Significance
Consumption transfers energy and materials through food chains and fundamentally influences ecosystem productivity. Therefore, mapping the distribution of consumer feeding intensity is key to understanding how environmental changes influence biodiversity, with consequent effects on trophic transfer and top–down impacts through food webs. Our global comparison of standardized bait consumption in shallow coastal habitats finds a peak in feeding intensity away from the equator that is better explained by the presence of particular consumer families than by latitude or temperature. This study complements recent demonstrations that changes in biodiversity can have similar or larger impacts on ecological processes than those of climate.
Keywords: latitudinal gradients, trophic processes, seagrass, biogeography, macroecology
Abstract
The global distribution of primary production and consumption by humans (fisheries) is well-documented, but we have no map linking the central ecological process of consumption within food webs to temperature and other ecological drivers. Using standardized assays that span 105° of latitude on four continents, we show that rates of bait consumption by generalist predators in shallow marine ecosystems are tightly linked to both temperature and the composition of consumer assemblages. Unexpectedly, rates of consumption peaked at midlatitudes (25 to 35°) in both Northern and Southern Hemispheres across both seagrass and unvegetated sediment habitats. This pattern contrasts with terrestrial systems, where biotic interactions reportedly weaken away from the equator, but it parallels an emerging pattern of a subtropical peak in marine biodiversity. The higher consumption at midlatitudes was closely related to the type of consumers present, which explained rates of consumption better than consumer density, biomass, species diversity, or habitat. Indeed, the apparent effect of temperature on consumption was mostly driven by temperature-associated turnover in consumer community composition. Our findings reinforce the key influence of climate warming on altered species composition and highlight its implications for the functioning of Earth’s ecosystems.
Latitudinal diversity gradients have stimulated decades of research, much of it invoking a decline from tropics to poles in rates of key biological processes and species interactions (1–3). General explanations for ecological patterns across latitude, however, remain elusive in part because so many environmental and biological variables change in parallel with latitude. As a result, the connections between ecological patterns and processes at global scales remain controversial (4–6). This uncertainty has recently been amplified by demonstrations that diversity of many modern and ancient lineages peaks at midlatitudes rather than at the equator, particularly in the ocean (7, 8).
Temperature is among the environmental factors that vary most consistently with latitude, and is a fundamental driver of biological processes. Metabolic theory mechanistically links environmental temperatures to a suite of biological processes, including metabolism and trophic transfer (9–12). For example, metabolic theory predicts that per-capita consumption rates of ectothermic consumers should follow increased metabolic needs and activity, and increase with rising temperature (13). But the traits of consumers, their abundance, and the resources available to them also change with temperature (14, 15), so total consumption rates may be poorly predicted by temperature alone. Separating these effects requires data on geographic variation in consumption.
Our understanding of global variation in top–down processes in marine systems is largely indirect, based on inferences from distributions of organismal traits such as body size and morphological defenses (e.g., refs. 2 and 16) and on comparisons of primary production, prey abundance, and predator abundance (e.g., refs. 17 and 18). The distribution and abundance of species respond to ecosystem productivity, reproductive rate, migration, mortality, and evolutionary history, all of which are modified by temperature (e.g., refs. 19 and 20). But while the spatial distribution of primary production and human predation (fishing) is well-documented (21, 22), we have only sparse empirical measurements of geographic variation in consumption by natural predators, which are needed to predict trophic transfer and prey abundance. Thus, we lack a global map linking the central ecological process of consumption to temperature and other drivers.
We approached this problem by measuring feeding intensity of generalist marine consumers across 42 sites around the globe representing two widespread coastal habitats: seagrass and unvegetated sediments. Seagrasses provide shelter and fuel primary and secondary production (23–25), and seagrass persistence is in turn linked to trophic processes, as midlevel carnivores consume herbivores that can facilitate or damage seagrass (26–30). Therefore, understanding consumption by midlevel predators is key to seagrass conservation and restoration efforts (30).
To compare consumption rigorously around the world’s coasts, we used a simple, standardized feeding assay, offering small (∼1 cm) discs of dried squid mantle as bait. Squid is attractive to many generalist marine predators, including midsized fishes and crustaceans, which we surveyed in both seagrass and unvegetated habitats. Importantly, standardizing bait allowed us to estimate consumption rates on a comparable basis while avoiding confounding influences of geographic variation in prey type, prey behavior, and prey defenses. Previous studies (e.g., refs. 31 and 32) have shown changes in feeding on standardized prey across latitude, but ours covers nearly the entire latitudinal range of seagrasses on four continents and multiple ocean basins in both the Northern and Southern Hemispheres (our study, 38°S to 67°N; seagrass, 45°S to 70°N). This allowed us to test the consistency of latitudinal gradients in consumption in two widespread habitats, and to begin disentangling the role of correlated drivers. Based on previous studies documenting broad-scale patterns in biodiversity (3, 33, 34), prey defense (2), trophic interactions (5, 35, 36), and metabolism (12), we hypothesized that rates of bait consumption would increase with temperature toward the equator.
Results and Discussion
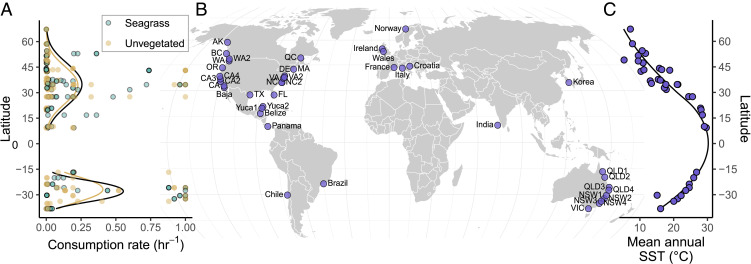
Contrary to our expectations, bait consumption peaked away from the equator in both hemispheres (25 to 35° north and south) and was consistent between seagrass and sediment habitats (Fig. 1A), despite slightly higher density, richness, and biomass of consumers in seagrass (SI Appendix, Fig. S1). Although our sampling near the equator was relatively sparse, the decline in measured bait consumption at the lowest latitudes was robust in two independent regions (SI Appendix, Fig. S2). This midlatitude peak was supported by a hump-shaped relationship between absolute latitude and consumption (comparison of models with and without a second-order polynomial; quadratic model Akaike weight wquad = 0.86). In contrast, satellite-derived mean annual sea surface temperature (SST) decreased monotonically with latitude (Fig. 1C), and the hump-shaped relation of SST to consumption was much stronger than that for latitude (wquad > 0.99). This nonlinear relationship between SST and consumption was also supported in two independent, well-sampled transects along the northwest Atlantic (wquad = 0.97) and southwest Pacific (wquad = 0.99) (SI Appendix, Fig. S2), strengthening the inference that the equatorial dip in consumption reflects a response to temperature, rather than some other correlate of latitude.
Fig. 1.
Distributions of bait consumption by generalist marine predators and temperature across the 42 sites in this study. (A) Consumption rate of tethered dried squid bait peaks at midlatitudes in both hemispheres. Point color represents habitat, and lines show independent quadratic generalized linear models fitted for each habitat type in each hemisphere. (B) Map of study sites. (C) Latitudinal pattern of mean annual SST.
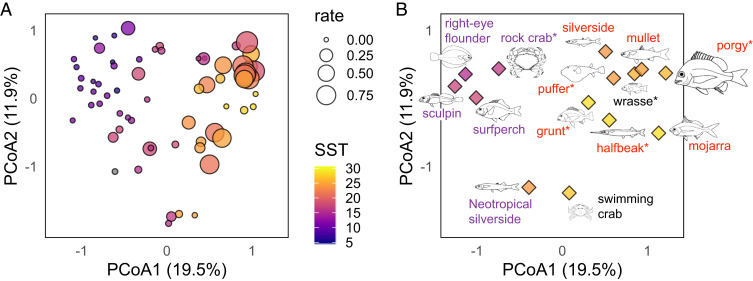
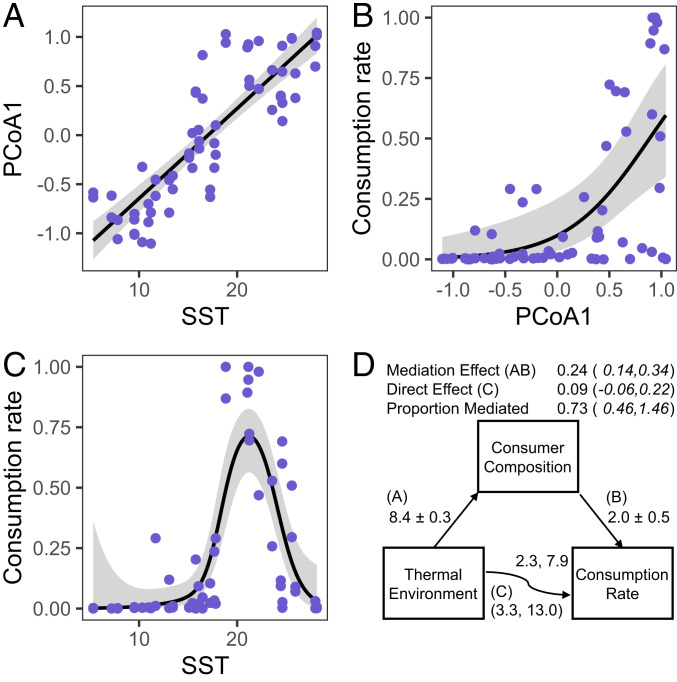
Fish and crabs were the main consumers of bait at all sites, and their taxonomic composition varied greatly across sites and with temperature (Fig. 2). Because species and genera of coastal animals differ markedly across ocean basins and hemispheres, we analyzed consumer composition at the level of taxonomic families, which allowed us to describe gradual shifts in global consumer biogeography across broad environmental gradients, while maintaining functional distinction among taxa. We used presence vs. absence rather than abundance data because we wanted to explore consumer composition and abundance separately. Mean annual sea surface temperature alone explained 16% of the dissimilarity in fish and crustacean assemblages across sites (canonical analysis coefficient 0.13, P = 0.001). However, a single unconstrained index of compositional dissimilarity (principal-coordinate analysis [PCoA] axis 1 in Fig. 2A) explained 19.5% of the total variation in consumer composition, and separated cool-water assemblages from warm assemblages (37). Indeed, this index of consumer composition was a stronger predictor of bait consumption than water temperature (either measured during the assays or using mean annual sea surface temperatures; SI Appendix, Fig. S3), latitude, consumer density and biomass, or estimates of ocean productivity, fishing pressure, or human population density (Table 1). Consumer density and biomass only became important predictors of consumption once we reduced the dataset to include only those consumer families whose presence was associated with increased consumption rates (Materials and Methods and SI Appendix, Table S1). When viewed as a simple network of causal relationships, the effect of thermal environment on consumption rate was largely indirect, being mediated by consumer community composition, and this remained true even when allowing for a nonlinear relationship between consumption rate and mean annual sea surface temperature (Fig. 3). Roughly three-quarters of the total effect of thermal environment on bait consumption flowed indirectly through differences in consumer taxa in different climates.
Fig. 2.
Composition of consumer assemblages reflects global gradients in environmental temperatures and consumption rate. (A) Principal-coordinate analysis, where locations of symbols reflect compositional differences among sites and habitats based on family-level presence–absence data. Symbol color represents mean annual sea surface temperature (°C), and symbol size corresponds to bait consumption rate. (B) The same ordination showing scores for consumer families driving differences in composition and consumption rate among sites. Symbol color represents average in situ temperature at sites where the predator family was observed; label color represents positive (red), negative (purple), or nonsignificant (black) correlations with consumption rate; and body length (width for crabs) is proportional to the magnitude of the correlation. Asterisks denote families that were seen feeding on bait in video footage.
Table 1.
Comparison of generalized linear mixed-effects models predicting bait consumption by generalist consumers in two shallow marine habitats
| Candidate model | K | AICc | ΔAICc | wi | R2 |
| Taxonomic composition | 3 | 70.3 | 0 | 0.932 | 0.51 |
| Selected abundance | 3 | 77.0 | 6.7 | 0.033 | 0.39 |
| Sea surface remperature2 | 4 | 79.2 | 8.9 | 0.011 | 0.66 |
| Selected biomass | 3 | 79.6 | 9.3 | 0.009 | 0.38 |
| Functional richness | 3 | 79.9 | 9.5 | 0.008 | 0.26 |
| Sea surface temperature | 3 | 80.5 | 10.2 | 0.006 | 0.39 |
| In situ temperature | 3 | 82.8 | 12.5 | 0.002 | 0.31 |
| Productivity | 3 | 90.5 | 20.2 | <0.001 | 0.12 |
| Proportion of active foragers | 3 | 90.5 | 20.2 | <0.001 | 0.10 |
| Body size | 3 | 90.6 | 20.3 | <0.001 | 0.10 |
| Consumer species richness | 3 | 90.8 | 20.5 | <0.001 | 0.05 |
| Functional evenness | 3 | 91.4 | 21.1 | <0.001 | 0.04 |
| Intercept-only | 2 | 92.0 | 21.7 | <0.001 | 0 |
| Trophic group | 6 | 92.1 | 21.8 | <0.001 | 0.27 |
| Functional group richness | 3 | 92.2 | 21.9 | <0.001 | 0.04 |
| Feeding type | 3 | 92.4 | 22.1 | <0.001 | 0.15 |
| Habitat | 3 | 93.1 | 22.8 | <0.001 | 0.01 |
| Lateral body shape | 6 | 93.1 | 22.8 | <0.001 | 0.23 |
| Total biomass | 3 | 93.2 | 22.9 | <0.001 | 0.02 |
| Effective number of species | 3 | 93.2 | 22.9 | <0.001 | 0.01 |
| Total abundance | 3 | 93.3 | 23.0 | <0.001 | 0.02 |
| Fishing pressure | 3 | 93.6 | 23.3 | <0.001 | 0.02 |
| Water column use | 6 | 93.7 | 23.4 | <0.001 | 0.20 |
| Human population density | 3 | 94.0 | 23.7 | <0.001 | <0.01 |
| Rao Q | 3 | 94.0 | 23.7 | <0.001 | <0.01 |
| Functional dispersion | 3 | 94.0 | 23.7 | <0.001 | <0.01 |
Taxonomic composition refers to the first axis from the PCoA of consumer assemblages (Fig. 2). Selected abundance and biomass refer to density or biomass of fish and decapod families selected through constrained ordination (Fig. 2B and SI Appendix, Table S1). Productivity refers to remotely sensed mean annual chlorophyll a. Habitat categorically relates seagrass and unvegetated habitats. Abundance, biomass, and human population density were log10-transformed. Body size, trophic group, lateral body shape, and water column use are community-weighted mean trait values by site and habitat. We provide marginal pseudo-R2 values for comparison of fixed effects. For model comparison, we only included data from sites with the full complement of predictors (27 of 42 sites).
Fig. 3.
Predator composition mediates the effect of thermal environment on consumption rates. (A–C) Bivariate relationships between consumer composition (PCoA1, Fig. 2A), thermal environment (SST), and consumption rate. Lines show predictions from models used in mediation analysis (A, linear regression; B, logistic regression; C, generalized additive modeling). (D) Paths represent causal hypotheses about relationships. Numbers next to paths leading to and from consumer composition are standardized regression coefficients and SEs. Numbers above and below the path from thermal environment to consumption rate are estimated degrees of freedom and χ2 values for the smooth term in the presence and absence of mediation, respectively. Numbers above the path diagram are estimates of the direct and indirect (mediation) effects with 95% bootstrapped CIs.
Locations with high consumption rates had consumer assemblages composed largely of invertivores and omnivores that actively forage on or just above the seafloor (SI Appendix, Figs. S3 and S4). Actively swimming foragers should consume bait faster due to increased encounter rates, all else being equal, and arguably consumption by these foragers might rise more rapidly with temperature than for more sedentary taxa. Video evidence confirmed the association of key families with high consumption. Porgies (family Sparidae), for example, removed bait at every site where they were observed in video footage (SI Appendix, Table S2) and the presence of this family showed the strongest association with consumption rate in our analysis of community composition (Fig. 2B and SI Appendix, Table S1).
The equatorial decline in bait consumption appears to be related to consumer community composition, as many of the actively foraging taxa associated with high consumption rates, including porgies, half-beaks (Hemiramphidae), and grunts (Haemulidae), were rare or absent at the sites closest to the equator (SI Appendix, Fig. S5). Some of these consumer families (e.g., porgies) are known from low-latitude waters but were not recorded in our surveys (38). It is possible that larger enemies reduce mesopredator abundance or restrict their foraging (39, 40) to a greater extent at low latitudes, but we do not have any direct evidence to support this hypothesis. Similarly, human harvest or other activities could have restricted the abundance of these key consumers, but we know of no reason to expect this to be more intense at low latitudes, as many of the mid- and high-latitude sites in this study are heavily influenced by human activities, including overfishing (41). Alternatively, environmental tolerances could limit consumer access or abundance in shallow seagrass habitats at low latitude (42). There is currently little evidence to evaluate these explanations.
Our finding that feeding intensity peaked at midlatitudes differs strongly from most previous studies on latitudinal gradients in species interactions (5, 12, 31, 36). Nonlinear ecological transitions between warm-temperate and subtropical locations might help explain this result. These regions feature rapid transition between thermal guilds of consumers with cool- vs. warm-water affinities (37) and these biogeographic transitions are correlated with shifts in the relative strength of bottom–up vs. top–down processes that are directly and indirectly related to temperature (17). We find it interesting that such transitions co-occur in similar climatic regions: transitions in consumption from this study (∼19 to 22 °C SST), transitions in dominant fish guilds [∼21 to 25 °C SST (37)], and transitions in top–down vs. bottom–up control [∼17 to 20 °C temperature 0 to 200 m (17)]. These comparisons suggest that zones of biogeographic and trophic transitions associated with climate are also areas of transition for consumptive pressure by small mesopredators.
The weak and inconsistent differences we found in consumption rates between seagrass and unvegetated sediment habitats (SI Appendix, Figs. S6 and S7) was surprising given decades of research showing that the structure provided by seagrasses and other foundation species can strongly influence predator–prey relationships (43–46). While we found no overall difference in consumer composition between seagrass and unvegetated habitats (permutation test, P = 0.75), consumer densities were generally higher inside than outside of seagrass habitat (SI Appendix, Fig. S1). Thus, any protection provided by seagrass structure may have been offset by consumer aggregation in seagrass. Yet the consistency of latitudinal patterns in consumption between the two habitats suggests that broad-scale environment and consumer biogeography had stronger influences on consumption than local differences in habitat structure.
Our feeding assay used identical bait at all sites to isolate the effect of consumer activity from the behavioral and morphological traits of prey, which vary widely across space. No single bait will attract all predators equally; ours targeted the small- to medium-sized generalists that dominate many shallow marine habitats. Thus, the consumption rates that we describe are relative measures of one-half of a predator–prey interaction (i.e., consumption in the absence of prey behavior and other trait variation). Whereas this design cannot completely characterize species interactions, standardization more rigorously estimates how potential consumption varies across the globe. Our assays did not measure top–down control per se, but the kind of information we gathered is critical to understanding trophic interactions, including cascading effects in seagrass ecosystems (30, 47, 48), because it measures the willingness of consumers to eat prey of a certain size. The consistency of our results across ocean basins and hemispheres, along with similar recent findings for pelagic top predators (49), suggests that the midlatitude peak in marine consumption is indeed general. The importance of particular predator taxa and traits in the geography of consumption we found parallels the outsized role of endothermy in the effectiveness of marine predators (e.g., refs. 50 and 51), including in some seagrass meadows (47, 48). We focused on the smaller ectothermic consumers that consume herbivorous invertebrates that can be critical to seagrass persistence (30), but these are potential prey of larger endotherms like fishing birds and small marine mammals, so endothermy might well influence the broader food webs we studied. However, given that many endothermic predators are most abundant and diverse in cooler regions of the world’s oceans (51), we would expect the distribution of their collective feeding intensity to differ from the pattern we observed.
Changing climate, overfishing, and global species introductions are altering the biogeography of marine life and the composition of communities (52, 53), with wide-ranging effects on ecosystems (54), including in seagrass habitats (55). Shifting biogeography of consumers can alter community and ecosystem structure and processes (56, 57) independent of temperature, as we show here. Simultaneously, warming can directly influence the physiology of ectothermic consumers [e.g., metabolic demand, activity (58)]. We show that spatial variation in water temperature influences marine trophic processes mainly indirectly by changing consumer community composition. The hump-shaped relationship between temperature and consumption we found suggests that predation and trophic transfer may intensify at middle to high latitudes and decline near the equator as the world’s oceans warm and species continue to shift their ranges. Such shifts in species ranges and biomass distributions could lead to large changes in consumption, with repercussions for community structure and trophic flows through marine food webs. It is already clear that many ectotherms are expanding or contracting their ranges with climate change (59, 60). Our findings suggest that such distributional shifts may affect ecological processes as much or more than those predicted based only on temperature effects on metabolism.
Materials and Methods
We assessed rates of consumption using a simple, standardized field assay (61). We tethered a 1- to 1.3-cm-diameter piece of dried squid mantle with monofilament to a fiberglass garden stake (hereafter, “squidpop”) that we inserted into the sediment such that the bait dangled 20 to 30 cm above the sediment surface in or just above the seagrass canopy. At most sites, we deployed 20 to 30 squidpops within a seagrass meadow and 20 to 30 squidpops in nearby unvegetated sediments (SI Appendix, Table S3). We checked the squidpops for the presence (1) or absence (0) of bait after 1 h and again after 24 h. Most sites repeated this assay for a total of three deployments in each of the two habitat types, and measured water temperature during each deployment.
To characterize variation in environments across the range of the study, we drew upon several publicly available datasets with global-scale variables of interest. We accessed sea surface temperature and chlorophyll records using Bio-ORACLE (62), which packages data collected by the Aqua-MODIS satellite. We used mean annual SST because it showed stronger relationships with consumption rate than maximum or minimum annual SST and it summarizes well the differences between thermal conditions across the globe (Fig. 1). Most assays were conducted during the summer, but differences in timing of assays generated variation in in situ temperature that altered the rank order of our estimates of the thermal environments compared with sea surface temperature, making nearby sites appear less similar environmentally (Fig. 3). We used mean annual chlorophyll a as a proxy for surface ocean primary productivity across sites. We also accessed data on human population densities from the Gridded Population of the World (63), which we used as a proxy for local human disturbance. Finally, we accessed fishing pressure data from the Sea Around Us project (64) using the R package seaaroundus (65).
At most sites (30 of 42), we also conducted consumer surveys in the areas adjacent to feeding assays. These surveys used hand-pulled seines in seagrass and unvegetated sediment habitats to sample epibenthic consumers (mainly fishes, but also large crustaceans) adjacent to feeding assays. All consumers were identified, counted, and released. The total lengths of the first 25 individual fish of each species were also measured. We used these data to estimate consumer density, size distribution, biomass, and diversity, as well as to generate a species list for each site. Species lists from five additional sites were added using data from video footage and diver transects (FL, India, Italy, Yuca1, Yuca2; Movie S1). Biomass estimates were calculated using length–weight regressions available in FishBase (66).
For each of the squidpop assays, we independently fitted an exponential decay model and estimated consumption rate (bait loss through time) using the slope parameter. We then used the resulting rate estimates as data points in subsequent analysis.
We predicted individual consumption rates in generalized linear mixed-effects models (logit link, random intercepts for sites) using a variety of potential abiotic and biotic drivers, and compared models using the Akaike information criterion corrected for small sample size (AICc) calculated using the R package bbmle (67). We also explored a variety of polynomial terms and LOESS (locally estimated scatterplot smoothing) curves to investigate possibilities of nonlinear relationships between temperature and consumption, although for model comparison we only included linear terms. We restricted the data used in model comparison to the 27 sites for which we had the full complement of explanatory variables. For simplicity, and because our analysis was largely exploratory using a large set of candidate explanatory variables, we compared models with individual predictor variables only. All mixed models were fitted using maximum likelihood in the package lme4 in R (68).
When estimating consumer species (alpha) diversity, we used both species richness and Hurlbert’s probability of interspecific encounter as effective numbers of species (69). We also wanted to investigate changes in consumer community composition across sites (beta diversity), but given the scale of our analysis and the large biogeographic gradients we captured, comparing composition in terms of species identity was not possible. Species-level overlap was low among sites, especially across ocean basins and hemispheres, so we chose to compare composition (presence–absence) at the level of families across sites using Raup–Crick dissimilarities. While this metric has been used to investigate small spatial scale differences in species composition within regions (70), we use it here to investigate global among-site turnover of consumers at higher taxonomic levels. In order to visualize and quantify major axes of community variation, we used principal-coordinate analysis to ordinate consumer communities based on their dissimilarities, and then assess how these dissimilarities related to the thermal environment and consumption rate. We used a combination of unconstrained (PCoA) and constrained (canonical analysis of principal coordinates) techniques in this analysis. Unconstrained ordination reduces dimensionality of the dataset by finding orthogonal axes of decreasing variation in the dataset, while constrained ordination uses a regression-based approach to define a set of axes of interest a priori based on explanatory variables (71). We used the resultant axes from unconstrained ordination (PCoA) as explanatory variables in the models described above because the unconstrained ordination does not require a priori assumptions about which factors are important. We also assessed relationships between consumer community composition and thermal environment by constraining the first ordination axis to SST or in situ temperature. Multivariate analyses were performed using the R package vegan (72).
In order to identify which consumer families were positively and negatively associated with consumption intensity across sites, we constrained the first axis of the ordination to align with our estimates of consumption rate. Then we selected families that mapped onto the positive side of this axis as candidate taxa driving spatial variation in consumption rate, and calculated the density and biomass of these consumers at sites with seining data (27 of 42 sites). Finally, we compared the results from multivariate analysis to direct observations of squidpop attacks and bait removal from video footage captured at 14 sites (SI Appendix, Table S2).
To explore which predator traits might explain feeding intensity in our assays, we scored six traits for each taxon in our dataset (416 morphospecies in 103 taxonomic families). Four traits were derived from FishBase [feeding habit, lateral body shape (73)] and the Reef Life Survey [trophic group, water column usage (74)]. A fifth binomial trait scored whether each taxon is an actively swimming forager or tends toward ambush or sit-and-wait behavior, either on the benthos or in the water column. We applied the most common value of this trait to all taxa in each family, but we acknowledge that variation in foraging activity can occur within families. Traits missing in these databases were filled using expert opinion of coauthors and available trait information from related taxa. A sixth continuous trait describing body size as the average total length of each taxon (carapace width for crabs) was calculated from our seining data. Whereas published total length estimates are available for many taxa, we opted to use length estimates from our own dataset because many taxa only utilize seagrass and other nearshore habitats for part of their development, when they may differ greatly from the species’ maximum size. Using the R package FD (75), we calculated community-level weighted means of trait values to derive estimates of average conditions for each of the six individual traits in each site and habitat combination in the dataset, and we calculated a variety of functional diversity metrics (functional richness, functional dispersion, functional evenness, functional diversity, and Rao’s Q) following published methods (75–77). For all consumer functional diversity metrics and all community-level weighted means except body size, we used presence–absence data instead of weighting by relative abundance so that we could include sites with seining and video data. We did weight mean consumer body size estimates by relative abundance because we only had size estimates from seine sampling. Weighting by abundance did not qualitatively change the results. We regressed each functional diversity metric and each community-level weighted mean trait against consumption rate individually using the linear mixed-effects models described above.
We tested whether consumer composition mediated the influence of mean annual SST on consumption rates using the package mediation in R (78). Because we found support for a hump-shaped relationship between SST and consumption rate, we tested whether consumer composition mediated the nonlinear relationship between temperature and consumption rate (using 33 of 42 sites with all three variables). We modeled the relationships using 1) smooth terms for SST on consumption rate and a linear term for composition (PCoA1) on consumption rate in a generalized additive model [GAM; logit link function; R package mgcv (79)] and 2) a generalized linear model for SST on composition. We report the standardized linear regression coefficients, estimated degrees of freedom for smoothed GAM terms (and associated χ2 statistic), estimates of the mediation effect and direct effect, and the proportion of the direct effect of SST mediated by composition for the second mediation analysis, along with 95% CIs around estimates of the direct effect, mediation effect, and proportion mediated. All models in mediation analysis used data that were averaged at the level of habitats within sites, which is the lowest level of pairwise comparisons we can make between squidpop assays and consumer composition.
All analyses were performed in R version 3.5.3 (80). Data and analyses for this project are available at https://doi.org/10.5281/zenodo.3998836.
Supplementary Material
Acknowledgments
We thank M. I. O’Connor, J. R. Bernhardt, and P. L. Thompson for helpful reviews of early versions of the manuscript. We are grateful for three anonymous reviewers who greatly improved the paper and for G. J. Vermeij, who provided several rounds of thoughtful review and suggested that high-level consumers might depress mesopredator consumption at low latitudes. We acknowledge funding from the Smithsonian Institution and the Tula Foundation. This is Contribution 63 from the Smithsonian’s MarineGEO and Tennenbaum Marine Observatories Network.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005255117/-/DCSupplemental.
Data Availability.
The original data reported in this paper (.csv files) have been deposited in Dryad (https://doi.org/10.5061/dryad.8cz8w9gnm) (81), and the code (R scripts) used to process and analyze the data have been deposited in Zenodo (https://doi.org/10.5281/zenodo.3998836).
References
- 1.Dobzhansky T., Evolution in the tropics. Am. Sci. 38, 208–221 (1950). [Google Scholar]
- 2.Vermeij G. J., Evolution and Escalation: An Ecological History of Life, (Princeton University Press, 1993). [Google Scholar]
- 3.Krug A. Z., Jablonski D., Valentine J. W., Roy K., Generation of Earth’s first-order biodiversity pattern. Astrobiology 9, 113–124 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Levin S. A., The problem of pattern and scale in ecology: The Robert H. MacArthur Award Lecture. Ecology 73, 1943–1967 (1992). [Google Scholar]
- 5.Schemske D. W., Mittelbach G. G., Cornell H. V., Sobel J. M., Roy K., Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269 (2009). [Google Scholar]
- 6.Moles A. T., Bonser S. P., Poore A. G. B., Wallis I. R., Foley W. J., Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct. Ecol. 25, 380–388 (2011). [Google Scholar]
- 7.Powell M. G., Beresford V. P., Colaianne B. A., The latitudinal position of peak marine diversity in living and fossil biotas: Latitudinal position of peak marine diversity. J. Biogeogr. 39, 1687–1694 (2012). [Google Scholar]
- 8.Chaudhary C., Saeedi H., Costello M. J., Bimodality of latitudinal gradients in marine species richness. Trends Ecol. Evol. 31, 670–676 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L., Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B., Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004). [Google Scholar]
- 11.Mittelbach G. G. et al., Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Schramski J. R., Dell A. I., Grady J. M., Sibly R. M., Brown J. H., Metabolic theory predicts whole-ecosystem properties. Proc. Natl. Acad. Sci. U.S.A. 112, 2617–2622 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor M. I., Gilbert B., Brown C. J., Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. Am. Nat. 178, 626–638 (2011). [DOI] [PubMed] [Google Scholar]
- 14.O’Connor M. I., Piehler M. F., Leech D. M., Anton A., Bruno J. F., Warming and resource availability shift food web structure and metabolism. PLoS Biol. 7, e1000178 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dell A. I., Pawar S., Savage V. M., Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J. Anim. Ecol. 83, 70–84 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Harfoot M. B. J. et al., Emergent global patterns of ecosystem structure and function from a mechanistic general ecosystem model. PLoS Biol. 12, e1001841 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyce D. G., Frank K. T., Worm B., Leggett W. C., Spatial patterns and predictors of trophic control in marine ecosystems. Ecol. Lett. 18, 1001–1011 (2015). [DOI] [PubMed] [Google Scholar]
- 18.van Denderen P. D., Lindegren M., MacKenzie B. R., Watson R. A., Andersen K. H., Global patterns in marine predatory fish. Nat. Ecol. Evol. 2, 65–70 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Bernhardt J. R., Sunday J. M., Thompson P. L., O’Connor M. I., Nonlinear averaging of thermal experience predicts population growth rates in a thermally variable environment. Proc. Biol. Sci. 285, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauly D., On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 39, 175–192 (1980). [Google Scholar]
- 21.Field C. B., Behrenfeld M. J., Randerson J. T., Falkowski P., Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 281, 237–240 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Watson R. A. et al., Global marine yield halved as fishing intensity redoubles. Fish Fish. 14, 493–503 (2013). [Google Scholar]
- 23.Cebrian J., Patterns in the fate of production in plant communities. Am. Nat. 154, 449–468 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Williams S. L., Heck K. L. Jr., “Seagrass community ecology” in Marine Community Ecology, Bertness M. D., Gaines S. D., Hay M. E., Eds. (Sinauer Associates, 2001), pp. 317–337. [Google Scholar]
- 25.Sievers M. et al., The role of vegetated coastal wetlands for marine megafauna conservation. Trends Ecol. Evol. 34, 807–817 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Valentine J. F., Duffy J. E., “The central role of grazing in seagrass ecology” in Seagrasses: Biology, Ecology and Conservation, Larkum A. W. D., Orth R. J., Duarte C. M., Eds. (Springer Netherlands, 2006), pp. 463–501. [Google Scholar]
- 27.Duffy J. E., Richardson J. P., France K. E., Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecol. Lett. 8, 301–309 (2005). [Google Scholar]
- 28.Baden S. P., Emanuelsson A., Pihl L., Svensson C.-J., Åberg P., Shift in seagrass food web structure over decades is linked to overfishing. Mar. Ecol. Prog. Ser. 451, 61–73 (2012). [Google Scholar]
- 29.Reynolds P. L., Richardson J. P., Duffy J. E., Field experimental evidence that grazers mediate transition between microalgal and seagrass dominance. Limnol. Oceanogr. 59, 1053–1064 (2014). [Google Scholar]
- 30.Duffy J. E., Hughes A. R., Moksnes P.-O., “Ecology of seagrass communities” in Marine Community Ecology and Conservation, Bertness M. D., Bruno J. F., Silliman B. R., Stachowicz J. J., Eds. (Sinauer Associates, ed. 2, 2013), pp. 271–297. [Google Scholar]
- 31.Reynolds P. L. et al., Latitude, temperature, and habitat complexity predict predation pressure in eelgrass beds across the Northern Hemisphere. Ecology 99, 29–35 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Musrri C. A. et al., Variation in consumer pressure along 2500 km in a major upwelling system: Crab predators are more important at higher latitudes. Mar. Biol. 166, 142 (2019). [Google Scholar]
- 33.Mannion P. D., Upchurch P., Benson R. B. J., Goswami A., The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. 29, 42–50 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Worm B., Tittensor D. P., A Theory of Global Biodiversity, (Princeton University Press, 2018). [Google Scholar]
- 35.Freestone A. L., Osman R. W., Ruiz G. M., Torchin M. E., Stronger predation in the tropics shapes species richness patterns in marine communities. Ecology 92, 983–993 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Roslin T. et al., Higher predation risk for insect prey at low latitudes and elevations. Science 356, 742–744 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Stuart-Smith R. D., Edgar G. J., Barrett N. S., Kininmonth S. J., Bates A. E., Thermal biases and vulnerability to warming in the world’s marine fauna. Nature 528, 88–92 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Carpenter K. E., “The Living Marine Resources of the Western Central Atlantic. Volume 3: Bony Fishes Part 2 (Opistognathidae to Molidae), Sea Turtles and Marine Mammals” in FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5, (FAO, 2002), pp. 1375–2127. [Google Scholar]
- 39.Micheli F., Peterson C. H., Estuarine vegetated habitats as corridors for predator movements. Conserv. Biol. 13, 869–881 (1999). [Google Scholar]
- 40.Madin E. M. P. et al., Multi-trophic species interactions shape seascape-scale coral reef vegetation patterns. Front. Ecol. Evol. 7, 102 (2019). [Google Scholar]
- 41.Eriksson B. K. et al., Effects of altered offshore food webs on coastal ecosystems emphasize the need for cross-ecosystem management. Ambio 40, 786–797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunday J. M., Bates A. E., Dulvy N. K., Global analysis of thermal tolerance and latitude in ectotherms. Proc. Biol. Sci. 278, 1823–1830 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hovel K. A., Lipcius R. N., Habitat fragmentation in a seagrass landscape: Patch size and complexity control blue crab survival. Ecology 82, 1814–1829 (2001). [Google Scholar]
- 44.Orth R. J., Heck K. L. Jr., van Montfrans J., Faunal communities in seagrass beds: A review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7, 339–350 (1984). [Google Scholar]
- 45.Main K. L., Predator avoidance in seagrass meadows: Prey behavior, microhabitat selection, and cryptic coloration. Ecology 68, 170–180 (1987). [Google Scholar]
- 46.Mattila J. et al., Increased habitat structure does not always provide increased refuge from predation. Mar. Ecol. Prog. Ser. 361, 15–20 (2008). [Google Scholar]
- 47.Hughes B. B. et al., Recovery of a top predator mediates negative eutrophic effects on seagrass. Proc. Natl. Acad. Sci. U.S.A. 110, 15313–15318 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang A. C., Essak M., O’Connor M. I., Top–down control by great blue herons Ardea herodias regulates seagrass‐associated epifauna. Oikos 124, 1492–1501 (2015). [Google Scholar]
- 49.Roesti M. et al., Pelagic fish predation is stronger at temperate latitudes than near the equator. Nat. Commun. 11, 1527 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe Y. Y., Goldman K. J., Caselle J. E., Chapman D. D., Papastamatiou Y. P., Comparative analyses of animal-tracking data reveal ecological significance of endothermy in fishes. Proc. Natl. Acad. Sci. U.S.A. 112, 6104–6109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grady J. M. et al., Metabolic asymmetry and the global diversity of marine predators. Science 363, eaat4220 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Pinsky M. L., Worm B., Fogarty M. J., Sarmiento J. L., Levin S. A., Marine taxa track local climate velocities. Science 341, 1239–1242 (2013). [DOI] [PubMed] [Google Scholar]
- 53.McCauley D. J. et al., Marine defaunation: Animal loss in the global ocean. Science 347, 1255641 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Estes J. A. et al., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Hyndes G. A. et al., Accelerating tropicalization and the transformation of temperate seagrass meadows. Bioscience 66, 938–948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeidberg L. D., Robison B. H., Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern north Pacific. Proc. Natl. Acad. Sci. U.S.A. 104, 12948–12950 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Côté I. M., Green S. J., Hixon M. A., Predatory fish invaders: Insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biol. Conserv. 164, 50–61 (2013). [Google Scholar]
- 58.Sinclair B. J. et al., Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372–1385 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Sagarin R. D., Barry J. P., Gilman S. E., Baxter C. H., Climate-related change in an intertidal community over short and long time scales. Ecol. Monogr. 69, 465–490 (1999). [Google Scholar]
- 60.Sanford E., Sones J. L., García-Reyes M., Goddard J. H. R., Largier J. L., Widespread shifts in the coastal biota of northern California during the 2014-2016 marine heatwaves. Sci. Rep. 9, 4216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duffy J. E., Ziegler S. L., Campbell J. E., Bippus P. M., Lefcheck J. S., Squidpops: A simple tool to crowdsource a global map of marine predation intensity. PLoS One 10, e0142994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyberghein L. et al., Bio-ORACLE: A global environmental dataset for marine species distribution modelling: Bio-ORACLE marine environmental data rasters. Glob. Ecol. Biogeogr. 21, 272–281 (2012). [Google Scholar]
- 63.Center for International Earth Science Information Network (CIESIN) Columbia University , Gridded Population of the World (Version 4 [GPWv4], Population Density, Revision 10, Columbia University, 2017).
- 64.Pauly D., Zeller D., Eds., Sea Around Us Concepts, Design and Data. http://www.seaaroundus.org. Accessed 13 December 2018.
- 65.Chamberlain S., Reis R. S., seaaroundus: Sea around Us API Wrapper. https://www.rdocumentation.org/packages/seaaroundus. Accessed 13 December 2018.
- 66.Froese R., Pauly D., FishBase, (World Wide Web Electronic Publication, 2019). [Google Scholar]
- 67.Bolker B. M., R Development Core Team, bbmle: Tools for General Maximum Likelihood Estimation. http://cran.r-project.org/package=bbmle. Accessed 3 February 2020.
- 68.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 69.Chase J. M., Knight T. M., Scale-dependent effect sizes of ecological drivers on biodiversity: Why standardised sampling is not enough. Ecol. Lett. 16 (suppl. 1), 17–26 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Chase J. M., Kraft N. J. B., Smith K. G., Vellend M., Inouye B. D., Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2, art24 (2011). [Google Scholar]
- 71.Anderson M. J., Willis T. J., Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 84, 511–525 (2003). [Google Scholar]
- 72.Oksanen J., et al. , vegan: Community Ecology Package. http://cran.r-project.org/package=vegan. Accessed 1 September 2019.
- 73.Boettiger C., Lang D. T., Wainwright P. C., rfishbase: Exploring, manipulating and visualizing FishBase data from R. J. Fish Biol. 81, 2030–2039 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Stuart-Smith R. D. et al., Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature 501, 539–542 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Laliberté E., Legendre P., A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010). [DOI] [PubMed] [Google Scholar]
- 76.Villéger S., Mason N. W. H., Mouillot D., New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Botta‐Dukát Z., Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 16, 533–540 (2005). [Google Scholar]
- 78.Tingley D., Yamamoto T., Hirose K., Keele L., Imai K., mediation: R package for causal mediation analysis. J. Stat. Softw. 59, 1–38 (2014).26917999 [Google Scholar]
- 79.Wood S. N., Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73, 3–36 (2011). [Google Scholar]
- 80.R Core Team , R: A Language and Environment for Statistical Computing, (R Foundation for Statistical Computing, 2019). [Google Scholar]
- 81.Whalen M., Whippo R., Duffy E., . MarineGEO_Bitemap_2016. Dryad. 10.5061/dryad.8cz8w9gnm. Deposited 3 October 2020. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data reported in this paper (.csv files) have been deposited in Dryad (https://doi.org/10.5061/dryad.8cz8w9gnm) (81), and the code (R scripts) used to process and analyze the data have been deposited in Zenodo (https://doi.org/10.5281/zenodo.3998836).