsy specimen.
Universal screening in CRC was recommended to increase detection of Lynch syndrome in the patient and cascade testing for their relatives, allowing initiation of intensive cancer screening to reduce cancer morbidity and mortality. While the prevalence of Lynch syndrome is lower in non-CRC GI cancers, identifying dMMR in the initial work-up of patients presenting with a GI cancer can help clinicians gauge prognosis and better select cancer therapies. Immune checkpoint inhibitor therapy is FDA approved for dMMR/MSI metastatic cancers irrespective of tissue origin based on several studies showing clinically significant efficacy after progression on initial systemic therapy (32–35). In CRC, single agent 5-FU is not recommended in the adjuvant setting based on data showing a possible detrimental effect on disease-free survival (10). In gastric and GEJ cancers, 2 retrospective reviews of perioperative and adjuvant trials have revealed concern regarding a potential detriment of chemotherapy in the dMMR/MSI subtype but has not yet influenced practice guidelines (36, 37). Currently, the National Comprehensive Cancer Network (NCCN) guidelines recommend that dMMR/MSI status be assessed in all gastric, esophageal andGEJ adenocarcinoma patients if metastatic disease is documented or suspected as they may be candidates for immune checkpoint inhibitor therapy (38). In the NCCN pancreatic cancer guidelines it is a category 2B recommendation to screen locally advanced adenocarcinomas with MMR by IHC or MSI by polymerase chain reaction (PCR) testing (39). Based on our data showing that 7.5% of GI malignancies harbor dMMR, we recommend considering universal screening for dMMR across all GI malignancies. Further cost-effectiveness analysis would be useful to support this recommendation and understand the path toward implementation in various practice settings.
We recognize that our study has several limitations. As a single-center retrospective review, the study is limited in its generalizability. With few double somatic cases we were not able to meaningfully determine whether any specific patient characteristics dominated in this group. We were also unable to establish the etiology for dMMR in all cases as some patients did not have germline testing or tumor material was not available to perform tumor sequencing, which further limited our analysis.
Our results suggest that there may be clinically significant value to universal screening for mismatch repair deficiency in all patients presenting with a GI malignancy and suggest that rates of dMMR within non-CRC GI cancer may be higher than previously known. Furthermore, our results suggest that whereas it is an uncommon occurrence, double somatic pathogenic variants can be a biologically significant pathway toward clinical dMMR particularly within pancreatic cancer. Further multicenter analyses characterizing dMMR in GI cancers as well as cost-effectiveness analyses would be recommended to gain more insight into dMMR GI malignancies.
Funding
This study was funded by Stanford University.
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to disclose.
Author contributions: All authors contributed to the study design, data collection, data analysis, and manuscript preparation process.
Data availability
Data is available upon request.
References
- 1. Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352(18):1851–1860. [DOI] [PubMed] [Google Scholar]
- 2. Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57(5):808–811. [PubMed] [Google Scholar]
- 3. Mensenkamp AR, Vogelaar IP, van Zelst-Stams WAG, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146(3):643–646.e8. [DOI] [PubMed] [Google Scholar]
- 4. Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147(6):1308–1316.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bae JM, Kim JH, Kwak Y, et al. Distinct clinical outcomes of two CIMP-positive colorectal cancer subtypes based on a revised CIMP classification system. Br J Cancer. 2017;116(8):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinicrope FA, Rego RL, Foster N, et al. Microsatellite instability accounts for tumor site-related differences in clinicopathologic variables and prognosis in human colon cancers. Am J Gastroenterology. 2006;101(12):2818–2825. [DOI] [PubMed] [Google Scholar]
- 8. Kakar S, Burgart LJ, Thibodeau SN, et al. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer. 2003;97(6):1421–1427. [DOI] [PubMed] [Google Scholar]
- 9. Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- 10. Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103(11):863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176–4187. [DOI] [PubMed] [Google Scholar]
- 12. Haraldsdottir S, Hampel H, Wu C, et al. Patients with colorectal cancer associated with Lynch syndrome and MLH1 promoter hypermethylation have similar prognoses. Genet Med. 2016;18(9):863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dudley JC, Lin M-T, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813–820. [DOI] [PubMed] [Google Scholar]
- 14. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu L, Li Z, Wang Y, Zhang C, Liu Y, Qu X. Microsatellite instability and survival in gastric cancer: a systematic review and meta-analysis. Mol Clin Oncol. 2015;3(3):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cortes-Ciriano I, Lee S, Park W-Y, Kim T-M, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8(1):15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lupinacci RM, Goloudina A, Buhard O, et al. Prevalence of microsatellite instability in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2018;154(4):1061–1065. [DOI] [PubMed] [Google Scholar]
- 18. Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24(6):1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laghi L, Beghelli S, Spinelli A, et al. Irrelevance of microsatellite instability in the epidemiology of sporadic pancreatic ductal adenocarcinoma. PLoS ONE. 2012;7(9):e46002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Colorectal (Version 1); 2014. [Google Scholar]
- 22. Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014;147(2):502–526. [DOI] [PubMed] [Google Scholar]
- 23. Pritchard CC, Smith C, Salipante SJ, et al. ColoSeq provides comprehensive Lynch and polyposis syndrome mutational analysis using massively parallel sequencing. J Mol Diagn. 2012;14(4):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pai RK, Pai RK. A practical approach to the evaluation of gastrointestinal tract carcinomas for Lynch syndrome. Am J Surg Pathol. 2016;40(4):e17–34. [DOI] [PubMed] [Google Scholar]
- 25. Shia J. Evolving approach and clinical significance of detecting DNA mismatch repair deficiency in colorectal carcinoma. Semin Diagn Pathol. 2015;32(5):352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hampel H, Pearlman R, Beightol M, et al. Assessment of tumor sequencing as a replacement for Lynch syndrome screening and current molecular tests for patients with colorectal cancer. JAMA Oncol. 2018;4(6):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pearlman R, Haraldsdottir S, de la Chapelle A, et al. Clinical characteristics of patients with colorectal cancer with double somatic mismatch repair mutations compared with Lynch syndrome. J Med Genet. 2019;56(7):462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fleisher AS, Esteller M, Wang S, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999;59(5):1090–1095. [PubMed] [Google Scholar]
- 29. O’Brien O, Ryan É, Creavin B, et al. Correlation of immunohistochemical mismatch repair protein status between colorectal carcinoma endoscopic biopsy and resection specimens. J Clin Pathol. 2018;71(7):631–636. [DOI] [PubMed] [Google Scholar]
- 30. Humphris JL, Patch A-M, Nones K, et al. Hypermutation in pancreatic cancer. Gastroenterology. 2017;152(1):68–74.e2. [DOI] [PubMed] [Google Scholar]
- 31. Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10(4):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2019;38(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marabelle A, Le DT, Ascierto PA. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3(9):1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi YY, Kim H, Shin S-J, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the classic randomized controlled study. Ann Surg. 2019;270(2):309–316. [DOI] [PubMed] [Google Scholar]
- 38.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Gastric Cancer (Version 2); 2019.
- 39.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Pancreatic Adenocarcinoma (Version 3); 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.