Significance
What are the principles of brain organization? In the motor domain, separate pathways were found for reaching and grasping actions performed by the hand. To what extent is this organization specific to the hand or based on abstract action types, regardless of which body part performs them? We tested people born without hands who perform actions with their feet. Activity in frontoparietal association motor areas showed preference for an action type (reaching or grasping), regardless of whether it was performed by the foot in people born without hands or by the hand in typically-developed controls. These findings provide evidence that some association areas are organized based on abstract functions of action types, independent of specific sensorimotor experience and parameters of specific body parts.
Keywords: brain development, hands, motor cortex, plasticity, actions
Abstract
Many parts of the visuomotor system guide daily hand actions, like reaching for and grasping objects. Do these regions depend exclusively on the hand as a specific body part whose movement they guide, or are they organized for the reaching task per se, for any body part used as an effector? To address this question, we conducted a neuroimaging study with people born without upper limbs—individuals with dysplasia—who use the feet to act, as they and typically developed controls performed reaching and grasping actions with their dominant effector. Individuals with dysplasia have no prior experience acting with hands, allowing us to control for hand motor imagery when acting with another effector (i.e., foot). Primary sensorimotor cortices showed selectivity for the hand in controls and foot in individuals with dysplasia. Importantly, we found a preference based on action type (reaching/grasping) regardless of the effector used in the association sensorimotor cortex, in the left intraparietal sulcus and dorsal premotor cortex, as well as in the basal ganglia and anterior cerebellum. These areas also showed differential response patterns between action types for both groups. Intermediate areas along a posterior–anterior gradient in the left dorsal premotor cortex gradually transitioned from selectivity based on the body part to selectivity based on the action type. These findings indicate that some visuomotor association areas are organized based on abstract action functions independent of specific sensorimotor parameters, paralleling sensory feature-independence in visual and auditory cortices in people born blind and deaf. Together, they suggest association cortices across action and perception may support specific computations, abstracted from low-level sensorimotor elements.
Performing an action such as reaching for a ball involves planning the goal and executing it with specific body parts. This rich movement information is encoded by a wide range of motor areas. Hand kinematic and muscle synergies during reaching and grasping actions are encoded by population neural responses in the primary motor cortex (1–4). These are largely organized into a large-scale somatotopic organization in the primary motor cortex, albeit lacking clear boundaries, with each brain area selectively representing a body part (5–10). Beyond the primary motor cortex, brain areas are sensitive to specific action types for a given body part. For example, a dorsomedial frontoparietal network is devoted to the act of reaching toward an object with one’s hand, as found in both humans and nonhuman primates (11–18). However, many common action goals can be achieved with different body parts or effectors. For example, one can reach for a ball with the hand or with the foot. Are there neural mechanisms that control each action type, independently of the effector ultimately used to perform the action?
Recent studies provide evidence that despite body-part preference, some association cortex regions can support action representations extending across body parts. During motor execution, activation patterns in the premotor cortex and parietal lobe represent more-abstract information, such as the target location and movement direction, regardless of which hand performed the action (19, 20). During motor planning, common brain areas, including the dorsal premotor cortex (PMd) and intraparietal sulcus (IPS), were involved when planning to direct the hands or the eyes toward an instructed direction (5, 21–24). Moreover, a few studies reported similarity in activation during planning of pointing movements across the hand and foot (5, 22, 23). These findings suggest a more abstract organization of the motor system based on action types or goals (25–27), which may be less dependent on the effector.
Despite evidence from past literature, the level of representation of action encoding and its transfer across body parts is not entirely clear. Although studies found representation of action type (reaching, grasping) shared by the two hands, it is unknown whether the effector-independence is limited to the hands or generalizable to other effector types (e.g., the foot). For the few studies that showed common activation for hand- and foot-pointing (5, 22, 23), pointing was not compared to other actions, leaving unknown what information the common activation represents. Finally, another potential confound in past findings is motor imagery. When typically developed individuals are asked to perform actions with the foot, because they are less experienced in acting with this limb, it is possible that they perform the action by engaging motor imagery of how they typically perform it with their hand. Motor imagery engages the same sensorimotor systems as actual motor execution (29–34). As a result, common activation found across effectors in prior work might partially stem from shared activation of motor execution and motor imagery of the same dominant hand. Together, these reservations raise the question of whether the common brain activation pattern across effectors truly reflects effector-independent motor representation, abstracted beyond the body part.
Here we address the question of whether the sensorimotor system is organized by effector-independent action-type representations, overcoming these confounds, by examining a unique population: People born without hands who use the feet as their primary effector (individuals with dysplasia, IDs). We scanned IDs while they performed reaching and grasping actions, actions that engage at least partially-separable neural networks for hand movements (35–38). If an area is selective for an action type both when IDs perform with their foot and controls with their hand, it would indicate that this area is involved in processing that action type independently of effector. Areas showing selectivity for foot actions in IDs as compared to hand actions in controls, or vice versa, would be characterized as effector-dependent. This paradigm thus allows us to directly test which components of the reaching and grasping networks are effector-dependent and specific to the hand and foot and which, if any, components are based on more abstract action-type processing, independent of the effector.
Results
We scanned four IDs, born without hands and arms, and a group of typically-developed control participants while they performed reaching (reach-to-touch) and grasping (reach-to-grasp) actions toward a centrally located object (see Fig. 1A and schematic in SI Appendix, Fig. S1). The IDs performed the actions with their feet, the body part that they use extensively and dexterously for daily actions (39–43). The controls performed the actions with their hands. Here we focus on actions of the dominant effector (right hand in controls and right foot in IDs) because it is most dexterous in reaching and grasping tasks, and reaching and grasping networks are most frequently reported for the dominant (right) hand (36, 37, 44–46).
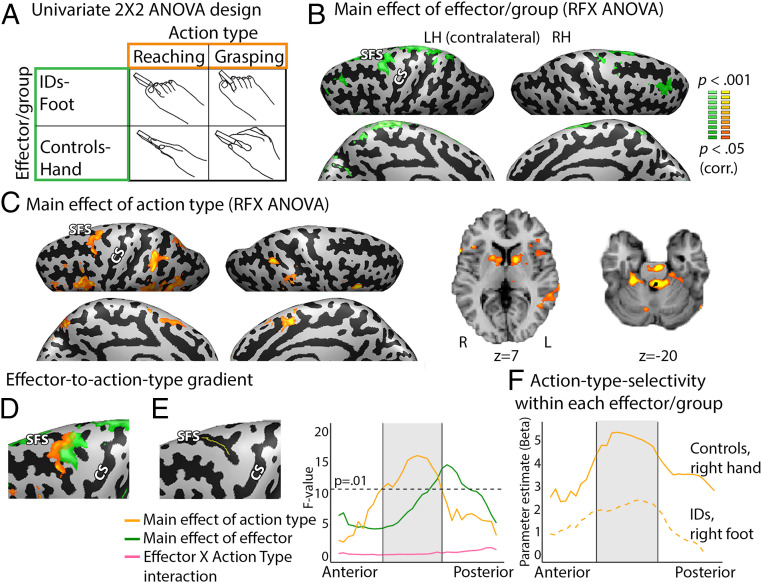
Fig. 1.
Effector-dependent and effector-independent action networks. Results from the RFX ANOVA analysis for the right hand in the controls and right foot in the IDs. (A) An RFX ANOVA was performed with action type as a within-subjects factor and effector/group as a between-subjects factor. (B) Main effect of effector/group. Difference between hand actions in controls and foot actions in IDs was found in the contralateral (Left) primary sensorimotor cortex foot area and PMd. An ROI analysis also revealed a difference between effectors in the primary sensorimotor hand area (SI Appendix, Fig. S2A). SFS, superior frontal sulcus; CS, central sulcus; LH, left hemisphere; RH, right hemisphere. (C) A main effect of action type (i.e., a consistent difference between reaching and grasping) across effectors/groups was found in contralateral (Left) PMd and PMv, SPOC, medial frontal gyrus (SMA and preSMA), and bilateral IPS. Subcortical activation was found in the bilateral caudate and cerebellum (lobules I–IV and V), displayed on axial slices. (D) Overlaying statistical maps of both effector- and action-type main effects together revealed a posterior–anterior shift from effector- to action-type–dependent activation along the PMd. (E) A line ROI along the SFS was created, along which the F-values of the main effect of action type and effector/group were plotted to demonstrate the selectivity gradient from effector- (green) to action-type–dependent (yellow) activation along the PMd. The gray window denotes spatial range showing a significant (P < 0.01) main effect of action type. (F) Parameter estimate (β-weights) of the GLM contrast between reaching and grasping was plotted for each group along the line ROI in the SFS, from anterior to posterior voxels, showing an increase in action-type preference in both groups along the SFS. The gray window denotes spatial range showing a significant (P < 0.01) main effect of action type in the ANOVA.
To examine the effect of action type and effector on brain activation, we first performed a random-effects (RFX) ANOVA with effector as a between-subjects factor (right foot in the IDs and right hand in the controls; the dominant effector for both), and action type (reaching and grasping) as a within-subjects factor (Fig. 1A). Importantly, this analysis allows us to infer effector-dependent and effector-independent mechanisms in the sensorimotor system. For example, areas demonstrating a main effect of action type show preference for one action type over the other across both effectors (hand and foot in their respective groups), indicating effector-independent organization. Post hoc analyses were then performed to investigate the direction of the main effects.
We first inspected which areas show a main effect of effector, signifying preference based on body part (i.e., stronger activation for hand movements versus foot movement or vice versa) regardless of the action type performed. We found a significant difference between hand (in controls) and foot (in IDs) actions in the medial left primary sensorimotor cortex where the foot area is located in the sensorimotor homunculus (7, 10), as well as in the left PMd (Fig. 1B). Consistent with a somatotopic organization in primary sensorimotor cortex, post hoc region-of-interest (ROI) analyses found higher activation in the foot area for foot movements in the IDs versus hand movements in the controls, and higher activation in the hand area (sampled from a motor localizer experiment; see Methods) for hand movements in the controls versus foot movements in the IDs [two-tailed t test, t(9) = 2.84, P = 0.020] (SI Appendix, Fig. S2A). This is consistent with recent evidence for somatotopic organization in the primary sensorimotor cortex in IDs despite compensatory foot use, indicating it adheres to somatotopic (as opposed to function-based) organization (39). Foot movements in IDs also elicited higher activation in the left PMd than hand movements in the controls (SI Appendix, Fig. S2A). These findings indicate effector-selective action representation across action types in these brain areas.
Next, we addressed the main question of whether there is effector-independent organization in sensorimotor cortex. Brain areas with a main effect of action type—that is, a consistent preference for an action type for both the hand actions in controls and foot actions in IDs—would indicate effector-independent representation. We found a main effect of action type, regardless of the effector used by the two groups, in a wide network of frontoparietal association sensorimotor cortices (Fig. 1C). These included left PMd and ventral premotor cortex (PMv), supplementary and presupplementary motor area (SMA and preSMA, respectively), middle-to-anterior IPS (midaIPS; found bilaterally), and superior parieto-occipital cortex (SPOC). Additionally, a preference based on action type was found in the basal ganglia in the head of the caudate nucleus, as well as in the anterior cerebellum (lobules I–IV and V), bilaterally. No significant interaction between effector/group and action type was found. We also performed this analysis on each ID, demonstrating the consistency of action-type preference across IDs (SI Appendix, Figs. S3 and S9). In testing the direction of this action-based preference, an overall preference for reaching over grasping was found across both groups (SI Appendix, Fig. S4A). Grasping activated the typical grasping network, including the premotor cortex and IPS (SI Appendix, Fig. S5), consistent with past studies (16, 20), although it did not generate higher activation than reaching. This likely results from the absence of visuomotor coordination (47) in our task, as the participants could not see their moving effectors or the target object (see Discussion). Preference for reaching with the feet in the typical hand-reaching PMd (19, 38, 48–50) was found consistently across the IDs (SI Appendix, Fig. S4 C, Right and SI Appendix, Fig. S4D). These findings show that nonprimary, association motor areas selectively represent action type for reaching, independent of specific sensorimotor parameters, such as muscle group, associated with the hand and foot, respectively.
How do these two types of representation, effector-dependent and effector-independent, relate to each other in cortical spatial organization? To investigate this question, we examined the transition areas between the two representation types. Plotting the two main effects together, we found a posterior–anterior gradient along the left PMd from effector- to action-type selectivity (Fig. 1D). This pattern is further demonstrated by a gradual shift between these main effects seen when sampling consecutive points along the posterior–anterior axis of the superior frontal sulcus (SFS) (Fig. 1E). Similar findings were found for the SFS gradient across effectors (the hand and foot) in the control group (SI Appendix, Fig. S6D). Although findings from the controls cannot rule out the potential confound of hand motor imagery, these findings are also consistent with abstract representations of action type in the PMd. Similar to the changes in the main effect of action type across the two groups, within each group generalized linear model (GLM) β-estimates for the contrast between the two action types also gradually increased and decreased from posterior to anterior in the PMd (Fig. 1F). Overall, these findings suggest a hierarchical gradient of abstraction in representing actions, from body-part to abstract action-type specificity in the PMd.
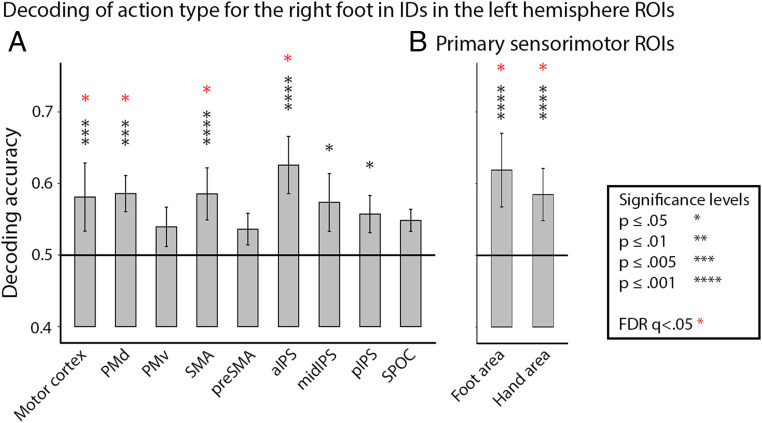
Does the differentiation based on action-type across groups and effectors also manifest in the regional pattern of activation? We performed multivariate pattern analysis (MVPA) to investigate whether action-related brain regions’ activity pattern represents information regarding action type (see Methods): That is, if we can decode what action is performed. We specifically tested whether we can decode foot action type in IDs in areas previously shown to represent action-type information for the hand (28) (see Methods for detailed information). It was previously reported that response patterns in the left PMd and anterior IPS (aIPS) differed between planned action types (reaching and grasping) for either hand (28). Here, we further show that response patterns in the aIPS and PMd also differed between action types performed by the foot (Fig. 2A). In addition, we successfully decoded foot action types in areas that were previously reported to represent action type only for the contralateral hand (motor cortex and SMA) (28). Finally, response pattern in the left hand-selective primary sensorimotor cortex could be used to decode action types for the foot in IDs (Fig. 2B). Therefore, action type representation for foot actions in IDs manifests in activity patterns in typical frontoparietal hand-action areas.
Fig. 2.
Effector-independent action-type decoding. Decoding of action type (grasp vs. reach) for foot actions in IDs was significant in action peak ROIs defined in the past literature (28) (A), as well as body-part–selective ROIs defined from a control motor experiment (B). Horizontal lines at 0.5 denote chance level. Error bars denote SE. Asterisks denote significance (red: FDR-corrected for multiple comparisons).
Discussion
Manual reaching and grasping actions were reported to engage partially separable neural pathways, indicating an organization of the sensorimotor network based on action type (35–38). We examined individuals born without hands who act with their feet to investigate to what extent this organization is specific to the hand or based on preference to the action type regardless of specific effectors. We found that frontal and parietal association sensorimotor areas, including the contralateral PMd, PMv, SMA/preSMA, midaIPS, and SPOC, preferentially responded to an action type (reaching/grasping), regardless of whether it was performed by the hand in typically-developed controls or by the foot in IDs. Moreover, using MVPA we found that in areas known to have distinct patterns for hand actions, distinct patterns of foot actions exist as well. These findings indicate that in these association areas, motor representations are based on action type without specified sensorimotor parameters, encoding information at a more abstract level than the effector-dependent computation.
Effector-Independent Action-Based Organization in Sensorimotor Cortices.
Studying IDs and their foot use allowed us to extend past findings on effector-independent action representation. There is evidence that response patterns in the aIPS, midIPS, PMd, and preSMA can differentiate planning or execution of reaching and grasping actions for both hands (19, 20, 28). Given that the two hands share highly-homologous muscle structures, it is possible that the abstraction level found in previous studies was hand-side–independent but still specific to the biomechanical structure of the hands (19) and does not generalize to the foot. In addition, hand motor areas share dense cross-hemispheric connections that mediate bimanual coordination (51–53), leading to each hemisphere being sensitive to movements of either hand. By testing individuals born without hands, our findings indicate effector-independence that is across biomechanical structures (i.e., between the hand and foot) and independent of direct interhemispheric cortico–cortical connection. Past studies also reported common activation between hand and foot pointing in the aIPS and SPOC (5, 22, 23), but did not discriminate between pointing and other action types. We provide evidence for effector-independent action-type representation in these areas. Moreover, shared representations when planning to direct either the hand (in reaching) or eye (in performing saccades) toward external locations has been found in the aIPS and posterior superior parietal lobule (22, 24), although the aIPS is primarily hand-selective (5, 23). One possibility is that these common motor representations are abstracted during hand–eye coordination in performing visually guided hand actions, whereas effector-independent motor organization in individuals born without hands precludes an account of hand-based coordination.
Finally, a few studies reported that areas in the posterior parietal cortex, including the aIPS and anterior superior parietal lobule, were equally activated by planning hand- and foot-pointing movements (5, 22, 23). Although supporting effector-independent organization, it is possible that performing foot actions involved mental imagery of analogous hand actions, a process that also engages the hand-action execution network (29–34). In contrast, by studying individuals born without hands who do not have prior experience with hand movements, our results are not confounded by manual motor (kinesthetic) imagery. Although IDs can still imagine viewing other people perform hand actions, visual action imagery does not reliably involve motor-associated areas and instead more strongly engages visual areas (54–56). Furthermore, action observation in ventral premotor areas and the inferior parietal lobule are associated primarily with action-execution and motor imagery functions (57, 58). Therefore, excluding kinesthetic motor imagery by testing individuals with dysplasia reveals the core role of these regions in action execution, and expands on previous findings in showing effector-independence beyond aIPS to additional regions, including the premotor cortex, basal ganglia, and cerebellum (see Effector-Independence in Subcortical Areas below).
This effector-independent organization suggests that at least for the nonprimary sensorimotor system, the organization may be better defined by action types rather than by body-part topography. A proposal of an action-type–based organization principle for the motor system has been made previously based on studies in nonhuman primates. Long-train (∼500 ms) microstimulation in the premotor cortex appears to generate coordinated actions that extend beyond a single body part to complete ethological goals (e.g., grasping, feeding; 15, 59–61). Despite their theoretical interest, these works were criticized, stating that long stimulation may lead to spread of activation and nonlocal recruitment (62). Here, using whole-brain neuroimaging showing distinct activation levels and spatial patterns between reaching and grasping, our findings lend support to a motor organization based on action-type and extend the level of abstraction across effectors.
Relationship between Different Abstraction Levels.
While we found a large-scale dissociation between effector-dependence in the primary sensorimotor cortex and effector-independence in association areas, our results also support a proposal that multiple organization principles, for body parts and for action-types, coexist in some motor areas (25). First, we found a gradient between effector-dependent and effector-independent preferences in the sensorimotor system, along the left PMd. Although these two seemingly-discrete clusters allow us to draw a line between more anterior areas whose activity is action-type–dependent and more posterior areas whose activity is effector-dependent, they may also be interpreted as a gradient of overlapping distributions, which includes transition voxels selective to both properties (see interaction of these two main effects in the controls) (SI Appendix, Fig. S6 C and D). Second, the primary motor area, while showing clear preference for a specific body part (Fig. 1B and SI Appendix, Fig. S2A), also showed distinct multivoxel response patterns for foot reaching and grasping actions in the IDs, indicating coexistence of representations at different abstraction levels. This apparent effector-invariance contrasts with past findings that during movement execution, the decoding between action types in the primary motor hand area is limb-specific, only for the contralateral hand and not for the ipsilateral hand (20, 28). However, a recent study reported differential responses to individual toes in IDs in the missing-hand area of the primary sensorimotor cortex, indicating this region also receives meaningful information about the foot in these individuals (63). Therefore, the role of the early sensorimotor cortex in effector-invariant action processing remains open to future explorations. Taken together, our findings suggest that instead of having discrete areas each representing action at a specific abstraction level, there may be multiple organization principles coexisting in sensorimotor areas, consistent with the proposal from past studies (25, 64, 65).
Effector-Independence in Subcortical Areas.
Our findings show effector-independent action-type preference not only in cortical frontoparietal areas but also in the cerebellum and basal ganglia (peaking in the caudate). A somatotopic organization in the anterior cerebellum is well characterized in humans, with an anterior–posterior distribution from lobule IV to VI between foot, hand, and lip movements (66–68). We found a main effect of action type in lobule IV to V, falling between typical hand- and foot-movement areas (SI Appendix, Fig. S7). Importantly, the role of the cerebellum extends beyond simple movements to various domains of motor control, including performing reaching and grasping actions (69–74) and motor learning (75–77). Although these cerebellar responses generally overlap with the cerebellar hand area, it remains a question whether these functions are specific to the hand or operate at an effector-independent level. Interestingly, a recent study investigated cerebellar organization in people born with one hand and found that the cerebellar missing-hand area is activated by multiple body parts (78). Although body-part selectivity in the cerebellum for compensatory effectors was not investigated in that case, it is also consistent with our evidence for function-based organization near the somatotopic hand area. Overall, our findings suggest that the cerebellum may also have effector-independent organization for action types, adding important insights for the understanding of the topography and motor hierarchy of the cerebellum.
The role of the basal ganglia in motor control has been extensively studied: Activity in the caudate nucleus was associated with skilled limb movements, including reaching, grasping, and self-feeding in animal models (79, 80). Functional neuroimaging studies on humans reported basal ganglia activity during motor learning and transferring (81–83). Interestingly, literature on motor-sequence learning proposed a division between anterior (associative) and posterior (sensorimotor) areas of the striatum (82). Learning new motor sequences activated associative (anterior) areas, including the caudate and anterior putamen (81, 82). Our finding of a main effect of action type supports the anterior striatal area engagement in encoding more-abstract movement rules, showing it can also support effector-independent abstract action representation.
Effector-Independence Across Motor Domains.
Whereas we examined ethological actions (e.g., reaching, grasping; 15, 65), the concept of effector-independent motor representation has long been discussed (84–87) and demonstrated in several additional domains, including rhythmic movements (88–90) and writing (91–95). One classic example is similar shape between text written by different body parts (94, 95), more recently explored by showing common activation in the PMd and PMv when individuals signed their names with either the index finger or big toe (92). Our findings add to previous discussion by testing IDs, a clean model that rules out cross-effector coordination and motor imagery confounds. Moreover, studies on motor learning and transfer also indicate effector-independent motor representation. There is evidence that when individuals learn a motor sequence or visuomotor adaptation with one hand, learning is transferred to the other hand (96–100), or between proximal (shoulder–elbow) and distal (wrist–index finger) effector systems either unilaterally or bilaterally (101–103). The ability to transfer motor skills from a trained effector to a novel effector indicates that movements are represented not only in terms of specific muscle patterns, but also in more abstract forms that can be flexibly translated into alternative muscle patterns. Consistent with our findings, such learning transference relies on the frontal motor areas and parietal lobe (96, 99, 104). Whereas these domains differ in time scale, from evolutionarily old (e.g., grasping) to more recent (e.g., writing) and to newly-trained visuomotor perturbations, studies on different domains indicate that effector-independent motor representation may be a common mechanism that supports flexible motor execution and learning across effectors.
Study Limitations.
It could be argued that the effects reported here could be limited to the IDs, resulting from a unique reorganization in their brains due to the absence of hands, undermining our data's usefulness in informing theories of the typically-developed brain. However, overall the IDs’ brain has shown remarkable consistency with typical organization for other visuomotor properties, including the action observation network (40, 43). Furthermore, the controls in our study also showed a difference between reaching and grasping in the SMA/preSMA, SPOC, PMv, and PMd for the right foot (SI Appendix, Fig. S8), indicating that action type affects responses also for foot actions even in typically-developed participants. Therefore, together with past evidence of effector-invariance in the association sensorimotor cortex in typically-developed individuals (19, 20, 28), it is likely that these areas are organized in an effector-invariant manner. Taken together, our findings indicate that effector-independence may be an innate organization principle of the sensorimotor system.
Our task emphasis on motor pattern rather than on visuomotor coordination generated some differences from past literature. Past studies that employed visually-guided reaching and grasping reported a larger network across the posterior parietal cortex (PPC) activated by reaching and grasping actions (16, 28, 44). Some of these were not found in our motor-driven task, which did not provide the participants with a view of the objects and effector. This design was created to match the inability to see the foot action in the scanner environment and isolate the motor reach and grasp component. The lack of PPC involvement in our design is therefore likely due to reduced need to transform object location from an eye-centered reference frame to an effector-based reference frame (11, 105–107). Similarly, whereas we found a preference for reaching in the PMd and SPOC, consistent with past findings (13, 16, 35, 37), no preference for grasping was found in the aIPS and PMv (35, 37, 44, 108). Notably, preference for grasping vs. reaching has only been reported when visual information was available (36, 37, 44) and has not been widely studied when visual information is removed from sighted individuals (20, 109). Hence, our results do not contradict past findings and instead likely reflect modulation by visual information of the reaching and grasping networks and tasks, for example, by affecting visuomotor coordination (47). Importantly, these differences do not influence our conclusion of the effector-independent action network. By removing common visual object representation between hand and foot actions, our findings demonstrate effector-independent action-type representation across muscle patterns that were not driven by shared visual representation mechanisms. Finally, we recognize that our findings may be limited in revealing the complete scale of effector-invariance organization given the study power and sample size. Therefore, while our study found reliable effector invariance based on motor coding in some regions, future studies may also reveal similar properties for visuomotor transformation processes and their neural substrates.
Implications for Brain Organization Principles.
Beyond addressing motor system organization and action representations, our findings also have broader implications on brain organization and plasticity. Specifically, our results add to past findings from blind and deaf individuals that showed typical functional specificity of the visual and auditory association cortices despite sensory deprivation (110). Such findings, of processing domain- and category-specific computations in the ventral and dorsal steams of the visual cortex in people born blind (110–114), suggested that visual cortex organization is independent of visual features and experience and can support a representation abstracted from such elements (110, 111, 115, 116). Visual association cortices also appear to be independent from motor experience for relevant visual percepts, as shown for hand- and action-perception areas in people born without hands (40, 43). Importantly, comparable findings of processing for localization, identity, and communication signals in the auditory cortex in deafness (117–122) indicate that this principle of sensory-modality independence is applicable to the association sensory cortex at large. Here we extend the same principle to the motor domain. We provide evidence that similar to sensory-independence, some parts of the association motor system are effector-independent, representing actions regardless of specific motor outputs on which the computation is carried out. Taken together, these findings suggest a common organization principle in association areas across multiple domains in cortical organization.
In summary, by examining individuals with dysplasia, we provide evidence that the association sensorimotor cortex represents abstract action types, reaching and grasping, regardless of whether performed by hands or feet. The present study extends past literature on effector-independent representation by controlling for potential manual motor imagery and identifies a level of abstraction beyond homologous muscle structure between the two hands. Effector-independent representation in motor domain adds to research in the domains of visual and auditory perception indicating that the organization principle in association areas is based on abstract function and computation, regardless of specific sensory inputs or motor effectors, for both perception and action.
Methods
Participants.
Four individuals born with severely shortened or completely absent upper limbs (individuals with upper limb dysplasia), and nine typically-developed control participants, matched for age (no group difference; P < 0.29), participated in the experiment. The IDs’ data in a series of behavioral and neuroimaging experiments have been reported previously (39, 40, 43). For the correspondence of participant codes across studies, see SI Appendix, Table S1. The causes of dysplasia were genetic, ototoxic medications (thalidomide), or unknown. See SI Appendix for a summary of the characteristics of the IDs. None of the IDs had a history of phantom limb sensations or movements, and all were adept at performing everyday actions with their feet. Two control participants were excluded from analyses due to missing data for technical reasons. All participants gave written informed consent in accordance with the Institutional Review Board of Harvard University that approved all experiments.
Experimental Design and Procedure.
The experiment was conducted in a block design, with each type of effector (feet in the IDs, hands and feet in the controls) tested in two runs of 255 repetition times (TRs) each. The order of hand and foot runs was randomized across control participants. Each run began with a baseline period of 10 TRs, followed by reaching and grasping trials. Participants began with the legs resting naturally on a triangular support pillow and the hands resting on the sides of the abdomen. A foam bar (1-cm wide, 4-cm tall, 6-cm deep) was placed ∼30 cm centrally in front of the hands or feet, equidistant from both sides (SI Appendix, Fig. S1). Each trial started with an auditory instruction on the effector side and action type (e.g., “right reach”). At the end of the 2-s instruction, participants began to perform the instructed action four times at auditory cues (metronome) spaced by 1.5-s intervals. At each auditory cue, participants completed the movement and returned the limb to the starting position. In each reaching movement, participants reached toward and touched the foam bar with the tips of the fingers (with the hand flat open) or toes (Fig. 1A). In each grasping movement, participants reached toward and grasped the foam bar between digit 1 and digit 2 of the hand or foot (Fig. 1A) (thumb and index finger for the hand). Trials were spaced by 4 s. Twenty-five percent of the trials were followed by a prolonged 16-s rest period to better model the baseline. Each combination of side and action type was repeated in eight trials within each run, with trial order pseudorandomized. All trials were visually inspected by an experimenter during their execution to make sure they were accurately performed.
Functional Imaging.
The blood-oxygen-level-dependent fMRI measures were obtained in a Siemens Trio 3T scanner at the Center for Brain Science at Harvard University. For acquisition details, see SI Appendix. Data that support the findings of this study are available from the corresponding authors upon reasonable request.
Data analyses were performed using the Brain Voyager QX 21.4 software package (Brain Innovation) using standard preprocessing procedures. The first two images of each scan (during the first baseline rest condition) were excluded from the analysis because of nonsteady-state magnetization. fMRI data preprocessing included head motion correction, slice scan time correction, and high-pass filtering (cutoff frequency: three cycles per scan) using temporal smoothing in the frequency domain to remove drifts and to improve the signal-to-noise ratio. No data included in the study showed translational motion exceeding 2 mm in any given axis, or had spike-like motion of more than 1 mm in any direction.
Functional and anatomical datasets for each participant were aligned and fit to standard Talairach space (123). Anatomical cortical reconstruction procedures included segmentation of the white matter using a grow-region function embedded in Brain Voyager. Analyses were conducted in the volumetric space and then superimposed to cortical space. Single-subject data were spatially smoothed by a 6-mm full-width half-maximum (FWHM) Gaussian kernel for the univariate analyses, and a 3-mm FWHM Gaussian kernel for MVPA.
Univariate Analyses.
We first performed RFX ANOVAs to examine the main effect of effector/group and action type. We then performed a GLM contrasting reaching and grasping within each group, using RFX GLM analyses (124) for the control group, and fixed-effect GLM for the IDs due to the sample size. These were complemented by additional probabilistic mapping of the overlap of significant activation across individuals (SI Appendix, Fig. S4D) and presentation of single subject data for the various ROIs (SI Appendix, Fig. S9), to illustrate the consistency of the findings within this group. Activation maps were thresholded at P < 0.01 and corrected for multiple-comparison at P < 0.05 using the spatial extent method, a set-level statistical inference correction (125, 126), implemented in Brain Voyager.
To investigate the effector-dependence of the sensorimotor hand area, which did not appear at a whole-brain group-level analysis, we sampled the sensorimotor cortex ROI using a functional ROI from a simple motor localizer conducted on the same participants (39). The typically-developed controls performed simple flexing/contraction movements of different body parts, including the right and left hand, foot and shoulder, as well as the bilateral mouth and abdomen in separate blocks. Data were analyzed using standard preprocessing as performed here, and analyzed in RFX GLM analysis. For full detail of the experimental design and analysis, see Striem-Amit et al. (39). For the purpose of the current analysis, a contrast was computed for right hand flexing as compared to the right foot, shoulder, and bilateral mouth and abdomen, at P < 0.05 corrected for multiple comparisons, to define the hand-selective ROI in the left cortex. A foot-selective ROI was also defined for each group separately for the multivariate analyses (see Multivariate Analyses below). For the controls, the foot ROI was computed by a contrast of right foot flexing vs. flexing the right hand, shoulder, and bilateral mouth and abdomen, thresholded at P < 0.001 to obtain a cluster constrained within the primary sensorimotor cortex. For the IDs, we calculated a contrast of right foot flexing vs. flexing of bilateral mouth and abdomen, thresholded at P < 1e-20, to achieve a similarly sized ROI.
Multivariate Analyses.
Whole-brain searchlight MVPA was performed using a linear support-vector machine classifier implemented in the CoSMoMVPA toolbox (43, 127), with each spherical searchlight containing 200 voxels. First, z-normalized β-weights were estimated for each trial using Brain Voyager. For within-effector decoding, classification accuracies were calculated using leave-one-trial-pair-out N-fold cross-validation (28): In each iteration, one trial from each action type was left out of the testing dataset and the classifier was trained on the remaining dataset. The final decoding accuracy was the average across all iterations. For each participant, 100 null maps were generated for each analysis by randomly shuffling action-type labels across trials. These chance-accuracy maps were then used at group level for multiple-comparison correction for ROI MVPA (10,000 simulation iterations) (43, 127).
To investigate if specific brain regions represent action-type information, we performed ROI MVPA. To assess hand-action regions independently from our experimental data, we sampled ROIs used to investigate action representation across hands in a previous study, based on reported peak Talairach coordinates (28) (Fig. 2A; see SI Appendix, Table S2 for ROI list and coordinates). Each ROI was an 8-mm radius sphere centered on the coordinate reported in SI Appendix, Table S2. Centers of the ROIs were shifted from the coordinates reported in the original study (28) within one SD when necessary to prevent overlap between ROIs. Body-part–selective ROIs sampled based on the control motor experiment were also examined (Fig. 2B). Within each ROI, decoding accuracy of each voxel was extracted from the searchlight MVPA results and then averaged across voxels. For each group and ROI, an average accuracy was calculated by averaging across all individuals. To determine the significance level of the decoding accuracy, a bootstrap permutation method was used. In each iteration, one of the null maps from each individual was sampled, then a new decoding accuracy was calculated for each group and ROI based on the null maps. The sampling procedure was repeated for 10,000 iterations, resulting in 10,000 decoding accuracies under the null hypothesis. Significance level was calculated by counting the number of null decoding accuracies that surpassed the actual decoding accuracy, with less than 5% (P < 0.05) deemed significant. For each effector-side and group, significance level of decoding accuracy was corrected for the number of ROIs at false-discovery rate (FDR) q < 0.05.
Control for Magnetic Field Distortion.
Performing arm movements in the scanner may distort the magnetic field and induce artifacts that occur instantaneously at the time of movement (44). To verify such movement-induced artifacts could not have driven our results, we reanalyzed our data by explicitly modeling instantaneous movement-induced artifacts using box-car functions. All regions showing a main effect of action type retained their effects after regressing out artifacts, indicating that our reported results reflect action hemodynamic response and not field distortions (SI Appendix, Fig. S9). Additionally, we conducted a control experiment in which a phantom was scanned while an experimenter performed the same reaching and grasping actions in the scanner. If our findings were results of movement-induced artifacts, we would also see an effect of action type on the phantom. We show that this is not the case (SI Appendix, Fig. S10), confirming that our results cannot be accounted for by such artifacts.
Supplementary Material
Acknowledgments
We thank the individuals with dysplasia who participated in our experiments; Jody Culham for her helpful discussions; Himanshu Bhat and Thomas Benner of Siemens Healthcare for the simultaneous multislice echo-planar imaging sequence; and Steven Cauley of Massachusetts General Hospital for modifications that enabled implementation of our protocols in a routine session. This work was supported by the Edwin H. Richard and Elisabeth Richard von Matsch Distinguished Professorship in Neurological Diseases (to E.S.-A.), the Società Scienze Mente Cervello–Fondazione Cassa di Risparmio di Trento e Rovereto, a grant from the Provincia Autonoma di Trento, and a Harvard Provostial postdoctoral fund (to A.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2017789117/-/DCSupplemental.
Data and Availability.
Study data are available upon request from the corresponding authors.
References
- 1.Gallego J. A. et al., Cortical population activity within a preserved neural manifold underlies multiple motor behaviors. Nat. Commun. 9, 4233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallego J. A., Perich M. G., Miller L. E., Solla S. A., Neural manifolds for the control of movement. Neuron 94, 978–984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overduin S. A., d’Avella A., Roh J., Carmena J. M., Bizzi E., Representation of muscle synergies in the primate brain. J. Neurosci. 35, 12615–12624 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leo A. et al., A synergy-based hand control is encoded in human motor cortical areas. eLife 5, e13420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heed T., Leone F. T. M., Toni I., Medendorp W. P., Functional versus effector-specific organization of the human posterior parietal cortex: Revisited. J. Neurophysiol. 116, 1885–1899 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeharia N., Hertz U., Flash T., Amedi A., Negative blood oxygenation level dependent homunculus and somatotopic information in primary motor cortex and supplementary motor area. Proc. Natl. Acad. Sci. U.S.A. 109, 18565–18570 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeharia N., Hertz U., Flash T., Amedi A., New whole-body sensory-motor gradients revealed using phase-locked analysis and verified using multivoxel pattern analysis and functional connectivity. J. Neurosci. 35, 2845–2859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filimon F., Nelson J. D., Huang R. S., Sereno M. I., Multiple parietal reach regions in humans: Cortical representations for visual and proprioceptive feedback during on-line reaching. J. Neurosci. 29, 2961–2971 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier J. D., Aflalo T. N., Kastner S., Graziano M. S., Complex organization of human primary motor cortex: A high-resolution fMRI study. J. Neurophysiol. 100, 1800–1812 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penfield W., Boldrey E., Somatic motor and sensory representation in man. Brain 60, 389–443 (1937). [Google Scholar]
- 11.Batista A. P., Buneo C. A., Snyder L. H., Andersen R. A., Reach plans in eye-centered coordinates. Science 285, 257–260 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Chang S. W. C., Snyder L. H., The representations of reach endpoints in posterior parietal cortex depend on which hand does the reaching. J. Neurophysiol. 107, 2352–2365 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly J. D., Andersen R. A., Goodale M. A., FMRI evidence for a ‘parietal reach region’ in the human brain. Exp. Brain Res. 153, 140–145 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Kaas J. H., Stepniewska I., Gharbawie O., Cortical networks subserving upper limb movements in primates. Eur. J. Phys. Rehabil. Med. 48, 299–306 (2012). [PMC free article] [PubMed] [Google Scholar]
- 15.Kaas J. H., Gharbawie O. A., Stepniewska I., Cortical networks for ethologically relevant behaviors in primates. Am. J. Primatol. 75, 407–414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konen C. S., Mruczek R. E. B., Montoya J. L., Kastner S., Functional organization of human posterior parietal cortex: Grasping- and reaching-related activations relative to topographically organized cortex. J. Neurophysiol. 109, 2897–2908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yttri E. A., Wang C., Liu Y., Snyder L. H., The parietal reach region is limb specific and not involved in eye-hand coordination. J. Neurophysiol. 111, 520–532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinkley L. B., Krubitzer L. A., Padberg J., Disbrow E. A., Visual-manual exploration and posterior parietal cortex in humans. J. Neurophysiol. 102, 3433–3446 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haar S., Dinstein I., Shelef I., Donchin O., Effector-invariant movement encoding in the human motor system. J. Neurosci. 37, 9054–9063 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turella L., Rumiati R., Lingnau A., Hierarchical action encoding within the human brain. Cereb. Cortex 30, 2924–2938 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Gallivan J. P., McLean D. A., Smith F. W., Culham J. C., Decoding effector-dependent and effector-independent movement intentions from human parieto-frontal brain activity. J. Neurosci. 31, 17149–17168 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heed T., Beurze S. M., Toni I., Röder B., Medendorp W. P., Functional rather than effector-specific organization of human posterior parietal cortex. J. Neurosci. 31, 3066–3076 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leoné F. T. M., Heed T., Toni I., Medendorp W. P., Understanding effector selectivity in human posterior parietal cortex by combining information patterns and activation measures. J. Neurosci. 34, 7102–7112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magri C., Fabbri S., Caramazza A., Lingnau A., Directional tuning for eye and arm movements in overlapping regions in human posterior parietal cortex. Neuroimage 191, 234–242 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Graziano M. S. A., Aflalo T. N., Rethinking cortical organization: Moving away from discrete areas arranged in hierarchies. Neuroscientist 13, 138–147 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Kaas J. H., Garraghty P. E., Hierarchical, parallel, and serial arrangements of sensory cortical areas: Connection patterns and functional aspects. Curr. Opin. Neurobiol. 1, 248–251 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Tresilian J. R., Abstract levels of motor control in prehension: Normal and pathological performance. Hum. Mov. Sci. 18, 219–239 (1999). [Google Scholar]
- 28.Gallivan J. P., McLean D. A., Flanagan J. R., Culham J. C., Where one hand meets the other: Limb-specific and action-dependent movement plans decoded from preparatory signals in single human frontoparietal brain areas. J. Neurosci. 33, 1991–2008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrsson H. H., Geyer S., Naito E., Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J. Neurophysiol. 90, 3304–3316 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Porro C. A. et al., Primary motor and sensory cortex activation during motor performance and motor imagery: A functional magnetic resonance imaging study. J. Neurosci. 16, 7688–7698 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solodkin A., Hlustik P., Chen E. E., Small S. L., Fine modulation in network activation during motor execution and motor imagery. Cereb. Cortex 14, 1246–1255, 1246–1255 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Hanakawa T. et al., Functional properties of brain areas associated with motor execution and imagery. J. Neurophysiol. 89, 989–1002 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Lacourse M. G., Orr E. L., Cramer S. C., Cohen M. J., Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 27, 505–519 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Schnitzler A., Salenius S., Salmelin R., Jousmäki V., Hari R., Involvement of primary motor cortex in motor imagery: A neuromagnetic study. Neuroimage 6, 201–208 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Culham J. C., Cavina-Pratesi C., Singhal A., The role of parietal cortex in visuomotor control: What have we learned from neuroimaging? Neuropsychologia 44, 2668–2684 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Cavina-Pratesi C. et al., Human neuroimaging reveals the subcomponents of grasping, reaching and pointing actions. Cortex 98, 128–148 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Cavina-Pratesi C. et al., Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J. Neurosci. 30, 10306–10323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabbri S., Strnad L., Caramazza A., Lingnau A., Overlapping representations for grip type and reach direction. Neuroimage 94, 138–146 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Striem-Amit E., Vannuscorps G., Caramazza A., Plasticity based on compensatory effector use in the association but not primary sensorimotor cortex of people born without hands. Proc. Natl. Acad. Sci. U.S.A. 115, 7801–7806 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Striem-Amit E., Vannuscorps G., Caramazza A., Sensorimotor-independent development of hands and tools selectivity in the visual cortex. Proc. Natl. Acad. Sci. U.S.A. 114, 4787–4792 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannuscorps G., Caramazza A., Conceptual processing of action verbs with and without motor representations. Cogn. Neuropsychol. 36, 301–312 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Vannuscorps G., Andres M., Pillon A., Is motor knowledge part and parcel of the concepts of manipulable artifacts? Clues from a case of upper limb aplasia. Brain Cogn. 84, 132–140 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Vannuscorps G., F Wurm M., Striem-Amit E., Caramazza A., Large-scale organization of the hand action observation network in individuals born without hands. Cereb. Cortex 29, 3434–3444 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Culham J. C. et al., Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp. Brain Res. 153, 180–189 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Frey S. H., Vinton D., Norlund R., Grafton S. T., Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res. Cogn. Brain Res. 23, 397–405 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Chapman H. et al., Posterior parietal cortex control of reach-to-grasp movements in humans. Eur. J. Neurosci. 15, 2037–2042 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Jeannerod M., Arbib M. A., Rizzolatti G., Sakata H., Grasping objects: The cortical mechanisms of visuomotor transformation. Trends Neurosci. 18, 314–320 (1995). [PubMed] [Google Scholar]
- 48.Beurze S. M., de Lange F. P., Toni I., Medendorp W. P., Integration of target and effector information in the human brain during reach planning. J. Neurophysiol. 97, 188–199 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Crawford J. D., Henriques D. Y., Medendorp W. P., Three-dimensional transformations for goal-directed action. Annu. Rev. Neurosci. 34, 309–331 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Gallivan J. P., Culham J. C., Neural coding within human brain areas involved in actions. Curr. Opin. Neurobiol. 33, 141–149 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Brus-Ramer M., Carmel J. B., Martin J. H., Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J. Neurosci. 29, 6196–6206 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardoso de Oliveira S., Gribova A., Donchin O., Bergman H., Vaadia E., Neural interactions between motor cortical hemispheres during bimanual and unimanual arm movements. Eur. J. Neurosci. 14, 1881–1896 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Liuzzi G., Hörniss V., Zimerman M., Gerloff C., Hummel F. C., Coordination of uncoupled bimanual movements by strictly timed interhemispheric connectivity. J. Neurosci. 31, 9111–9117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guillot A. et al., Brain activity during visual versus kinesthetic imagery: An fMRI study. Hum. Brain Mapp. 30, 2157–2172 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stinear C. M., Byblow W. D., Steyvers M., Levin O., Swinnen S. P., Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp. Brain Res. 168, 157–164 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Neuper C., Scherer R., Reiner M., Pfurtscheller G., Imagery of motor actions: Differential effects of kinesthetic and visual-motor mode of imagery in single-trial EEG. Brain Res. Cogn. Brain Res. 25, 668–677 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Gallese V., Fadiga L., Fogassi L., Rizzolatti G., Action recognition in the premotor cortex. Brain 119, 593–609 (1996). [DOI] [PubMed] [Google Scholar]
- 58.Fabbri-Destro M., Rizzolatti G., Mirror neurons and mirror systems in monkeys and humans. Physiology (Bethesda) 23, 171–179 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Graziano M. S., Taylor C. S., Moore T., Complex movements evoked by microstimulation of precentral cortex. Neuron 34, 841–851 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Graziano M. S., Aflalo T. N., Cooke D. F., Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J. Neurophysiol. 94, 4209–4223 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Baldwin M. K. L., Cooke D. F., Goldring A. B., Krubitzer L., Representations of fine digit movements in posterior and anterior parietal cortex revealed using long-train intracortical microstimulation in macaque monkeys. Cereb. Cortex 28, 4244–4263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankowska E., Padel Y., Tanaka R., The mode of activation of pyramidal tract cells by intracortical stimuli. J. Physiol. 249, 617–636 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dempsey-Jones H., Wesselink D. B., Friedman J., Makin T. R., Organized toe maps in extreme foot users. Cell Rep. 28, 2748–2756.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aflalo T. N., Graziano M. S., Possible origins of the complex topographic organization of motor cortex: Reduction of a multidimensional space onto a two-dimensional array. J. Neurosci. 26, 6288–6297 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graziano M. S. A., Ethological action maps: A paradigm shift for the motor cortex. Trends Cogn. Sci. 20, 121–132 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Rijntjes M., Buechel C., Kiebel S., Weiller C., Multiple somatotopic representations in the human cerebellum. Neuroreport 10, 3653–3658 (1999). [DOI] [PubMed] [Google Scholar]
- 67.Nitschke M. F., Kleinschmidt A., Wessel K., Frahm J., Somatotopic motor representation in the human anterior cerebellum. A high-resolution functional MRI study. Brain 119, 1023–1029 (1996). [DOI] [PubMed] [Google Scholar]
- 68.Boillat Y., Bazin P. L., van der Zwaag W., Whole-body somatotopic maps in the cerebellum revealed with 7T fMRI. Neuroimage 211, 116624 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Jacobs S., Danielmeier C., Frey S. H., Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. J. Cogn. Neurosci. 22, 2594–2608 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Grafton S. T., Fagg A. H., Woods R. P., Arbib M. A., Functional anatomy of pointing and grasping in humans. Cereb. Cortex 6, 226–237 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Fu Q. G., Flament D., Coltz J. D., Ebner T. J., Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J. Neurophysiol. 78, 478–491 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Kitazawa S., Kimura T., Yin P. B., Cerebellar complex spikes encode both destinations and errors in arm movements. Nature 392, 494–497 (1998). [DOI] [PubMed] [Google Scholar]
- 73.Espinoza E., Smith A. M., Purkinje cell simple spike activity during grasping and lifting objects of different textures and weights. J. Neurophysiol. 64, 698–714 (1990). [DOI] [PubMed] [Google Scholar]
- 74.Dugas C., Smith A. M., Responses of cerebellar Purkinje cells to slip of a hand-held object. J. Neurophysiol. 67, 483–495 (1992). [DOI] [PubMed] [Google Scholar]
- 75.Imamizu H. et al., Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403, 192–195 (2000). [DOI] [PubMed] [Google Scholar]
- 76.Doyon J., Benali H., Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 15, 161–167 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Penhune V. B., Doyon J., Cerebellum and M1 interaction during early learning of timed motor sequences. Neuroimage 26, 801–812 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Hahamy A., Makin T., Reorganization in cerebral and cerebellar cortices is not restricted by proximity between body-part representations. J. Neurosci. 39, 9328–9342 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dolbakyan E., Hernandez-Mesa N., Bures J., Skilled forelimb movements and unit activity in motor cortex and caudate nucleus in rats. Neuroscience 2, 73–80 (1977). [DOI] [PubMed] [Google Scholar]
- 80.Buser P., Pouderoux G., Mereaux J., Single unit recording in the caudate nucleus during sessions with elaborate movements in the awake monkey. Brain Res. 71, 337–344 (1974). [DOI] [PubMed] [Google Scholar]
- 81.Jueptner M., Frith C. D., Brooks D. J., Frackowiak R. S. J., Passingham R. E., Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J. Neurophysiol. 77, 1325–1337 (1997). [DOI] [PubMed] [Google Scholar]
- 82.Lehéricy S. et al., Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc. Natl. Acad. Sci. U.S.A. 102, 12566–12571 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seidler R. D., Noll D. C., Chintalapati P., Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp. Brain Res. 175, 544–555 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Schmidt R. A., Lee T. D., Motor Control and Learning: A Behavioral Emphasis, (Human Kinetics, Champaign, IL, 1988). [Google Scholar]
- 85.Bernstein N. A., The Co-ordination and Regulation of Movements: Conclusions Towards the Study of Motor Co-ordination, (Pergamon Press, 1967). [Google Scholar]
- 86.Rosenbaum D. A., Human movement initiation: Specification of arm, direction, and extent. J. Exp. Psychol. Gen. 109, 444–474 (1980). [DOI] [PubMed] [Google Scholar]
- 87.Lashley K. S., Basic neural mechanisms in behavior. Psychol. Rev. 37, 1–24 (1930). [Google Scholar]
- 88.Bengtsson S. L., Ehrsson H. H., Forssberg H., Ullén F., Effector-independent voluntary timing: Behavioural and neuroimaging evidence. Eur. J. Neurosci. 22, 3255–3265 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Ivry R. B., Keele S. W., Diener H. C., Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp. Brain Res. 73, 167–180 (1988). [DOI] [PubMed] [Google Scholar]
- 90.Ehrsson H. H. et al., Simultaneous movements of upper and lower limbs are coordinated by motor representations that are shared by both limbs: A PET study. Eur. J. Neurosci. 12, 3385–3398 (2000). [DOI] [PubMed] [Google Scholar]
- 91.Rapp B., Caramazza A., From graphemes to abstract letter shapes: Levels of representation in written spelling. J. Exp. Psychol. Hum. Percept. Perform. 23, 1130–1152 (1997). [DOI] [PubMed] [Google Scholar]
- 92.Rijntjes M. et al., A blueprint for movement: Functional and anatomical representations in the human motor system. J. Neurosci. 19, 8043–8048 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kadmon Harpaz N., Flash T., Dinstein I., Scale-invariant movement encoding in the human motor system. Neuron 81, 452–462 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Merton P. A., How we control the contraction of our muscles. Sci. Am. 226, 30–37 (1972). [DOI] [PubMed] [Google Scholar]
- 95.Raibert M. H., “Motor control and learning by the state space model,” PhD dissertation, Massachusetts Institute of Technology, Cambridge, MA (1977).
- 96.Perez M. A. et al., Neural substrates of intermanual transfer of a newly acquired motor skill. Curr. Biol. 17, 1896–1902 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Schulze K., Lüders E., Jäncke L., Intermanual transfer in a simple motor task. Cortex 38, 805–815 (2002). [DOI] [PubMed] [Google Scholar]
- 98.van Mier H. I., Petersen S. E., Intermanual transfer effects in sequential tactuomotor learning: Evidence for effector independent coding. Neuropsychologia 44, 939–949 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Wiestler T., Waters-Metenier S., Diedrichsen J., Effector-independent motor sequence representations exist in extrinsic and intrinsic reference frames. J. Neurosci. 34, 5054–5064 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kovacs A. J., Mühlbauer T., Shea C. H., The coding and effector transfer of movement sequences. J. Exp. Psychol. Hum. Percept. Perform. 35, 390–407 (2009). [DOI] [PubMed] [Google Scholar]
- 101.Vangheluwe S., Puttemans V., Wenderoth N., Van Baelen M., Swinnen S. P., Inter- and intralimb transfer of a bimanual task: Generalisability of limb dissociation. Behav. Brain Res. 154, 535–547 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Aune T. K., Aune M. A., Ingvaldsen R. P., Vereijken B., Transfer of motor learning is more pronounced in proximal compared to distal effectors in upper extremities. Front. Psychol. 8, 1530 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grafton S. T., Hazeltine E., Ivry R. B., Abstract and effector-specific representations of motor sequences identified with PET. J. Neurosci. 18, 9420–9428 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar A., Panthi G., Divakar R., Mutha P. K., Mechanistic determinants of effector-independent motor memory encoding. Proc. Natl. Acad. Sci. U.S.A. 117, 17338–17347 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Graziano M. S., Is reaching eye-centered, body-centered, hand-centered, or a combination? Rev. Neurosci. 12, 175–185 (2001). [DOI] [PubMed] [Google Scholar]
- 106.Desmurget M. et al., Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat. Neurosci. 2, 563–567 (1999). [DOI] [PubMed] [Google Scholar]
- 107.Snyder L. H., Batista A. P., Andersen R. A., Coding of intention in the posterior parietal cortex. Nature 386, 167–170 (1997). [DOI] [PubMed] [Google Scholar]
- 108.Binkofski F. et al., Human anterior intraparietal area subserves prehension: A combined lesion and functional MRI activation study. Neurology 50, 1253–1259 (1998). [DOI] [PubMed] [Google Scholar]
- 109.Ariani G., Wurm M. F., Lingnau A., Decoding internally and externally driven movement plans. J. Neurosci. 35, 14160–14171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heimler B., Striem-Amit E., Amedi A., Origins of task-specific sensory-independent organization in the visual and auditory brain: Neuroscience evidence, open questions and clinical implications. Curr. Opin. Neurobiol. 35, 169–177 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Bi Y., Wang X., Caramazza A., Object domain and modality in the ventral visual pathway. Trends Cogn. Sci. 20, 282–290 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Cecchetti L., Kupers R., Ptito M., Pietrini P., Ricciardi E., Are supramodality and cross-modal plasticity the yin and yang of brain development? From blindness to rehabilitation. Front. Syst. Neurosci. 10, 89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Renier L., De Volder A. G., Rauschecker J. P., Cortical plasticity and preserved function in early blindness. Neurosci. Biobehav. Rev. 41, 53–63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ricciardi E., Bonino D., Pellegrini S., Pietrini P., Mind the blind brain to understand the sighted one! Is there a supramodal cortical functional architecture? Neurosci. Biobehav. Rev. 41, 64–77 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Pascual-Leone A., Hamilton R., The metamodal organization of the brain. Prog. Brain Res. 134, 427–445 (2001). [DOI] [PubMed] [Google Scholar]
- 116.Peelen M. V., Downing P. E., Category selectivity in human visual cortex: Beyond visual object recognition. Neuropsychologia 105, 177–183 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Bola Ł. et al., Task-specific reorganization of the auditory cortex in deaf humans. Proc. Natl. Acad Sci. U.S.A. 114, E600–E609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lomber S. G., What is the function of auditory cortex when it develops in the absence of acoustic input? Cogn. Dev. 42, 49–61 (2017). [Google Scholar]
- 119.Benetti S. et al., Functional selectivity for face processing in the temporal voice area of early deaf individuals. Proc. Natl. Acad. Sci. U.S.A. 114, E6437–E6446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cardin V. et al., Dissociating cognitive and sensory neural plasticity in human superior temporal cortex. Nat. Commun. 4, 1473 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Cardin V. et al., Monitoring different phonological parameters of sign language engages the same cortical language network but distinctive perceptual ones. J. Cogn. Neurosci. 28, 20–40 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Newman A. J., Supalla T., Fernandez N., Newport E. L., Bavelier D., Neural systems supporting linguistic structure, linguistic experience, and symbolic communication in sign language and gesture. Proc. Natl. Acad. Sci. U.S.A. 112, 11684–11689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Talairach J., Tournoux P., Co-Planar Stereotaxic Atlas of the Human Brain, (Thieme, New York, 1988). [Google Scholar]
- 124.Friston K. J., Holmes A. P., Worsley K. J., How many subjects constitute a study? Neuroimage 10, 1–5 (1999). [DOI] [PubMed] [Google Scholar]
- 125.Forman S. D. et al., Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn. Reson. Med. 33, 636–647 (1995). [DOI] [PubMed] [Google Scholar]
- 126.Friston K. J., Worsley K. J., Frackowiak R. S. J., Mazziotta J. C., Evans A. C., Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1, 210–220 (1994). [DOI] [PubMed] [Google Scholar]
- 127.Oosterhof N. N., Connolly A. C., Haxby J. V., CoSMoMVPA: Multi-modal multivariate pattern analysis of neuroimaging data in Matlab/GNU Octave. Front. Neuroinform. 10, 27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data are available upon request from the corresponding authors.