Significance
Social rank along a hierarchy determines physiological state and behavioral performance. A ubiquitous feature of social hierarchies is the communication of rank through nonphysical signaling systems (e.g., coloration) and aggression, traits that correlate with the reproductive status of an individual. Despite the links identified between social status, physiology, and behavior, the molecular basis of social status is not known. Here, we genetically dissect social status in the African cichlid fish Astatotilapia burtoni using CRISPR/Cas9 gene editing. We discovered that two paralogous androgen receptor genes control social status in a highly modular manner. This type of coordination of social status may be fundamental across species that rely on social information to optimally guide physiology and behavior.
Keywords: social status, CRISPR/Cas9 gene editing, androgen receptor, genome duplication
Abstract
Social hierarchies are ubiquitous in social species and profoundly influence physiology and behavior. Androgens like testosterone have been strongly linked to social status, yet the molecular mechanisms regulating social status are not known. The African cichlid fish Astatotilapia burtoni is a powerful model species for elucidating the role of androgens in social status given their rich social hierarchy and genetic tractability. Dominant A. burtoni males possess large testes and bright coloration and perform aggressive and reproductive behaviors while nondominant males do not. Social status in A. burtoni is in flux, however, as males alter their status depending on the social environment. Due to a teleost-specific whole-genome duplication, A. burtoni possess two androgen receptor (AR) paralogs, ARα and ARβ, providing a unique opportunity to disentangle the role of gene duplication in the evolution of social systems. Here, we used CRISPR/Cas9 gene editing to generate AR mutant A. burtoni and performed a suite of experiments to interrogate the mechanistic basis of social dominance. We find that ARβ, but not ARα, is required for testes growth and bright coloration, while ARα, but not ARβ, is required for the performance of reproductive behavior and aggressive displays. Both receptors are required to reduce flees from females and either AR is sufficient for attacking males. Thus, social status in A. burtoni is inordinately dissociable and under the modular control of two AR paralogs. This type of nonredundancy may be important in facilitating social plasticity in A. burtoni and other species whose social status relies on social experience.
Social animals often organize into hierarchies, wherein social status or rank influences many physiological and behavioral traits (1). Successful navigation through a social hierarchy requires the constant integration of social cues to optimize chances of survival and reproductive opportunities (2). Within social hierarchies dominant and nondominant individuals exist (1). Dominant individuals typically behave more aggressively and have more mating opportunities than nondominant individuals. Dominant animals may also express conspicuous signals that show their status to others. For many species, dominance is also marked by an activated reproductive system as indicated by their large gonads. On the other hand, nondominant individuals are not aggressive and have very few, if any, chances to mate. Nondominant individuals may appear inconspicuous to avoid confrontations from higher-ranking individuals and possess small gonads. For some species, social hierarchies are in flux as nondominant animals can ascend to dominant rank given the social opportunity (3). Despite what is known about the importance of social hierarchies in controlling traits that relate to reproduction, the molecular mechanisms underlying social status are unclear.
One candidate molecular substrate for regulating social status is the steroid testosterone, an androgen that can be converted to both estrogenic and androgenic metabolites (4). Dominant animals tend to have higher levels of testosterone compared to nondominant animals. Pharmacological manipulations across species suggest that testosterone signaling is required to enhance the motivation to seek higher social status (4–6). However, results of studies using pharmacology to tease apart the molecular mechanisms of social status are limited. For instance, pharmacological agents have off-target effects that in many cases are difficult, if not impossible, to control for (7). Moreover, variability exists across species in the number of receptors that bind the same steroid (8), making pharmacological studies in diverse species difficult to interpret. Due to a whole-genome duplication (WGD) (9), many teleosts have two distinct androgen receptors (ARs) that differ from one another in amino acid sequence and ligand binding affinity, while other vertebrates have one AR (10–12). For these reasons, genetic manipulations may be preferred over pharmacological ones to dissect the molecular basis of complex physiological and behavioral functions. However, genetic models of social status have until recently not been available for species in which rich social hierarchies can be controlled in the laboratory.
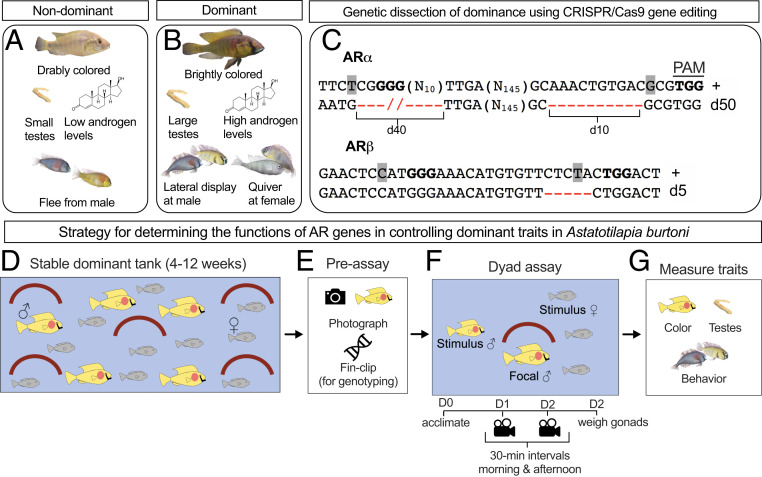
The African cichlid fish Astatotilapia burtoni is a powerful model species for the genetic dissection of social status (3, 13, 14). In the laboratory, as in nature, male A. burtoni exist as either nondominant or dominant, exhibiting clear variation in testes mass, coloration, and behavior (Fig. 1 A and B). Pharmacological studies suggest AR signaling enhances reproductive behavior while estrogen receptor (ER) signaling enhances aggressive behavior associated with social dominance in A. burtoni (6, 15). Specifically, pharmacological ER activation stimulates aggression in nondominant and dominant fish, while pharmacological AR activation stimulates reproductive behaviors only in dominant fish (15). Furthermore, pharmacological blockade of AR signaling prevents the rise of reproductive but not aggressive behaviors during social ascent to dominance in A. burtoni (6). These results suggest an important link between androgen signaling specifically and social dominance in A. burtoni.
Fig. 1.
Understanding the control of social dominance by ARs in A. burtoni. (A) Nondominant and (B) dominant male A. burtoni differ in terms of a variety of traits that reflect their social status. (C) We used CRISPR/Cas9 gene editing to generate A. burtoni that possess frameshift ARα or ARβ alleles. (D–G) Experimental strategy for testing the functions of ARα and ARβ in the control of social dominance. Predicted Cas9 cleavage sites are located to the left of letters highlighted in gray. Bolded three-letter sequences indicate a protospacer adjacent motif (PAM) sequence. +, wild type, d, deletion. (N#) indicates the number of bases not shown in actual gene sequence for clarity.
Due to the teleost-specific WGD mentioned above (9), A. burtoni possess two AR paralogs, ARα and ARβ (SI Appendix, Table S1), and several ER paralogs (16, 17), making pharmacological results difficult to interpret and providing a unique opportunity to disentangle the role of gene duplication in the evolution of social systems (18). Finally, recent work used CRISPR/Cas9 gene editing to generate mutant A. burtoni. With this in mind, as a first attempt to characterize the role of hormone signaling in regulating social dominance in A. burtoni we used gene editing to produce A. burtoni AR mutants and performed a suite of experiments interrogating the molecular basis of social dominance. We chose to generate A. burtoni AR mutants instead of ER mutants because of the link between androgen signaling and social dominance cited above and the increased experimental tractability of generating and studying AR mutants (two paralogs) compared to ER mutants (several paralogs).
Results
Genetic Dissection of Social Dominance in A. burtoni Using CRISPR/Cas9 Gene Editing.
We generated mutant fish possessing frameshift ARα or ARβ alleles using CRISPR/Cas9 gene editing (19). Two single-guide RNAs (sgRNAs) against ARα or ARβ and Cas9 messenger RNA (mRNA) (20, 21) were injected into embryos at the single-cell stage (22) after which frameshift mutant alleles were identified for both genes. ARα mutants possessed a total deletion of 50 base pairs (bp) (ARαd50) and ARβ mutants possessed a deletion of 5 bp (ARβd5) (Fig. 1C). For the wild-type and mutant alleles we determined the predicted amino acid sequences and protein tertiary structure (SI Appendix, Fig. S1) (23). Both mutant alleles contained premature stop codons that yielded predicted truncated amino acid sequences compared to wild types (SI Appendix, Fig. S1 A and B). Analysis of the predicted tertiary structure of wild-type and mutant ARα and ARβ revealed profound differences, with mutant versions of each protein totally lacking the complex tertiary structure observed in the wild-type versions (SI Appendix, Fig. S1 C and D). Thus, the frameshift alleles we generated for ARα and ARβ are highly likely to be nonfunctional. G0 injected fish were outcrossed with wild-type fish to generate heterozygous mutants (that were subsequently intercrossed). Heterozygous mutants of either genotype (ARαd50/+ or ARβd5/+) were crossed to yield offspring of homozygous wild types (ARα+/+ or ARβ+/+), heterozygous mutants (ARαd50/+ or ARβd5/+), and homozygous mutants (ARαd50/d50 or ARβd5/d5).
Do AR mutants display altered patterns of social dominance? To address this, we housed adult male fish from each specific genotype cross (i.e., all males from the ARα cross were housed together in a stable dominant tank, while genotypes from the ARβ cross were housed in a separate stable dominant tank) for 4 to 12 wk in stable dominant tanks (Fig. 1D), a housing environment that has been shown previously to reliably permit the full suite of social dominance traits (6, 24, 25). Fish were then assayed for three key traits related to social dominance: testes mass, coloration, and behavior (3) (Fig. 1 E–G). First, individuals were removed and photographed (Fig. 1E) followed by fin-clip removal (for subsequent genotyping). Each fish was then placed into a dyad assay tank (Fig. 1 E and F). In a dyad assay a focal fish (in this case, a fish from the stable dominant tank) is housed with a smaller stimulus male, three females, and a terra cotta pot simulating a potential mating site (26) (Fig. 1F). For the next 2 days fish were recorded from 8 AM to 2 PM. At 2 PM on the second day, focal fish were removed from the dyad assay tank and standard length, body mass, and testes mass were recorded. Blood was also collected for analysis of androgen levels. There was no effect of ARα or ARβ genotype on standard length or body mass (SI Appendix, Fig. S2).
ARα and ARβ Are Necessary for Distinct Aspects of Social Dominance.
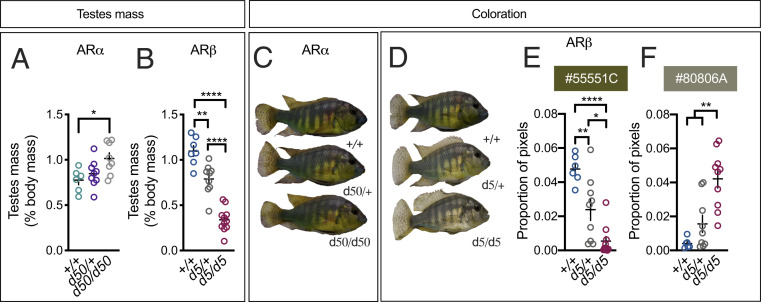
Variation in testes mass was measured first. We found that ARα and ARβ are required for normal testes mass, but in different directions. For instance, ARαd50/d50 males had testes that were larger than those seen in ARα+/+ males (Fig. 2A). On the other hand, ARβd5/d5 and ARβd5/+ males had smaller testes than ARβ+/+ males and ARβd5/+ males had larger testes than ARβd5/d5 males (Fig. 2B). Based on these findings, we predicted that ARβ mutants would lack dominant-typical coloration (16).
Fig. 2.
ARα and ARβ play distinct roles in the control of testes mass and coloration. (A) ARα+/+ males have smaller testes than ARαd50/d50 males, while ARαd50/+ males were not different from either group. (B) ARβ+/+ males have larger testes than ARβd5/+ males, which have larger testes than ARβd5/d5 males. (C) ARα mutant males do not look different from ARα+/+ males (D) while ARβ mutant males lack the dominant-typical yellow coloration seen in ARβ+/+ males. (E) ARβ+/+ males possessed more “very dark yellow” (#55551C) pixels than ARβd5/+ males, which had more dark yellow pixels than ARβd5/d5 males, (F) while ARβd5/d5 males had more “dark grayish yellow” (#80806A) pixels than both ARβd5/+ and ARβd5/d5 males. +, wild type; d, deletion. Circles represent data points for individual fish. Crosses represent mean ± SEM; ****P < 0.0001; **P < 0.01; *P < 0.05.
Dominant male A. burtoni typically express bright yellow or blue coloration while nondominant males appear drab (3) (Fig. 1 A and B). Visual inspection showed that, while ARαd50/+ and ARαd50/d50 males looked no different from ARα+/+ males (Fig. 2C), ARβ mutant males looked profoundly different from ARβ+/+ males, which possessed dominant-typical yellow coloration (3) (Fig. 2D). Hierarchical clustering confirmed these observations (SI Appendix, Figs. S3 and S4). To determine what specific colors were different between ARβ mutant males and ARβ+/+ males, we performed quantitative analysis on images of each fish. ARβ+/+ males differed significantly from ARβ mutant males for several colors (Fig. 2 E and F and SI Appendix, Fig. S5). For example, ARβ+/+ males possessed more “very dark yellow” (hex color code: #55551C) pixels than ARβd5/+ males, which had more dark yellow pixels than ARβd5/d5 males (Fig. 2E), while ARβd5/d5 males had more “dark grayish yellow” (#80806A) pixels than both ARβd5/+ and ARβd5/d5 males (Fig. 2F). Therefore, ARβ is required for dominant coloration, while ARα is not.
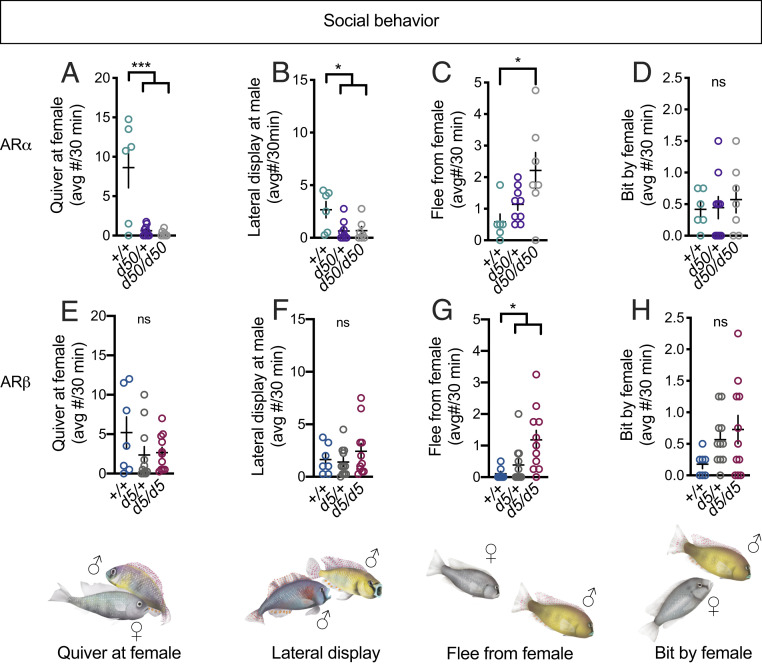
Given the above observations we formed several hypotheses for what to expect from the behavior analysis of mutant fish. Specifically, we anticipated that ARαd50/d50 and ARαd50/+ males should exhibit normal levels of dominant behavior, while ARβd5/+ and ARβd5/d5 fish should exhibit decreased levels of dominant behavior (3). Strikingly, the exact opposite was true. All reproductive behaviors in ARα mutant males were virtually abolished (Fig. 3A and SI Appendix, Fig. S6 A–D). ARα mutant males performed significantly fewer aggressive displays than ARα+/+ males (Fig. 3B), while attacks directed toward males were unaffected in ARα mutant males (SI Appendix, Fig. S6 E and F). ARα mutant males did not differ from ARα+/+ males in the number of times they fled from the stimulus male (SI Appendix, Fig. S6G); however, ARαd50/d50 males fled significantly more from females compared to ARα+/+ males (Fig. 3C). Importantly, there was no effect of ARα genotype on how often the stimulus females bit the focal male (Fig. 3D), suggesting the effects on fleeing from females were not due to higher aggression from the stimulus female toward the AR mutant focal males. ARβ mutant fish did not differ from ARβ+/+ males for all behaviors (Fig. 3 E and F and SI Appendix, Fig. S6) except for one, flee from female. Specifically, ARβ mutants fled from females more than ARβ+/+ males (Fig. 3G), coinciding with the findings for this behavior in ARα mutants (Fig. 3C). As with ARα, there was no effect of ARβ on how often the stimulus females bit the focal male (Fig. 3H). Thus, both ARα and ARβ are required to reduce flees from females.
Fig. 3.
Dissociable roles of ARα and ARβ in the regulation of social behavior. (A) ARα mutant males quivered at females significantly less than ARα+/+ males. (B) ARα mutant males performed lateral displays at males significantly less than ARα+/+ males. (C) ARαd50/d50 males fled from females significantly more than ARα+/+ males, (D) but there was no effect of ARα genotype on the number of times focal males were bit by females. (E and F) There was no effect of ARβ genotype on quiver at female or lateral display at female, (G) but ARβ mutants fled from females more than ARβ+/+ males. (H) There was no effect of ARβ genotype on the number of times focal males were bit by females. +, wild type; d, deletion. Circles represent data points for individual fish. Crosses represent mean ± SEM; ***P < 0.001; *P < 0.05. ns, not statistically significant.
Analysis of AR Double Mutants Reveals Either ARα or ARβ Is Sufficient for Attacking Males.
We were surprised that ARα was required for the performance of an aggressive display, but neither it nor ARβ was required for attacking other males. This suggests 1) either AR is sufficient for attacking other males or 2) an AR-independent mechanism controls attacks directed toward males. We were able to test the first hypothesis by generating AR double mutants through breeding strategies. Given that some ARα mutant males were severely injured in the dyad assay (SI Appendix, Supplementary Results), we used a modified dyad assay in which the focal fish was isolated physically from the three females and stimulus male using two transparent, perforated plastic barriers on either side (SI Appendix, Fig. S7A). This approach is sufficient for testing our hypothesis about the role of either AR in controlling aggression specifically given previous work (6, 24) showing males perform aggressive displays at males on the other side of a barrier and also attack the stimulus male through the barrier (SI Appendix, Fig. S7 B and C).
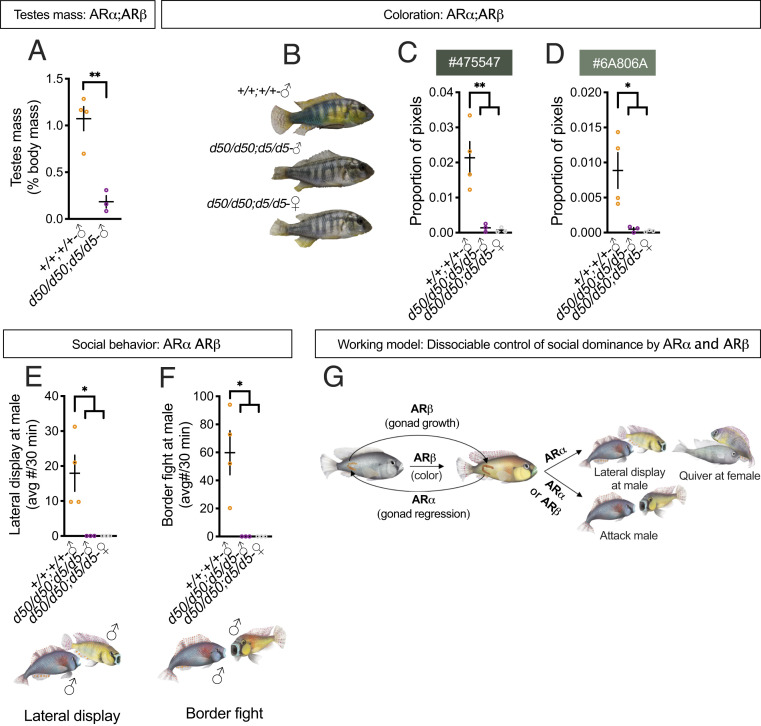
We assayed four AR wild-type (ARα+/+;ARβ+/+) males and six AR double-mutant (ARαd50/d50;ARβd5/d5) fish. Three of the ARαd50/d50;ARβd5/d5 fish were found to be female, which was not determined until dissection where the presence of ovaries was confirmed. ARα+/+;ARβ+/+ males weighed more than ARαd50/d50;ARβd5/d5 males and females (SI Appendix, Fig. S8A) and were greater in standard length than ARαd50/d50;ARβd5/d5 males (SI Appendix, Fig. S8B), suggesting that AR regulates body size in line with findings in AR mutant mice (18). Like ARβ mutant males, ARαd50/d50;ARβd5/d5 males had extremely small testes compared to ARα+/+;ARβ+/+ males (Fig. 4A). As with ARβ mutants, ARαd50/d50;ARβd5/d5 males lacked the dominant-typical coloration seen in ARα+/+;ARβ+/+ males and were indistinguishable from ARαd50/d50;ARβd5/d5 females (Fig. 4B). Hierarchical clustering confirmed this observation (SI Appendix, Fig. S9). Quantitative image analysis revealed that ARα+/+;ARβ+/+ males differed from ARαd50/d50;ARβd5/d5 males and females for several colors (Fig. 4 C and D and SI Appendix, Fig. S10). For example, ARα+/+;ARβ+/+ males possessed more “very dark grayish lime green” (#475547) and “dark grayish lime green” (#6A806A) pixels than ARαd50/d50;ARβd5/d5 males and females, which were indistinguishable from one another (Fig. 4 C and D).
Fig. 4.
Analysis of AR double mutants highlights the modular control of social dominance by ARα and ARβ. (A) ARαd50/d50;ARβd5/d5 males had smaller testes than ARα+/+;ARβ+/+ males. (B) ARαd50/d50;ARβd5/d5males and females lacked the dominant-typical coloration observed in ARα+/+;ARβ+/+ males. (C) ARα+/+;ARβ+/+ males possessed more “very dark grayish lime green” (#475547) (D) and “dark grayish lime green” (#6A806A) pixels than ARαd50/d50;ARβd5/d5 males and females, which were indistinguishable from one another. (E) ARα+/+;ARβ+/+ males performed more lateral displays (F) and border fights directed toward the stimulus male than ARα Δ/Δ;ARβd5/d5males and females. (G) A summary of the current findings in the form of a working model. +, wild type; d, deletion. Circles represent data points for individual fish. Crosses represent mean ± SEM; **P < 0.01; *P < 0.05.
In addition to the effects described above, we observed striking differences in aggressive behavior between ARα+/+;ARβ+/+ and ARαd50/d50;ARβd5/d5 fish (Fig. 3 E–H). ARα+/+;ARβ+/+ males performed significantly higher levels of aggressive displays and attacks directed toward the stimulus male compared to ARαd50/d50;ARβd5/d5 males and females (Fig. 4 H and I). Indeed, none of the ARαd50/d50;ARβd5/d5 fish performed these behaviors. Surprisingly, both ARαd50/d50;ARβd5/d5 males and females performed lateral displays directed toward females, while none of the ARα+/+;ARβ+/+ males performed this behavior (SI Appendix, Fig. S11A). ARαd50/d50;ARβd5/d5 males also directed attacks toward females more than ARα+/+;ARβ+/+ males (SI Appendix, Fig. S11B), but this difference did not reach statistical significance (P = 0.06). Previous work has shown that female A. burtoni perform these acts of aggression toward one another (27). Indeed, the stimulus females in our assays were observed attacking and performing lateral displays at one another. Therefore, the aggressive behaviors performed by ARαd50/d50;ARβd5/d5 males and females appear to be female-typical. Our findings support our first hypothesis that either AR is sufficient for male-directed attacks, demonstrating further evidence for highly modular control of social dominance traits by AR genes.
Observed Effects on Social Dominance Are Not Due to the Absence of Circulating Androgens.
To determine whether observed effects on dominance traits in AR mutant A. burtoni could have been be due to abnormally low levels of androgens (28, 29), we measured levels of testosterone and 11-ketotestosterone (11-KT) in all fish. ARαd50/d50 and ARαd50/+ males had levels of testosterone and 11-KT that were indistinguishable from ARα+/+ males (SI Appendix, Fig. S12 A and B). ARαd50/d50 and ARαd50/+ males had levels of testosterone that were not different from ARα+/+ males (SI Appendix, Fig. S12C). ARβd5/d5 males possessed higher levels of 11-KT compared to ARβd5/+ and ARβ+/+ males (SI Appendix, Fig. S12D), which is in line with previous work in AR mutant female mice that have intact gonads and significantly higher levels of 5-α dihydrotestosterone, a metabolite of testosterone found in mammals, compared to wild types (30).
ARαd50/d50;ARβd5/d5 males and females had levels of testosterone that were not different from ARα+/+;ARβ+/+ males (SI Appendix, Fig. S12E). Like ARβd5/d5 males, ARαd50/d50;ARβd5/d5 males had significantly higher levels of 11-KT compared to ARα+/+;ARβ+/+ males, while ARαd50/d50;ARβd5/d5 females were not different from either group (SI Appendix, Fig. S12F). These findings collectively indicate that the observed perturbations in dominance traits in AR mutants are not due to abnormally low levels of androgens.
Discussion
These data present coherent evidence for distinct modules of social status that are controlled by paralogous AR genes in male A. burtoni. For instance, we show a stark double dissociation in the regulation of social status, wherein ARα is required for most dominant behaviors and testes regression but not dominant coloration, while ARβ is required for dominant coloration and testes growth, but not dominant behaviors. At the same time, both ARα and ARβ are required for reducing flees from females and either AR is sufficient for males to attack other males (results are summarized in Fig. 4J). Previous work has shown the important role of paralogous genes in controlling different phenotypes. One of the best-known examples of paralogous genes controlling distinct aspects of a trait are the Hox genes, which are critical for different components of body patterning and axial morphogenesis in vertebrates (31). In Drosophila, paralogous genes are important for regulating distinct aspects of juvenile-hormone signaling, which governs metamorphosis and reproduction (32). Our work expands on this body of literature by revealing the different roles of two paralogous genes—in this case ARα and ARβ—in controlling the different components that make up a complex trait, social status. Our findings have important implications for understanding social status and social behavior in A. burtoni and other species.
Multiple factors could contribute to the different functions of ARα and ARβ in the modular regulation of social status in A. burtoni. For example, while both ARα and ARβ are expressed in the testes (33), they show distinct expression patterns in the brain (10), suggesting our results may be partially explained by regionally specific expression of either gene. Furthermore, ARα and ARβ differ in the amino acid sequence encoding their respective DNA binding domains, suggesting they may activate distinct molecular cascades, a hypothesis supported by previous work (34). Finally, work in mosquitofish (Gambusia affinis), another teleost, shows the AR paralogs have different binding affinities to testosterone and 11-KT (11). Future work in A. burtoni utilizing region-specific manipulations of ARα and ARβ as well as molecular and biochemical analyses of each receptor is needed to tease apart how they govern specific social dominance traits.
We were surprised to find that AR mutants had deficits in aggression. Indeed, as noted above, previous work using pharmacological manipulations suggest that ER, but not AR, is required for aggression in A. burtoni (6, 15). However, important differences exist between our current work and previous work on aggression in A. burtoni. For instance, previous work manipulated androgen or estrogen signaling in adult A. burtoni, while in our current work the ARs have been functionally disabled genetically throughout life. It is therefore possible that the early disabling of AR affects aggression in males, while manipulation only in adulthood does not. This suggests that AR may be required for organizing aggression circuits early on that are in turn activated by estrogen signaling in adulthood. Pursuing this question should be a goal of future studies.
Social information determines when a male A. burtoni will attempt to ascend to social dominance (2). Previous work in A. burtoni has shown males uncouple specific dominance traits as a function of variation in social information (2, 3). For instance, nondominant males will turn on bright colors and perform dominant behaviors in the absence of large testes when larger dominant males cannot see them but immediately turn off their colors and cease dominant behaviors when the dominant male can see them (35). Dominant males also uncouple coloration and behavior from physiological state to prevent neighboring males that are larger than them from attacking (36). The ability to dissociate coloration, physiology, and behavior depending on the social environment reflects a sophisticated social decision-making process that optimizes chances of survival and reproduction (2). Our results suggest that these social decisions may be controlled by distinct AR genes. Moreover, the current findings provide further support for theories stating that androgen signaling mediates complex suites of physiological and behavioral responses that contribute to successful social interactions (4, 6, 37, 38).
Through genetic dissection using CRISPR/Cas9 gene editing, we provide evidence suggesting that ARα and ARβ may have been subfunctionalized, the process by which each duplicated gene retains a subset of ancestral functions (18). In zebrafish (Danio rerio), which possess a single AR gene, mutation of AR produces male fish that possess small testes and reduced color and perform fewer courtship behaviors compared to wild types (39, 40). The results in zebrafish parallel those found in AR mutant mice, which also possess a single AR gene (28). In A. burtoni, we have shown that the control of these classes of traits is distributed over AR paralogs. To definitively determine whether and how ARα and ARβ have been subfunctionalized will require future studies of the relevant traits in fish species possessing the putative ancestral AR (e.g., the basal teleost silver arowana, Osteoglossum bicirrhosum) and numerous other teleosts (11). Deeper investigations into this question may be fruitful, considering AR gene duplication has been hypothesized to play an important role in the evolutionary divergence of sexual traits in teleost fish (9, 11). Given the rise of gene editing tools like CRISPR/Cas9 gene editing, novel insights into the roles of gene duplications and subfunctionalization in the evolution of social systems are more possible now than ever.
Based on our findings, we propose a working framework where the type of dissociable control of social status observed here occurs in other species that rely on social and environmental information to optimize physiology and behavior. In this framework, independent mechanisms integrate social cues and regulate distinct aspects of traits that relate to reproduction. This regulation may begin with androgen signaling, which acts on separate molecular and neural pathways that govern distinct dominance traits in a nonoverlapping manner. In this way, our framework is fundamentally similar to the hormonal control of courtship birdsong, wherein discrete steroid-sensitive molecular and neural pathways that regulate different features of song are activated by androgen signaling during the breeding season (37). This framework can be usefully applied to studies aiming to understand how nondominant and dominant animals rapidly alter social behavior in a way that is seemingly disconnected from their reproductive state, and vice versa (1, 2). By performing rich mechanistic studies in a genetically tractable model species of social status, we have yielded fundamental insights into the nature of social behavior.
Materials and Methods
Generation of Frameshift Alleles for ARα and ARβ Using CRISPR/Cas9 Gene Editing.
Fish were bred and used at Stanford University from a colony derived from Lake Tanganyika (41) in accordance with Association for Assessment and Accreditation of Laboratory Animal Care standards. All experimental procedures were approved by the Stanford University Administrative Panel for Laboratory Animal Care (Protocol #9882).
We generated fish with mutant ARα (accession number, NW_005179415) or ARβ (accession number, NW_005179497) alleles using an approach similar to that of Juntti et al. (22). Mutations of either gene were induced by injection of two sgRNAs simultaneously targeting regions upstream from the DNA-binding domain and ligand-binding domain within exon 1 for ARα and exon 2 for ARβ. For ARα, we designed sgRNAs targeting sequence ARα-A, 5′-ACTGTGGCGGATACTTCTCG-3′ and sequence ARα-B, 5′-GGTGCGCAAACT-GTGACGCG-3′, whose cut sites were separated by 178 bp. For ARβ, we designed sgRNAs targeting sequence ARβ-A, 5′- GGGAAACATGTGTTCTCT-AC-3′ and ARβ -B, 5′- GGGGGAAAGAAGAACTCCAT-3′, whose cut sites were separated by 21 bp. We generated each sgRNA using cloning-free sgRNA synthesis (42). For instance, to synthesize sgRNA targeting ARα-A (gARα-A) we annealed oligonucleotide-ARα-A (oligo-ARα-A), which contained the ARα-A target sequence, and oligo-2, a generic oligo that we used for all sgRNA synthesis reactions (see SI Appendix, Table S2 for oligo sequences). gARα-B, gARβ-A, and gARβ-B were synthesized in the same manner.
We waited for 30 min of fertilization and then injected single-cell embryos with the two sgRNAs targeting ARα or ARβ. We delivered ∼1 nL of each sgRNA, 60 ng/mL nls-zCas9-nls mRNA, and 0.3% Texas Red-conjugated dextran (3,000 molecular weight; Life Technologies). In ∼5-wk embryos injected with gARα-A and gARα-B, we PCR-amplified a 536-bp amplicon spanning the ARα-A and ARα-B target sites with the primers ARαFlankF, 5′-CCCAGTGCACTCTAACTCCG-3′ and ARαFlankR, 5′-TTTAAGGGTACGACCTCG-GC-3′ and Sanger-sequenced the product with ARαFlankR (MCLabs). We performed the same procedure for embryos injected with gARβ-A and gARβ-B by PCR amplifying a 642-bp amplicon with the primers ARbFlankF, 5′-CCA-TCCCACCTCCAAGAGTC-3′ and ARβFlankR, 5′-GAGGACAGGCCGATGATGAA-3′ and Sanger-sequenced the product with ARβFlankF (MCLabs). We saved fish showing evidence of mutations in ARα or ARβ and crossed these fish to wild types. These G1 offspring carried a variety of indel alleles, so we selectively propagated an allele for each gene predicted to result in a loss of function (i.e., a 50-bp deletion for ARα and a 5-bp deletion for ARβ). We intercrossed G1 fish from different founders to obtain biallelic ARα mutants (ARαd50/d50), heterozygous ARα mutants (ARαd50/+), and ARα wild types (ARα+/+) or biallelic ARβ mutants (ARβd5/d5), heterozygous ARβ mutants (ARβd5/+), and ARβ wild types (ARβ+/+).
Establishing Stable Dominant Tanks.
Social dominance is reliably induced when males have ample opportunity to establish a territory and access to females to mate with (6, 43). This social opportunity can be established using stable dominant tanks, wherein five to eight size-matched males are housed in a 121-liter tank with 10 to 15 females and five potential mating sites that are represented by halved terra cotta pots. We housed males from either cross in separate stable dominant tanks for 4 to 12 wk. Each stable dominant tank contained males that were matched by the particular cross they arose from. The age of males included in the dyad assays ranged from 6 to 10 mo. Two or three age- and size-matched males from a given stable dominant tank were run through separate dyad assays simultaneously.
Photography.
Fish were removed from their tank and placed for ∼20 s on a white paper towel to dry them off. Then, fish were immediately placed onto a white paper towel within a light chamber that contained a ruler for scale and photographed using a Sony camera (Sony Alpha NEX-C3 16 MP; shutter speed = 1/80; aperture = 4.5; white balance = +0.0) mounted on a tripod. Six to 10 photos were taken to increase the likelihood of capturing an image during which the fish were not operculating. Fish were fin-clipped (Fin Clipping and DNA Extraction) and immediately moved to their dyad assay tank. This whole process took ∼45 s. Images were transferred from the camera SD card to a computer (Mac). We were unable to take photos of one ARβ+/+ fish, two ARβd5/+ fish, and one ARβd5/d5 fish.
Quantitative Image Analysis.
JPEG images with the highest resolution and where the fish was not operculating were chosen for analyses. To perform quantitative analysis of photos, we first cropped the fish out of the rest of the image using the Lasso Selection tool in Preview. Cropped images were then analyzed using R code (colordistance package: https://cran.r-project.org/web/packages/colordistance/index.html), which computes the proportion of pixels in an image occupied by each color. For ARα fish, if 15 or more fish (two-thirds of fish analyzed) had a zero value for a given color, this color was not considered for further analysis. For ARβ fish, if 16 or more fish had a zero value for a given color, this color was not considered for further analysis. For ARα;ARβ fish, if 7 or more fish had a zero value for a given color, this color was not considered for further analysis. We measured the proportion of pixels occupied by 84 colors for ARα fish, 83 colors for ARβ fish, and 76 colors for ARα;ARβ fish.
Fin Clipping and DNA Extraction.
After photographing the fish, they were immediately fin-clipped. Using ethanol-cleaned scissors, a 1- to 2-mm portion of the anal fin was excised and placed into an individual PCR tube. This was repeated for the rest of the fish run on a given day and the scissors were cleaned thoroughly with ethanol between each fin clipping. To extract DNA, 180 mL of NaOH (50 mM) was added to the sample, which was incubated at 94 °C for 15 min. Then, 20 mL of Tris⋅HCl (pH 8) was added directly into the sample, which was then vortexed and spun down using a minicentrifuge for 5 s. The samples were then placed at −20 °C for at least 15 min before PCR amplification of mutated regions of ARα or ARβ (Generation of Frameshift Alleles for ARα and ARβ Using CRISPR/Cas9 Gene Editing).
Dyad Assay Setup.
Each dyad assay was conducted in 30-liter tanks with enough gravel spread evenly to cover the bottom of the tank and half a terra cotta pot (simulating a potential mating site) in the middle. An air stone supplying oxygen to the water was present in each tank. Each dyad assay tank was visually isolated from nearby tanks using black plastic barriers between and behind tanks. After fish were photographed and fin-clipped (Fin Clipping and DNA Extraction) they were immediately transferred to the tank. Three stimulus females and one stimulus male were collected from community tanks and then added to the dyad assay tank. We aimed to always include gravid females, but if this could not be accomplished one or two females that were obviously not brooding were included. Stimulus males were chosen based on being smaller than the focal male based on visual inspection. The standard length of the stimulus male was measured after each assay and ranged from being 8 to 25% smaller than the focal male. Notably, before the assay the stimulus females had never interacted visually, chemically, or physically with the stimulus or focal males nor had the focal and stimulus male interacted with each other. This process was then repeated for all of the other males for which dyad assays were ran. Two or three dyad assays were run simultaneously.
A Wifi-enabled camcorder (Canon VIXIA HF R80) was then mounted on tripod placed in front of each tank. Recording began the next day (day 1) at 9 AM and was started remotely using the Wifi function, preventing any disturbance from the experimenter to start recording. Recordings were stopped remotely at 2 PM when the fish were fed. The day after (day 2), recording commenced in the same fashion except at 2 PM focal fish were removed and tissue was harvested for physiological measurements and blood was collected.
Scoring Behavior.
Behavior was scored during 30-min intervals on days 1 and 2. The first 30 min of scoring for day 1 and day 2 started from the first observation of behavior performed by the focal male after lights on. If an hour elapsed and the fish did not perform a behavior, scoring in the morning was stopped and zero occurrences was recorded for all behaviors for that time point. On days 1 and 2, the final 30 min before lights on—from 1:30 to 2:00 PM—were also scored. Based on previous work, multiple types of behavior were quantified (41): subordinate behavior (flee from male or flee from female); territorial or agonistic behaviors (lateral display, chase male, bite male, and bite female); and reproductive behaviors (chase female, quiver, lead swim, pot entry, and dig). Fleeing was defined as a rapid retreat swim from an approaching fish. Lateral displays are aggressive displays classified as presentations of the side of the body to another fish with erect fins, flared opercula, and trembling of the body. Biting was defined as the male lunging a short distance toward a fish and biting it on its side and floating backward a short distance. Chase was defined as a rapid swim directed toward a fish. Chase male, bite male, and bite female are considered attack behaviors. Chase female was grouped with reproductive behaviors because they are a normal component of the courtship repertoire. Quiver was defined as a rapid vibration of the body by the male with presentation of the anal fin egg spots to a female, and lead swim was defined as swimming toward the shelter accompanied by back-and-forth motions of the tail (waggles) as the male attempted to lead a female toward the pot. We defined pot entry as any time the focal male entered the half terra cotta pot and digging as any time the male scooped gravel from inside its pot or around its pot into its mouth and subsequently released it around its pot. Videos were scored in Scorevideo (MATLAB). The results of scoring videos were saved into log files that were subjected to a variety of analyses using custom R software (log files and code are available at https://github.com/AlwardLab). Behaviors across the four 30-min intervals were averaged (average number of behaviors per 30 min) and statistical analyses were performed on these values.
Modified Dyad Assay Setup and Scoring.
Fish included in the modified dyad assay setup were handled in the same way as in the normal dyad assay setup in terms of photography and fin clipping. The tanks were set up differently, however. Two perforated, transparent, acrylic barriers were placed in the 30-liter tank to separate the focal fish from three females on one side and one stimulus male on the other. We scored multiple aggressive behaviors in this setup. As with the normal dyad assay setup, we scored aggressive displays: lateral displays that directed at the stimulus male or the stimulus females. We also scored attack behaviors called border fights, which involved the focal fish attacking the stimulus fish across the acrylic barrier and is typified by head-on lunges and rams against the barrier with an open mouth.
Morphological and Steroid Hormone Analyses.
Focal fish were assessed for standard length, body mass, and testes mass (corrected for body mass). Blood samples were also collected with capillary tubes from the caudal vein and centrifuged for 10 min at 5,200 × g, and the plasma was removed and stored at −80 °C until assayed. Immediately after blood collection fish were killed by cervical transection. Testes were removed and weighed. Testes mass could not be recorded for one ARαd50/+ and one ARαd50/d50 male that died before the end of the assay. Standard length, body mass, and testes mass were not recorded for two ARβd5/+ males.
Plasma testosterone and 11-KT levels were measured using commercially available enzyme immunoassay (EIA) kits (Cayman Chemical Co.) as previously described and validated for this species (24). Briefly, for testosterone and 11-KT assays, a 1- to 5-μL sample of plasma from each subject was extracted three times using 200 μL of ethyl ether and evaporated under a fume hood before reconstitution in EIA assay buffer. EIA kit protocols were then strictly followed, plates were read at 405 nm using a microplate reader (UVmax Microplate Reader; Molecular Devices), and steroid concentrations were determined based on standard curves. All samples were assayed in duplicate. Two ARβd5/d5 males could not be assayed for testosterone or 11-KT due to possible contamination and blood was not collected for one ARβd5/+ male. One ARαd50/+ and one ARαd50/d50 male could not be assayed for testosterone or 11-KT because they died before the end of the assay. One ARα+/+ and one ARαd50/d50 male could not be assayed for testosterone or 11-KT because their plasma was mistakenly discarded.
Statistics and Clustering.
All statistical tests were performed in the R statistical computing environment or Prism 8.3. We used one-way ANOVAs for all traits tested. Following a significant main effect for an ANOVA, Tukey’s post hoc tests were used for pairwise comparisons. An individual t test was used to compare testes mass between ARα+/+;ARβ+/+ males and ARαd50/d50;ARβd5/d5 males. Differences were considered significant at P ≤ 0.05.
To cluster fish based on their coloration, we computed the Euclidean distances between all animals using the R function dist. We input these distances into the R function hclust. Complete linkages were used to build the hierarchical dendrograms, as shown in SI Appendix, Figs. S3, S4, and S10).
Supplementary Material
Acknowledgments
We thank Danielle Blakkan for assistance with the experimental setup and Andrew Hoadley for assistance with hormone extractions and EIAs. We thank Anne Fernald and Andrew Hoadley for providing feedback on an earlier version of this manuscript. This work was supported by an Arnold O. Beckman Fellowship to B.A.A., a University of Houston–National Research University Fund grant R0503962 to B.A.A., and NIH grants NS034950, MH101373, and MH096220 to R.D.F. S.A.J. is supported by NSF grant IOS-1825723 and the Human Frontiers in Science Program.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2008925117/-/DCSupplemental.
Data Availability.
Data (behavior log files, fish photographs, and R code) have been deposited in GitHub (https://github.com/AlwardLab) and are publicly accessible.
References
- 1.Sapolsky R. M., The influence of social hierarchy on primate health. Science 308, 648–652 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Fernald R. D., Cognitive skills needed for social hierarchies. Cold Spring Harb. Symp. Quant. Biol. 79, 229–236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernald R. D., Maruska K. P., Social information changes the brain. Proc. Natl. Acad. Sci. U.S.A. 109 (suppl. 2), 17194–17199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenegger C., Haushofer J., Fehr E., The role of testosterone in social interaction. Trends Cogn. Sci. 15, 263–271 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Dreher J. C., et al. , Testosterone causes both prosocial and antisocial status-enhancing behaviors in human males. Proc. Natl. Acad. Sci. U.S.A. 113, 11633–11638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alward B. A., Hilliard A. T., York R. A., Fernald R. D., Hormonal regulation of social ascent and temporal patterns of behavior in an African cichlid. Horm. Behav. 107, 83–95 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight Z. A., Shokat K. M., Chemical genetics: Where genetics and pharmacology meet. Cell 128, 425–430 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Douard V., et al. , The fate of the duplicated androgen receptor in fishes: A late neofunctionalization event? BMC Evol. Biol. 8, 336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brawand D., et al. , The genomic substrate for adaptive radiation in African cichlid fish. Nature 513, 375–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbott L. K., Burmeister S. S., White R. B., Vagell M., Fernald R. D., Androgen receptors in a cichlid fish, Astatotilapia burtoni: Structure, localization, and expression levels. J. Comp. Neurol. 504, 57–73 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogino Y., Katoh H., Kuraku S., Yamada G., Evolutionary history and functional characterization of androgen receptor genes in jawed vertebrates. Endocrinology 150, 5415–5427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsu Y., et al. , In vitro assessment of transcriptional activation of the estrogen and androgen receptors of mosquitofish, Gambusia affinis affinis. Mol. Cell. Endocrinol. 276, 10–17 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Stevenson T. J., et al. , The value of comparative animal research: Krogh’s Principle facilitates scientific discoveries. Policy Insights Behav. Brain Sci. 5, 118–125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson G. E., Fernald R. D., Clayton D. F., Genes and social behavior. Science 322, 896–900 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connell L. A., Hofmann H. A., Social status predicts how sex steroid receptors regulate complex behavior across levels of biological organization. Endocrinology 153, 1341–1351 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Maruska K. P., Zhang A., Neboori A., Fernald R. D., Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J. Neuroendocrinol. 25, 145–157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafont A. G., Rousseau K., Tomkiewicz J., Dufour S., Three nuclear and two membrane estrogen receptors in basal teleosts, Anguilla sp.: Identification, evolutionary history and differential expression regulation. Gen. Comp. Endocrinol. 235, 177–191 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Ohno S., Evolution by Gene Duplication (Springer, 1970). [Google Scholar]
- 19.Doudna J. A., Charpentier E., The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1–9 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Jao L. E., Wente S. R., Chen W., Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. U.S.A. 110, 13904–13909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varshney G. K., et al. , High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 25, 1030–1042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juntti S. A., et al. , A neural basis for control of cichlid female reproductive behavior by prostaglandin F2α. Curr. Biol. 26, 943–949 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley L. A., Mezulis S., Yates C. M., Wass M. N., Sternberg M. J., The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruska K. P., Fernald R. D., Behavioral and physiological plasticity: Rapid changes during social ascent in an African cichlid fish. Horm. Behav. 58, 230–240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burmeister S. S., Jarvis E. D., Fernald R. D., Rapid behavioral and genomic responses to social opportunity. PLoS One 3, e363 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcazar R. M., Becker L., Hilliard A. T., Kent K. R., Fernald R. D., Two types of dominant male cichlid fish: Behavioral and hormonal characteristics. Biol. Open 5, 1061–1071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renn S. C. P., Fraser E. J., Aubin-Horth N., Trainor B. C., Hofmann H. A., Females of an African cichlid fish display male-typical social dominance behavior and elevated androgens in the absence of males. Horm. Behav. 61, 496–503 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juntti S. A., et al. , The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66, 260–272 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato T., et al. , Brain masculinization requires androgen receptor function. Proc. Natl. Acad. Sci. U.S.A. 101, 1673–1678 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X. B., et al. , Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice. Am. J. Physiol. Endocrinol. Metab. 305, E717–E726 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Maconochie M., Nonchev S., Morrison A., Krumlauf R., Paralogous Hox genes: Function and regulation. Annu. Rev. Genet. 30, 529–556 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Jindra M., Palli S. R., Riddiford L. M., The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Maruska K. P., Fernald R. D., Plasticity of the reproductive axis caused by social status change in an african cichlid fish: II. Testicular gene expression and spermatogenesis. Endocrinology 152, 291–302 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorin T., Salzburger W., Böhne A., Evolutionary fate of the androgen receptor-Signaling pathway in ray-finned fishes with a special focus on cichlids. G3 (Bethesda) 5, 2275–2283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desjardins J. K., Hofmann H. A., Fernald R. D., Social context influences aggressive and courtship behavior in a cichlid fish. PLoS One 7, e32781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C. C., Fernald R. D., Visual information alone changes behavior and physiology during social interactions in a cichlid fish (Astatotilapia burtoni). PLoS One 6, e20313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alward B. A., Cornil C. A., Balthazart J., Ball G. F., The regulation of birdsong by testosterone: Multiple time-scales and multiple sites of action. Horm. Behav. 104, 32–40 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Carré J. M., Archer J., Testosterone and human behavior: The role of individual and contextual variables. Curr. Opin. Psychol. 19, 149–153 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Crowder C. M., Lassiter C. S., Gorelick D. A., Nuclear Androgen receptor regulates testes organization and oocyte maturation in zebrafish. Endocrinology 159, 980–993 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong L., Thet Z., Zhu Y., Genetic editing of the androgen receptor contributes to impaired male courtship behavior in zebrafish. J. Exp. Biol. 220, 3017–3021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernald R. D., Hirata N. R., Field study of Haplochromis burtoni: Quantitative behavioural observations. Anim. Behav. 25, 964–975 (1977). [Google Scholar]
- 42.Varshney G. K., et al. , A high-throughput functional genomics workflow based on CRISPR/Cas9-mediated targeted mutagenesis in zebrafish. Nat. Protoc. 11, 2357–2375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox H. E., White S. A., Kao M. H., Fernald R. D., Stress and dominance in a social fish. J. Neurosci. 17, 6463–6469 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data (behavior log files, fish photographs, and R code) have been deposited in GitHub (https://github.com/AlwardLab) and are publicly accessible.