Huang et al. (1) describe a supposed mechanism of water extraction from gypsum by cyanobacteria sampled from endoliths inhabiting Ca sulfates in the Atacama Desert, and cultivated in the laboratory. The authors claim that the phase transformation from gypsum (CaSO4·2H2O) to anhydrite (CaSO4) (G→A) occurred under “dry conditions” in the contact zone between a “dry biofilm” and the gypsum, where only {011} planes of gypsum are transformed to anhydrite, supposedly providing water for cyanobacteria.
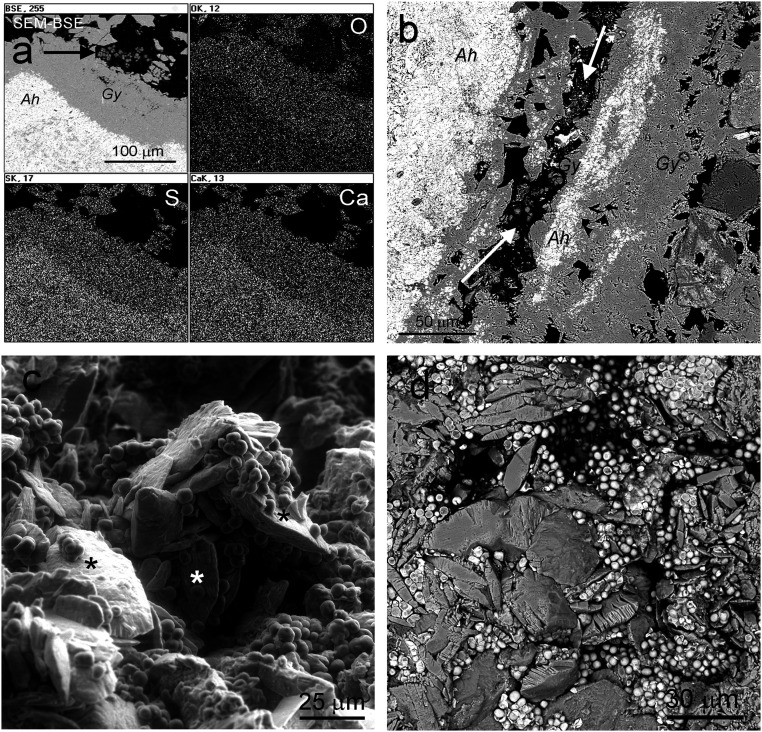
This work (1) has a number of major conceptual problems, as follows. First, the authors show the presence of gypsum and/or anhydrite in the inoculated Ca sulfate samples using X-ray diffraction and, incompletely, by Fourier transform infrared spectroscopy techniques (2), and not by Raman (3) or transmission electron microscopy. Selected area electron diffraction (SAED) data in ref. 1 do not allow any mineral identification, due to their differences with referenced reciprocal distances data (Table 1) for gypsum and anhydrite. Consequently, the authors neglect the fact that both gypsum and anhydrite phases were already included as natural components in the gypsum samples from the Tarapacá region (refs. 4 and 5 and Fig. 1 A and B). Second, their experimental design is not reproducible because the small Ca sulfate holders prepared for cultivated cyanobacteria would dissolve in 0.2 mL of aqueous medium. Third, even if the holders were bigger, the G→A transformation is theoretically supported by only one report (reference 20 in ref. 1), which demonstrates that the essential conditions required for the G→A transformation are the presence of 1.5 M H2SO4 (pH = 0.18) and a temperature of 80 °C (or higher). Obviously, these theoretical conditions are not met in the experiments described in ref. 1, and are unachievable within the dry (or moist) biofilm. Fourth, any dissociation and liberation of H+ is only possible in liquid water and not under “dry conditions.” Similarly, the supposed dissolution of gypsum in acid conditions is only possible in the liquid phase, and it is noteworthy that the pH of the BG11 medium is ±7.5. Fifth, the methodological and analytical approaches used in ref. 1 make absolutely impossible the detection of the supposed G→A transformation as a result of microbiological action. And sixth, the “preferred” attachment of “biofilms” to {011} gypsum planes was not supported by any statistical data. On the contrary, our results (Fig. 1 C and D) show that the attachment of cyanobacteria to different gypsum planes is actually randomized. Also, the concept of biofilm and its separation from cyanobacteria aggregates in ref. 1 is erroneous.
Table 1.
Comparison of theoretical d spacing corresponding to gypsum and anhydrite and those directly measured on figures 4 B, D, F, and H in Huang et al. (1)
| SAED patterns | Indicated | Theoretical d* | Measured | Δ Theoretical − Measured |
| Figure 4B | 021 gypsum | 0.428 | 0.570 | −0.142 |
| Figure 4D | n.d. anhydrite | 0.248 | 0.255 | −0.007 |
| Figure 4F | 200 anhydrite | 0.350 | 0.412 | −0.062 |
| Figure 4H | 200 anhydrite | 0.350 | 0.378 | −0.028 |
| Figure 4H | 002 anhydrite | 0.312 | 0.303 | +0.009 |
Not determined, n.d.
Reference codes 00-021-0816 and 00-033-0311 for gypsum and Inorganic Crystal Structure Database 016382 for anhydrite.
Fig. 1.
Scanning electron microscopy (SEM) images and energy-dispersive X-ray spectroscopy (EDS) maps from gypsum (Atacama Desert) colonized by endolithic microorganisms. (A) SEM in backscattered electron mode (SEM-BSE) contrast image of gypsum (Gy) and anhydrite (Ah) (Tarapacá region) and EDS distribution maps of oxygen, sulfur, and calcium, confirming the nature of these Ca sulfates; arrow, endoliths surrounded only by gypsum. (B) SEM-BSE contrast image of Gy and Ah (Tarapacá region); arrows, endoliths surrounded only by gypsum. (C) Environmental SEM image shows randomized attachment of cyanobacteria to the different gypsum planes; asterisks indicate {010} gypsum planes. (D) SEM-BSE image showing randomized oriented gypsum crystals surrounded by endolithic cyanobacteria, which are attached to all possible gypsum planes.
Contrary to the incorrect results and their invalid interpretation presented in ref. 1, our results (Fig. 1), and the results of previous works surprisingly not cited in ref. 1 in the context of their hypothesis (4–10), definitively confirm that the transformation of gypsum to anhydrite and the liberation of crystalline water in gypsum do not occur in the natural interface between gypsum and endolithic cyanobacteria. As an additional consequence, the authors (1) do not provide insights into potential adaptations of life on Mars.
Acknowledgments
This work was supported by the following grants: PGC2018-094076-B-I00 MCIU/AEI (Spain) and FEDER (UE) to J.W., O.A., and C.A.; ERC-CoG 818602 to A.G.F. and A.A.-B.; LM2018123-CzeCOS (Czech Republic) to P.V.; and RGY0066/2018 to A.A.-B.
Footnotes
The authors declare no competing interest.
References
- 1.Huang W. et al., Mechanism of water extraction from gypsum rock by desert colonizing microorganisms. Proc. Natl. Acad. Sci. U.S.A. 117, 10681–10687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad P. S. R., Krishna Chaitanya V., Shiva Prasad K., Rao D. N., Direct formation of the γ-CaSO4 phase in dehydration process of gypsum: In situ FTIR study. Am. Mineral. 90, 672–678 (2005). [Google Scholar]
- 3.Liu Y., Wang A., Freeman J. J., Raman, MIR, and NIR spectroscopic study of calcium sulfates: Gypsum, bassanite, and anhydrite. Lunar Planet. Sci. Conf. 40, 2128 (2009). [Google Scholar]
- 4.Wierzchos J. et al., Microbial colonization of Ca-sulfate crusts in the hyperarid core of the Atacama Desert: Implications for the search for life on Mars. Geobiology 9, 44–60 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Vítek P. et al., Phototrophic community in gypsum crust from the Atacama Desert studied by Raman spectroscopy and microscopic imaging. Geomicrobiol. J. 30, 399–410 (2013). [Google Scholar]
- 6.Dong H., Rech J. A., Jiang H., Sun H., Buck B. J., Endolithic cyanobacteria in soil gypsum: Occurrences in Atacama (Chile), Mojave (United States), and Al-Jafr Basin (Jordan) Deserts. J. Geophys. Res. 112, G02030 (2007). [Google Scholar]
- 7.DiRuggiero J. et al., Microbial colonisation of chasmoendolithic habitats in the hyper-arid zone of the Atacama Desert. Biogeosciences 10, 2439–2450 (2013). [Google Scholar]
- 8.Wierzchos J. et al., Adaptation strategies of endolithic chlorophototrophs to survive the hyperarid and extreme solar radiation environment of the Atacama Desert. Front. Microbiol. 6, 934 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vítek P., Ascaso C., Artieda O., Wierzchos J., Raman imaging in geomicrobiology: Endolithic phototrophic microorganisms in gypsum from the extreme sun irradiation area in the Atacama Desert. Anal. Bioanal. Chem. 408, 4083–4092 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Meslier V. et al., Fundamental drivers for endolithic microbial community assemblies in the hyperarid Atacama Desert. Environ. Microbiol. 20, 1765–1781 (2018). [DOI] [PubMed] [Google Scholar]