Significance
Dysregulation of cytoskeletal remodeling could result in defective GSIS and cause type 2 diabetes. Previous studies have reported the role of small GTPases including Rac1 and Cdc42 in the regulation of F-actin remodeling, whereas the upstream regulatory pathway remains poorly understood. Here, we identify the adaptor protein APPL2 as an upstream regulator of Rac1 activation. APPL2 promotes F-actin remodeling by antagonizing the inhibitory effect of RacGAP1 on Rac1 activation, which eventually enhances GSIS. Our findings fill the overall puzzle of F-actin remodeling with a crucial piece and provide insights into type 2 diabetes with disrupted actin dynamics.
Keywords: glucose-stimulated insulin secretion, type 2 diabetes, Rac1, F-actin depolymerization, APPL2
Abstract
Filamentous actin (F-actin) cytoskeletal remodeling is critical for glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells, and its dysregulation causes type 2 diabetes. The adaptor protein APPL1 promotes first-phase GSIS by up-regulating soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein expression. However, whether APPL2 (a close homology of APPL1 with the same domain organization) plays a role in β-cell functions is unknown. Here, we show that APPL2 enhances GSIS by promoting F-actin remodeling via the small GTPase Rac1 in pancreatic β-cells. β-cell specific abrogation of APPL2 impaired GSIS, leading to glucose intolerance in mice. APPL2 deficiency largely abolished glucose-induced first- and second-phase insulin secretion in pancreatic islets. Real-time live-cell imaging and phalloidin staining revealed that APPL2 deficiency abolished glucose-induced F-actin depolymerization in pancreatic islets. Likewise, knockdown of APPL2 expression impaired glucose-stimulated F-actin depolymerization and subsequent insulin secretion in INS-1E cells, which were attributable to the impairment of Ras-related C3 botulinum toxin substrate 1 (Rac1) activation. Treatment with the F-actin depolymerization chemical compounds or overexpression of gelsolin (a F-actin remodeling protein) rescued APPL2 deficiency-induced defective GSIS. In addition, APPL2 interacted with Rac GTPase activating protein 1 (RacGAP1) in a glucose-dependent manner via the bin/amphiphysin/rvs-pleckstrin homology (BAR-PH) domain of APPL2 in INS-1E cells and HEK293 cells. Concomitant knockdown of RacGAP1 expression reverted APPL2 deficiency-induced defective GSIS, F-actin remodeling, and Rac1 activation in INS-1E cells. Our data indicate that APPL2 interacts with RacGAP1 and suppresses its negative action on Rac1 activity and F-actin depolymerization thereby enhancing GSIS in pancreatic β-cells.
GSIS is a highly regulated and dynamic process in pancreatic β-cells. Glucose enters the β-cell and is, subsequently, metabolized in mitochondria to produce ATP. The increased ATP/ADP ratio leads to the closure of ATP-sensitive potassium (KATP) channels, resulting in membrane depolarization, calcium influx, and ultimate insulin secretion (also known as first-phase GSIS) (1). This rapid insulin secretion is followed by a gradual and prolonged second-phase GSIS which requires multiple coupling factors and translocation of insulin granules from intracellular storage pools to the plasma membrane for exocytosis (2). Both first- and second-phase GSIS are diminished in type 2 diabetes (1), yet the underlying pathogenic pathways remain poorly understood.
Secretory vesicle trafficking and cytoskeleton reorganization are essential for GSIS in pancreatic β-cells (2). Higher microtubule density and compromised cellular cytoskeletal structure have been observed in islet β-cells of diabetic mice and patients with type 2 diabetes, respectively (3, 4). Early studies indicated that cortical F-actin acts as a barrier to prevent fusion of the insulin granule with the plasma membrane under basal glucose condition (<5 mM) (5). In response to high glucose stimulation, F-actin is depolymerized to allow movement and fusion of insulin granules for exocytosis (6). The guanosine triphosphatases (GTPase) of the Rho family, including cell division cycle 42 (Cdc42) and Rac1, are key players to control insulin granule trafficking via F-actin remodeling and required for GSIS (7, 8).
Adaptor proteins containing the NH2-terminal BAR domain, a central PH domain, and a COOH-terminal phosphotyrosine-binding domain (PTB) 1 and 2 (APPL1 and APPL2), a pair of endosomal and signaling molecules with the same domain organization and high protein sequence identity, are originally identified as the interacting partners of the small GTPase Rab5 (9). Subsequent studies showed that APPL1 and APPL2 positively and negatively, respectively, control glucose homeostasis via adiponectin and insulin signaling (10–12). In pancreatic β-cells, APPL1 augments first-phase GSIS by up-regulating SNARE protein expression through the insulin signaling cascade (13). APPL1 also enhances the potentiating effect of adiponectin on GSIS (14). The loss-of-function APPL1 mutants are identified in the family with a high prevalence of diabetes, and APPL1 expression in the human pancreatic islet is positively correlated with GSIS (15). In addition, APPL1 protects pancreatic β-cells from apoptosis and inflammation in the type 1 diabetic mouse model by inhibiting nuclear factor NF-κB activation (16).
Although the protective effects of APPL1 on β-cells are well established, the role of its close homolog APPL2 on β-cell function has never been explored. By using the β-cell specific APPL2 knockout (KO) mouse model and the insulinoma cell line, we, here, show that APPL2 is essential for both first- and second-phase GSIS in pancreatic β-cells. Mechanistically, APPL2 regulates F-actin remodeling and Rac1 activity by interacting with RacGAP1 (also known as MgcRacGAP, CYK-4, or RacGAP50C), a GTPase-activating protein (GAP) that inactivates Rac1 during cytokinesis (17, 18).
Results
β-Cell Specific Deletion of APPL2 Results in Glucose Intolerance and Impaired GSIS.
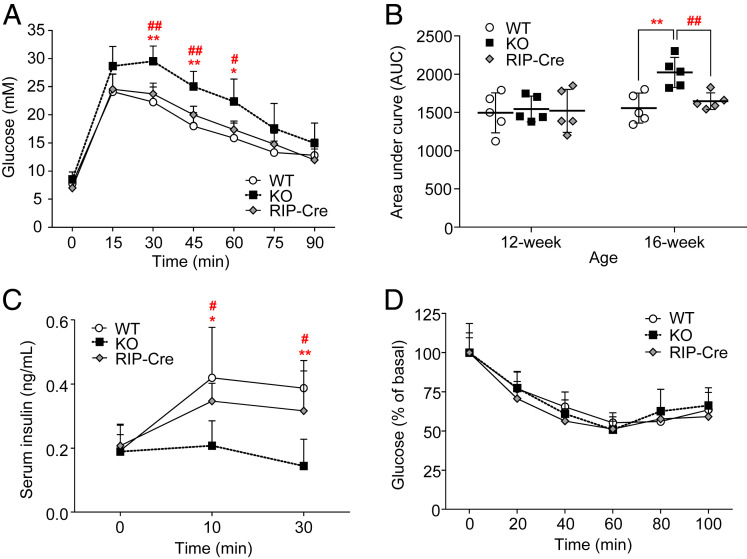
Our recent study demonstrated that APPL2 is expressed in both pancreatic islets and exocrine cells (19), but whether this adaptor protein plays a role in β-cell function, in particular, in the regulation of GSIS, is unknown. To address this question, we generated β-cell specific APPL2 KO mice (so-called RIP-APPL2 KO mice) by crossing APPL2floxed/floxed mice with the transgenic mice expressing Cre recombinase under the control of rat insulin promoter (19). Six-wk-old RIP-APPL2 KO mice, their wild-type (WT) littermates, and RIP-Cre controls were fed with a standard chow (STC) or a high fat diet (HFD) for 10 wk. Although STC-fed RIP-APPL2 KO mice displayed normal glucose tolerance at the age of 12 wk, they showed delayed glucose excursion and diminished serum level of insulin during a glucose tolerance test (GTT) at age 16 wk when compared to WT littermates and RIP-Cre controls (Fig. 1 A–C). Insulin sensitivity did not differ among the three groups of mice at age 14 wk (Fig. 1D). The above data indicate that glucose intolerance in RIP-APPL2 KO mice was primarily due to defective GSIS. Furthermore, an earlier and severe glucose intolerance was observed in RIP-APPL2 KO mice when they were fed with HFD (SI Appendix, Fig. S1 A and B). Similar to the observation in the STC-fed group, HFD-fed RIP-APPL2 KO mice also displayed defective GSIS but similar insulin sensitivity when compared to their WT littermates and RIP-Cre controls (SI Appendix, Fig. S1 C and D). These data suggest that APPL2, like APPL1, is essential for GSIS in pancreatic β-cells. Since WT littermates and RIP-Cre controls displayed similar glucose tolerance and insulin secretory ability, we only included WT littermates as controls for all of the subsequent analyses.
Fig. 1.
β-cell specific deletion of APPL2 impairs glucose-stimulated insulin secretion and induces glucose intolerance. Male RIP-APPL2 KO mice, their WT littermates, and RIP-Cre controls fed with STC were used. (A) GTT, 2g/kg in 6 h fasted 16-wk-old mice. (B) The area under the curve (AUC) of GTT performed at different ages. (C) Insulin secretion during GTT in A. (D) Insulin tolerance test in the 14-wk-old mice. n = 5 for each group. All data are presented as the mean ± SD. Significance was determined using one-way ANOVA with Bonferroni correction. *P < 0.05, **P < 0.01 (KO vs. WT), #P < 0.05, and ##P < 0.01 (KO vs. RIP-Cre).
Both First- and Second-Phase Glucose-Stimulated Insulin Secretions Are Abolished in APPL2 Deficient Islets.
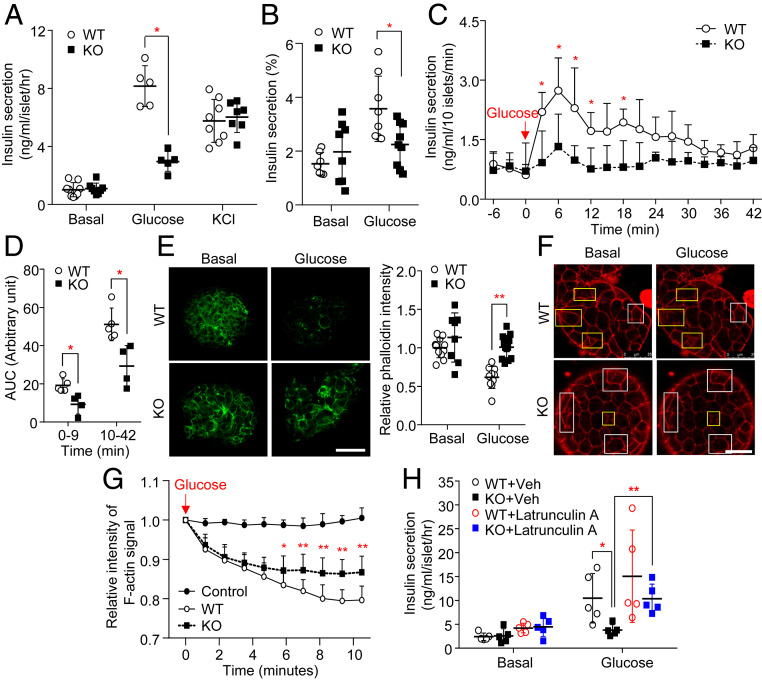
We next assessed the effect of APPL2 deficiency on insulin secretion using ex vivo approaches. Insulin secretion in response to low concentration of glucose stimulation (2.8 mM, basal) did not differ between the islets isolated from RIP-APPL2 KO mice and WT controls fed with STC or HFD (Fig. 2 A and B and SI Appendix, Fig. S1E). In contrast, under high glucose condition (16.7 mM), the islets from RIP-APPL2 KO mice fed with STC or HFD exhibited a dramatic reduction of insulin secretion when compared to those isolated from WT littermates (Fig. 2 A and B and SI Appendix, Fig. S1E). On the other hand, potassium chloride ([KCl], which directly induces membrane depolarization and subsequent insulin secretion)-induced insulin secretion was similar between the two genotypes (Fig. 2A and SI Appendix, Fig. S1E), indicating that the defect is specific to glucose stimulation and/or at the downstream of calcium influx. Next, we determined whether APPL2 deficiency affects first- and/or second-phase GSIS in isolated islets using a perfusion system as we previously described (13, 20). Similar to the observation in the static insulin secretion assay, dynamic insulin secretion in response to low glucose stimulation (2.8 mM) was comparable between islets from RIP-APPL2 KO mice and their WT controls (Fig. 2C). Under high glucose condition (16.7 mM), insulin secretion from APPL2 deficient islets during first- (first 9 min of high glucose stimulation) and second-phase (from 10 to 42 min of high glucose stimulation) was significantly diminished (Fig. 2 C and D).
Fig. 2.
The defective glucose-stimulated insulin secretion in APPL2 deficient islets is attributed to impaired glucose-induced F-actin remodeling. Pancreatic islets isolated from 16-wk-old male RIP-APPL2 KO mice and their WT littermates under a STC diet were used. (A) Static insulin secretion under basal (2.8 mM), glucose (16.7 mM), and KCl (50 mM) treatment conditions. n = 5–9. (B) The statics GSIS was repeated as A, and insulin secretion normalized with total intracellular insulin content. n = 7–9. (C) Dynamic insulin secretion in response to glucose stimulation (16.7 mM). Note that the islets were maintained in the conditioned medium with 2.8-mM glucose as the basal level. n = 4–5. (D) AUC for the first phase (0–9 min) and second phase (10–42 min) of insulin secretion in C. n = 4–5. (E) The islets were stimulated with glucose (16.7 mM) for 10 min, followed by staining with Alexa Fluor 488 phalloidin (green). The Right is a quantification of fluorescence intensity of the phalloidin signal relative to WT basal. n = 8–12. (Scale bar: 50 μM.) (F–H) The islets were incubated in Krebs buffer containing 2.8-mM glucose (basal) and SPY555-actin probe for 90 min, followed by 16.7-mM glucose (glucose) stimulation for 10 min. (F) Representative images of F-actin visualized by SPY555-actin probe (red) in the islets at the basal level and after high glucose stimulation for 10 min. Yellow rectangles indicate the regions with most obvious difference, while white rectangles indicate the regions without obvious difference before and after glucose stimulation. (Scale bar: 25 μM.) (G) Quantification of the dynamic change in the SPY555-actin signal intensity in the islets upon glucose stimulation. The control means the WT islets without high glucose stimulation. (H) The islets were pretreated with latrunculin A or vehicle for 1 h, followed by stimulation with 16.7-mM glucose for 30 min. Insulin secretion in the conditioned medium was measured. n = 5. All data are presented as the mean ± SD. Significance was determined using Student’s t test or one-way ANOVA with Bonferroni correction. *P < 0.05 and **P < 0.01. Representative images were shown.
APPL2 Regulates GSIS by Modulating F-Actin Remodeling.
Diminished GSIS could be due to reduced β-cell mass and insulin content, changes in β- and α-cell areas, defective glucose metabolism, and/or its downstream signal transduction in β-cells. First, we examined islet morphology and mass as well as β- and α-cell areas by hematoxylin and eosin staining and immunofluorescence staining of insulin and glucagon, respectively. There was no difference in islet morphology and mass as well as β- and α-cell proportion between RIP-APPL2 KO mice and WT controls (SI Appendix, Fig. S2 A–E). In addition, APPL2 deficiency did not affect glucose-stimulated ATP production and calcium influx in the isolated islets (SI Appendix, Fig. S3 A and B). As APPL2 transduces signals via the early endosome and the cell membrane, we examined the effect of glucose stimulation on APPL2 subcellular localization in MIN6 β-cells. Our confocal imaging results showed that APPL2 distributed in the cytosol and nucleus of MIN6 cells (SI Appendix, Fig. S4 A–C). A small portion of APPL2 was found to be localized in the early endosomes (labeled by the Rab5 antibody), insulin granules (labeled by the insulin antibody), and not much APPL2 could be found at the plasma membrane (labeled by the Cellmask Green Plasma Membrane Stain) (SI Appendix, Fig. S4 A–C). Glucose stimulation had no obvious effect on the subcellular localization of APPL2 in MIN6 cells (SI Appendix, Fig. S4 A–C).
Although APPL1 deficiency reduces SNARE protein expression and the number of insulin granules docking to the plasma membrane (13), the islets from RIP-APPL2 KO mice displayed similar expressions of both APPL1 and SNARE proteins including synaptosomal-associated protein 25, vesicle-associated membrane protein 2 , and syntaxin-1 when compared to those isolated from WT controls (SI Appendix, Fig. S3 C and D). Electron microscope analysis revealed that the islets from RIP-APPL2 KO mice had more insulin granules with smaller diameters, and the proportion of immature insulin granules was slightly increased (SI Appendix, Fig. S5 A–E). The total number of docked insulin granules in APPL2 deficient islets was comparable to their WT controls. Further analysis showed that there was a significant increase in immature docked insulin granules and a trend of decrease in mature docked insulin granules in APPL2 deficient islets compared to those in WT controls (SI Appendix, Fig. S5 F and G). In addition, the volume density of mature and immature docked insulin granules was significantly reduced and increased, respectively, in APPL2 deficiency islets (SI Appendix, Fig. S5 F and H). These data indicate that APPL2 regulates GSIS via a mechanism differing from that controlled by APPL1.
We and others previously demonstrated that APPL2 regulates small GTPase activity through direct interaction with small GTPase, such as Rab5 and Rab31 or the GAP protein TBC1 domain family member 1 (TBC1D1) (21, 22). On the other hand, the BAR domain containing proteins are known to regulate actin remodeling (23). Thus, we hypothesized that APPL2 regulates insulin secretion via GTPase activity and F-actin remodeling. To test this, we examined the distribution of F-actin in islets from RIP-APPL2 KO mice by phalloidin staining and live-cell imaging. Consistent with previous studies (6, 24), phalloidin staining revealed that intensity of F-actin was dramatically reduced in islets isolated from WT mice in response to high glucose stimulation (Fig. 2E), whereas genetic deletion of APPL2 abolished this glucose-induced effect (Fig. 2E). To further confirm this finding, we monitored F-actin remodeling in islets using a real-time live-cell imaging approach. The islets from RIP-APPL2-KO mice and WT controls were incubated with a SPY555-actin probe, a membrane permeable fluorescent probe that specifically binds to endogenous F-actin filaments. We first validated this method by observing the change in F-actin in WT islets treated with latrunculin A, a F-actin depolymerization compound. Consistent with previous studies (25, 26), islets isolated from WT controls exhibited obvious morphological changes and a small cluster of F-actin signal upon stimulation with latrunculin A for an hour (SI Appendix, Fig. S6A). In line with our phalloidin staining data, the intensity of the F-actin signal was decreased in WT islets upon glucose stimulation in a time-dependent manner, but this glucose action was less pronounced in APPL2 deficiency islets (Fig. 2 F and G). Notably, the F-actin signal did not obviously change in islets maintained at a low glucose condition for 10 min (Fig. 2G and SI Appendix, Fig. S6B), indicating that the reduction of the F-actin signal in WT and APPL2 deficiency islets was not due to photobleaching. These findings suggest that the defective GSIS observed in APPL2 deficient islets may be due to aberrant F-actin depolymerization. To confirm this, we treated the islets from RIP-APPL2 KO mice and WT controls with the F-actin depolymerizing compounds latrunculin A or cytochalasin B. In line with the above data, defective GSIS was observed in APPL2 deficient islets, but this defect could be partially rescued by treatment with latrunculin A or cytochalasin B (Fig. 2H and SI Appendix, Fig. S7A).
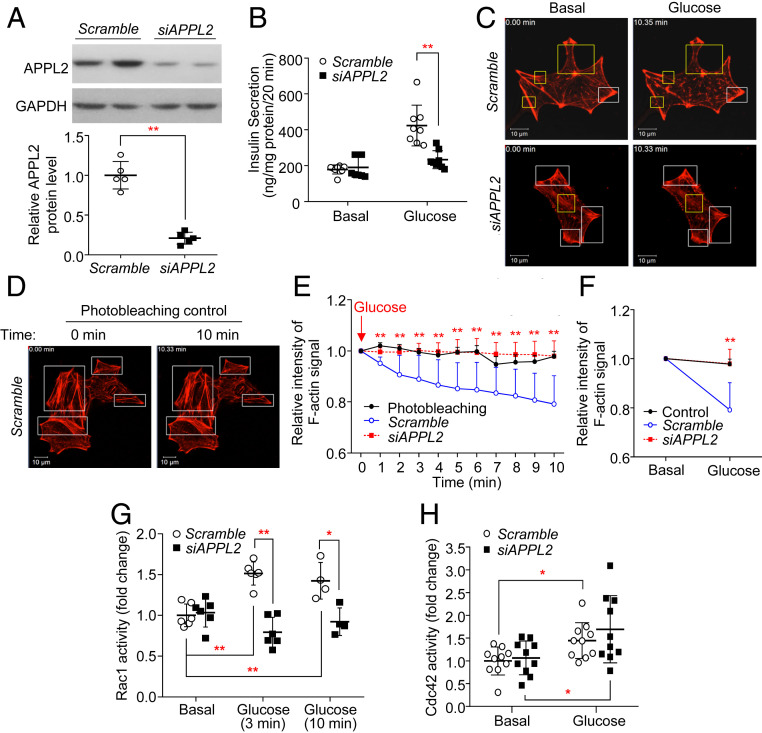
We also ascertained whether the inhibitory effects of APPL2 silencing on the β-cell function also occurs in INS-1E pancreatic insulinoma cells. We found that small interfering RNA (siRNA)-mediated knockdown of APPL2 expression abolished the effect of glucose on insulin secretion (Fig. 3 A and B), accompanied by defective F-actin depolymerization as determined by live-cell imaging using a SPY555-actin probe and phalloidin staining (Fig. 3 C–E and SI Appendix, Fig. S8A). Previous studies showed that the F-actin remodeling protein gelsolin promotes GSIS by inducing F-actin depolymerization in β-cells (27). We tested whether induction of F-actin depolymerization by overexpression of gelsolin is able to rescue the defective GSIS in INS-1E cells with APPL2 silencing. To this end, we cotransfected the plasmid encoding green fluorescent protein (GFP)-tagged gelsolin or a GFP empty vector together with siRNA against APPL2 or scrambled control into INS-1E cells for 48 h, followed by static GSIS assay. This analysis showed that overexpression of gelsolin was able to revert APPL2 deficiency-induced defective GSIS (SI Appendix, Fig. S7 B and C). Taken together, the findings from islets and INS-1E cells suggest that APPL2 deficiency impairs GSIS by abrogating F-actin remodeling.
Fig. 3.
Knockdown of APPL2 attenuates glucose-stimulated Rac1 activation and subsequent F-actin remodeling in INS-1E cells. (A–F) INS-1E cells were transfected with siRNA against scramble or APPL2 for 48 h. (A) Immunoblotting of APPL2 in the transfected cells. The Lower is the densitometric analysis for the relative abundance of APPL2 normalized with GAPDH. n = 5. (B) Static GSIS in the transfected cells. n = 7–8. (C) Representative images from live-cell imaging in INS-1E cells labeled with a SPY555-actin probe (red) under a basal condition (2.8 mM glucose) and upon glucose (16.7 mM) stimulation for 10 min were shown. (D) Representative images of the photobleaching experiment in INS-1E cells under low glucose (2.8 mM) condition for 10 min. (C, D) Yellow rectangles indicate the regions with the most obvious difference while white rectangles indicate the regions without obvious difference before and after glucose stimulation. (Scale bar: 10 µm.) (E) Quantification of the dynamic change in SPY555 signal intensity in an individual cell upon glucose stimulation and in the photobleaching control group in C and D. (F) Quantification of the relative fluorescence signal intensity in an individual cell before and after glucose stimulation for 10 min. (E, F) n = 8 for the control group and n = 14–25 for the other groups. (G) Rac1 activity in the transfected cells under the basal condition or after high glucose (16.7 mM) stimulation for 3 or 10 min. n = 4–6. (H) Cdc42 activity in the transfected cells under the basal level or after high glucose stimulation for 3 min. n = 10. All data are presented as the mean ± SD. Student’s t test or one-way ANOVA with Bonferroni correction for multiple comparisons was used. *P < 0.05 and **P < 0.01. Representative images were shown.
Activation of Rac1 Rescues the Defective GSIS and F-Actin Remodeling in APPL2-Deficient β-Cells.
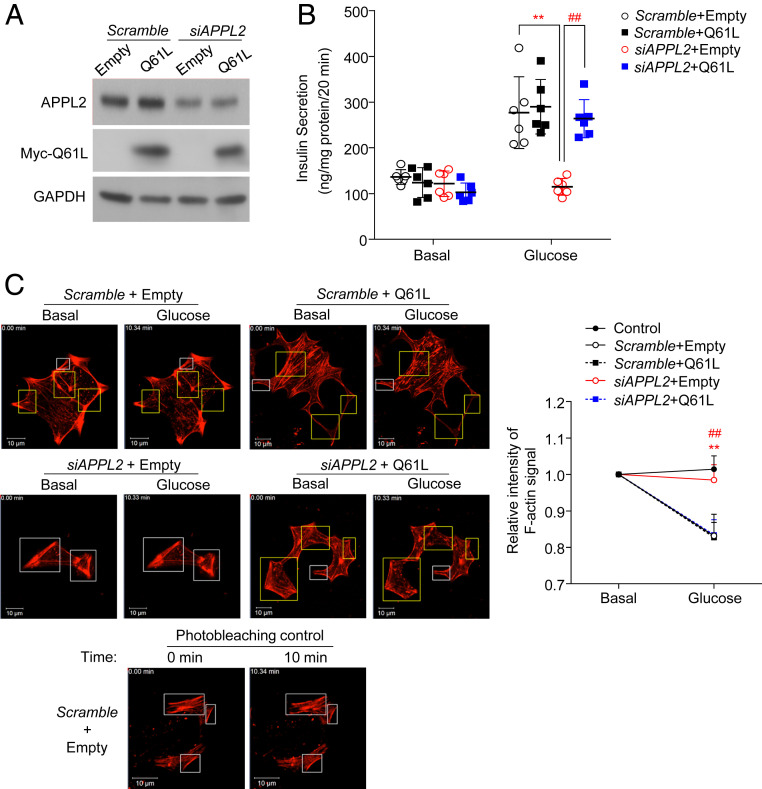
Since F-actin remodeling is tightly regulated by Rac1 and Cdc42 (6), we measured the activity of Rac1 and Cdc42 in INS-1E cells with down-regulation of APPL2 using pull-down activation assays as recently described (28). Consistent with previous studies (7, 29), glucose significantly increased both Rac1 and Cdc42 activities in INS-1E cells transfected with the scrambled control (Fig. 3 G and H). However, knockdown of APPL2 expression resulted in an impairment of Rac1 activation but had no effect on Cdc42 activity upon glucose stimulation (Fig. 3 G and H). To confirm the defective GSIS and F-actin remodeling are due to defective Rac1 activation, we transfected APPL2-knockdowned INS-1E cells with a vector expressing a constitutively active Rac1 mutant (Q61L) or an empty vector (as a control) (Fig. 4A). Rac1-Q61L is not responsible for its upstream GAP and hence exhibits a constitutively GTP-bound form (30). Consistently, knockdown of APPL2 expression diminished glucose-stimulated F-actin depolymerization and insulin secretion, but such unresponsiveness to glucose was largely reversed by ectopic expression of the constitutively active Q61L mutant (Fig. 4 B and C and SI Appendix, Fig. S8B). These data suggest that APPL2 appears to control GSIS via Rac1-mediated F-actin remodeling.
Fig. 4.
APPL2 regulates glucose-stimulated insulin secretion and F-actin remodeling via Rac1 activation. (A–C) INS-1E cells were cotransfected with siRNA against the scrambled control or APPL2 and an empty vector or plasmid encoding Rac1-Q61L mutant (active form of Rac1) for 48 h. The transfected cells were subjected to immunoblotting analysis (A), static GSIS (B), and (C) live-cell imaging of F-actin remodeling using a SPY555-actin probe (red) as described in Fig. 3. The chart on the Right is the quantification of the relative fluorescence signal intensity in an individual cell before (basal) and after glucose stimulation (16.7 mM) for 10 min. (B) n = 6. (C) n = 10 for the photobleaching control group, and n = 12–14 for the other groups. (Scale bar: 10 µm.) All data are presented as the mean ± SD. Significance was determined using one-way ANOVA with Bonferroni correction. **P < 0.01 (Scramble + Empty vs. siAPPL2 + Empty) and ##P < 0.01 (siAPPL2 + Empty vs. siAPPL2 + Q61L). Representative images were shown.
The Interaction between APPL2 and RacGAP1 Is Crucial for GSIS and F-Actin Remodeling.
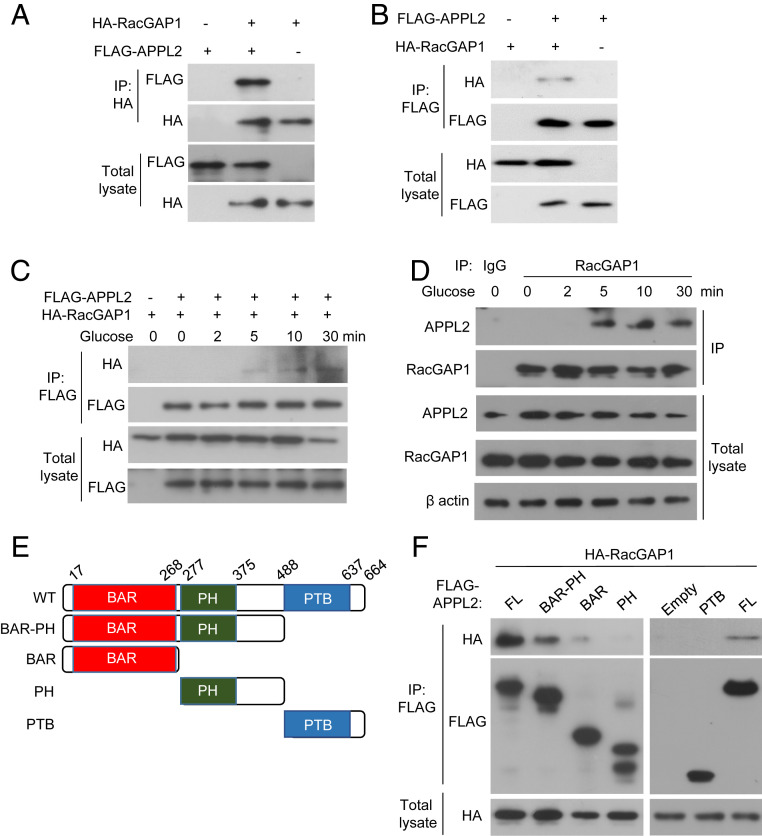
In our previous study, we have employed co-immunoprecipitation (IP) and mass spectrometry analysis to identify the interacting partners of APPL2 in the HEK293 cells (21). Interestingly, we found that APPL2 was coimmunoprecipitated (co-IP) with Rac GTPase activating protein 1 (RacGAP1), a GAP known to inactivate Rac1 during cytokinesis (17, 18). In addition, a recent proteomics analysis indicates that the interaction between Rac1 and RacGAP1 is observed in INS-1E cells, and RacGAP1 is expressed in human islets (31). Therefore, we hypothesized that APPL2 regulates Rac1 activity by antagonizing GAP activity of RacGAP1 via the protein–protein interaction. We first validated and characterized the APPL2-RacGAP1 interaction by co-IP and immunoblotting analyses in cells cotransfected with FLAG-tagged APPL2 and HA-tagged RacGAP1. IP of FLAG-tagged APPL2 protein resulted in co-IP of HA-tagged RacGAP1 protein in HEK293 cells and vice versa (Fig. 5 A and B). In addition, the APPL2-RacGAP1 interaction also occurred in INS-1E cells and was enhanced by glucose stimulation in a time-dependent manner (Fig. 5 C and D). Further analysis indicated that the BAR-PH domain of APPL2 was the functional unit for the RacGAP1 interaction, while the BAR, the PH, or the PTB domain alone only displayed a very weak or even no interaction with the RacGAP1 protein (Fig. 5 E and F). Apart from the interaction with RacGAP1, our co-IP assay revealed that APPL2 also interacted with Rac1 (SI Appendix, Fig. S9 A and B).
Fig. 5.
APPL2 interacts with RacGAP1 in a glucose-dependent manner in INS-1E cells. HEK293 cells (A, B) or INS-1E cells (C) were cotransfected with plasmids encoding FLAG-tagged APPL2 and HA-tagged RacGAP1 for 48 h. INS-1E cells were stimulated with glucose (16.7 mM) for the indicated time points. The transfected cells were subjected to IP using an antibody against HA tag (A) or FLAG tag (B and C). (D) INS-1E cells stimulated with glucose (16.7 mM) for the indicated time points were subjected to IP using an anti-RacGAP1 antibody or nonspecific IgG as a negative control, followed by immunoblotting analysis as indicated. (E) Schematic of FLAG-tagged full-length (FL) APPL2 and its truncated mutants. (F) HEK293 cells were transfected with indicated APPL2 plasmids and HA-RacGAP1, followed by IP and immunoblotting analysis. Representative images were shown from three independent experiments.
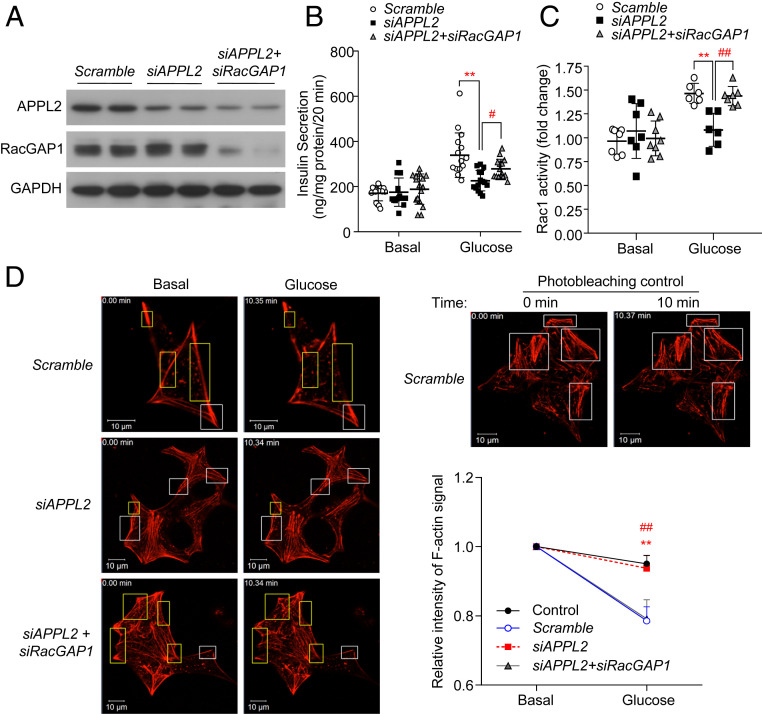
To delineate whether APPL2 regulates GSIS and Rac1 activation via RacGAP1, we employed siRNAs for concomitant knockdown of APPL2 and RacGAP1 in INS-1E cells. Protein expression of APPL2 and RacGAP1 were markedly decreased in INS-1E cells transfected with siRNA against APPL2 and/or RacGAP1 compared with the cells transfected with the scrambled control (Fig. 6A). As expected, knockdown of APPL2 expression led to impairment of GSIS, Rac1 activation, and F-actin depolymerization, whereas these impairments were largely reversed when RacGAP1 expression was concomitantly down-regulated (Fig. 6 B–D and SI Appendix, Fig. S8C). These data suggest that APPL2 regulates GSIS and Rac1-mediated F-actin remodeling by suppression of RacGAP1.
Fig. 6.
APPL2 regulates Rac1 activity and F-actin remodeling via RacGAP1. (A–D) INS-1E cells were cotransfected with siRNA against APPL2, RacGAP1, and/or the scrambled control for 48 h, followed by immunoblotting analysis of APPL2, RacGAP1, and GAPDH (A), static GSIS (B), measurement of Rac1 activity at the basal level or after glucose stimulation for 3 min (C), and live-cell staining of F-actin (D) as described in Fig. 3. Images taken from INS-1E cells transfected with the scrambled control kept in a Krebs buffer with 2.8-mM glucose for 10 min were used as the photobleaching control. The chart on the Right is the quantification of the relative fluorescence signal intensity in an individual cell before (basal) and after glucose stimulation (16.7 mM) for 10 min. (B) n = 11–16. (C) n = 6–8. (D) n = 8 for the photobleaching control group and n = 14–25 for the other groups. (Scale bar: 10 µm.) All data are presented as mean ± SD. Significance was determined using one-way ANOVA with Bonferroni correction. **P < 0.01 (Scramble vs. siAPPL2), #P < 0.05, and ##P < 0.01 (siAPPL2 vs. siAPPL2 + siRacGAP1). Representative images were shown.
Discussion
Dysregulation of cytoskeletal remodeling causes impairment of fusion and trafficking of insulin granules to the plasma membrane, leading to defective GSIS in type 2 diabetes. The small GTPase Rac1 positively controls GSIS via F-actin cytoskeletal remodeling, but its upstream regulatory mechanism remains poorly characterized. In this study, we show that the BAR-PH domain containing protein APPL2 regulates Rac1-mediated F-actin depolymerization and insulin secretion by interacting with RacGAP1 in pancreatic β-cells (SI Appendix, Fig. S10). APPL2 deficiency in pancreatic β-cells leads to a dramatic reduction of GSIS and glucose intolerance in mice, and such impairment is more pronounced in the dietary-induced obese mouse model.
APPL1 and APPL2 are a pair of Yin and Yang proteins that positively and negatively regulate insulin and adiponectin actions in multiple cell types, respectively (10, 32, 33). APPL1 and APPL2 form a heterodimer and/or homodimer via their BAR-PH domains and have distinct structures and electrostatic surfaces (22, 34). Both APPL1 and APPL2 can bind to the adiponectin receptors but exert opposite effects on adiponectin-induced AMPK activation and subsequent glucose uptake and nitric oxide production in myotubes and endothelial cells, respectively (11, 32, 33). As an insulin sensitizer, APPL1 enhances Akt activation by blocking the interaction between Akt and its endogenous inhibitor Tribble 3 (13, 35). On the other hand, overexpression of APPL2 suppresses insulin-stimulated glucose uptake in myotubes at a step downstream of Akt (21). On the contrary, in pancreatic β-cells, both APPL1 and APPL2 exert a promoting effect on insulin secretion. Our previous study showed that APPL1 deficiency impairs first- but not second-phase GSIS by downregulating SNARE proteins via an Akt-dependent pathway in pancreatic β-cells, whereas the current study indicates that APPL2 deficiency abolishes both first- and second-phase GSIS, at least, in part, via modulating the RacGAP1-Rac1 pathway. APPL1 has also been shown to increase the promoting effect of adiponectin on GSIS (14). In the current study, we show that APPL2 deficiency has no obvious effect on SNARE protein expression in pancreatic islets. Therefore, the regulatory pathways of APPL2 and APPL1 on GSIS appear to be distinct and independent of each other, although the effect of APPL2 deficiency on insulin and adiponectin signaling in pancreatic β-cells remains to be further determined.
Rac1 is a master regulator of cytoskeletal remodeling and is important for insulin granule fusion and trafficking for subsequent secretion in pancreatic β-cells (2). Inactivation of Rac1 by genetic ablation, the pharmacological inhibitor, or siRNA abolishes GSIS in insulinoma cell lines or islets (8, 29, 36). The activity of Rac1 is regulated by its upstream regulators including guanine exchange factors ([GEFs], positive regulator), Rho GDP-dissociation inhibitors ([Rho-GDIs], negative regulator) and GAPs (negative regulator), respectively. Two GEFs including T-lymphoma invasion and metastasis-inducing protein 1 (Tiam1), and guanine nucleotide exchange factor (Vav2) have been shown to promote GSIS via Rac1 activation in pancreatic β-cells (37–39). Rho-GDIα and Rho-GDI-β are expressed in INS-1E cells (40) and exhibit distinct effects on GSIS, although both of them inhibit Rac1 and Cdc42 activation (40–42). To date, no GAP has been identified to inactivate Rac1 and control GSIS in β-cells. Although a recent proteomics study identified RacGAP1 as a potential interacting partner of Rac1 in INS-1E 832/13 cells (31), its role in β-cell function remains unknown. RacGAP1 has been shown to be abundantly expressed in human and rodent islets and is known to inactivate Rac1 by promoting hydrolysis of GTP bound to GDP during cytokinesis (17, 18, 31, 43). Similar to the pancreatic β-cells lack of Rac1 (8), APPL2 deficiency only affects glucose- but not KCl-induced insulin secretion, and such a defect can be reversed by treatment with latrunculin A, cytochalasin B, or overexpression of gelsolin. Knockdown of APPL2 expression impairs Rac1 activation and F-actin remodeling induced by glucose. The defects in APPL2 knockdown cells are largely reversed by simultaneous down-regulation of RacGAP1 or ectopic expression of the constitutively active Rac1 mutant. Upon glucose stimulation, the interaction between APPL2 and RacGAP1 is enhanced in INS-1E cells. In addition, it is worth noting that Rac1 deficient islets display impairment of second- but not first-phase GSIS (8). On the contrary, genetic deletion of APPL2 abolishes both first- and second-phase GSIS in pancreatic islets. Further investigation on whether activation of Rac1 or inactivation of RacGAP1 is able to rescue the defective first- and second-phase GSIS in APPL2 deficient islets is required. Although concomitant knockdown of RacGAP1 almost completely rescues the defective Rac1 activation and F-actin depolymerization in APPL2 down-regulated INS-1E cells (Fig. 6), its rescue effect on insulin secretion is partial. These data indicate that an additional pathway, such as insulin and adiponectin signaling, might mediate the APPL2 actions in pancreatic β-cells. Taken in conjunction, our data suggest that APPL2 orchestrates GSIS, at least, in part, via the RacGAP1-Rac1 signaling axis-mediated F-actin remodeling.
The BAR domain proteins control multiple biological pathways including F-actin remodeling, activity of GTPases, and vesicle trafficking and fusion (44, 45). We and others have demonstrated that the BAR domain proteins affect the activity of GAP via protein–protein interaction (21, 46). Deletion of BAR domain in Arf-GAP with the SH3 domain, ANK repeat, and PH domain-containing protein 1 (ASAP1) increases its GAP activity (46). We previously reported that APPL2 prevents insulin-elicited phosphorylation of the GAP domain of TBC1D1 at threonine 596 via protein–protein interaction, which, in turn, inhibits glucose uptake in skeletal muscles (21). Our current study indicates that the BAR-PH domain but not the BAR, the PH, or the PTB domain alone mediates the interaction of APPL2 with RacGAP1. We speculated that the BAR-PH domain of APPL2 is able to antagonize the inhibitory effect of RacGAP1 on Rac1 activation via protein–protein interaction. Apart from APPL1 and APPL2, several other BAR domain proteins including protein interacting with C-kinase 1, islet cell autoantigen 69 kDa, and arfaptin-1 have been implicated in insulin secretion by controlling biogenesis, maturation, and trafficking of insulin granules (47–49). Deletion of these BAR domain containing proteins leads to generation of small nonfunctional and immature insulin granules in pancreatic β-cells, which in turn causes glucose intolerance in mouse models (19, 47–49). Of note, APPL2, Arfaptin-1, ICA69, and PICK1 belong to the classical crescent-shape BAR domain subfamily. Indeed, we also found that APPL2 somehow affects the docking of mature insulin granules to the plasma membrane. This change might explain the defective first-phase GSIS in the APPL2 deficiency islet, yet further experiments are required to delineate the underlying cause. Collectively, these findings indicate that the BAR domain protein family plays an essential role in the regulation of insulin secretion via multiple pathways and levels.
To summarize, our current study highlights the importance of APPL2 in the regulation of GSIS in pancreatic β-cells. In response to glucose, APPL2 interacts with RacGAP1, which in turn inhibits the conversion of active GTP-bound Rac1 to inactive GDP-bound Rac1 (SI Appendix, Fig. S10). Activation of Rac1 depolymerizes F-actin, allowing fusion and trafficking of insulin granules to the plasma membrane for exocytosis. Therefore, the APPL2-RacGAP-Rac1 signaling axis is essential for tight regulation of GSIS and subsequent glucose homeostasis.
Materials and Methods
Animal Studies.
All animals were sex and age matched, and littermates were used, as indicated in the figure legends. Animals were allocated to their experimental groups according to their genotypes. Therefore, no randomization was used unless otherwise noted. The investigators were not blinded to the experimental groups. Generation of APPL2floxed/floxed mice, RIP-APPL2 KO mice, and RIP-Cre control mice have been described in our previous publications (19, 21). All mice were kept in cages in a 12h/12h light/dark cycle and had free access to water and either STC (Purina) or 45% HFD (Cat. no. D12451, Research Diets). GTT and GSIS were performed in 6-h-fasted mice (fed with STC) and overnight-fasted mice (fed with HFD) after intraperitoneal (i.p.) injection of d-glucose (2 g/kg) as previously described (13, 20). For the insulin tolerance test, mice were i.p. injected with human recombinant insulin (Actrapid HM Novo Nordisk) after 6-h fasting. Blood samples were taken from the tail vein for the measurement of glucose and insulin levels using a glucose meter and an insulin enzyme-linked immunosorbent assay (ELISA) kit (Cat. no. 32380, Antibody and Immunoassay Services, The University of Hong Kong [HKU]), respectively. All animal experimental protocols were approved by the animal ethics committee of HKU and The Hong Kong Polytechnic University (PolyU).
Islet Isolation and Insulin Secretion Assay.
Mice were fasted for 4 h and killed by cervical dislocation. The pancreas was perfused with collagenase P (1.4 mg/mL, Cat. no. 11213865001, Roche) via the bile common duct and subsequently digested at 37 °C for 20 min. The digested pancreas was then filtered through 500- and 70-μm cell strainers. The captured islets at 70-μm cell strainers were washed with solution G (Hanks’ balanced salt solution [Cat. no. 14065056, ThermoFisher Scientific] with 0.1% bovine serum albumin [BSA]) and cultured in Roswell Park Memorial Institute (RPMI) 1640 with 10% fetal bovine serum (FBS) overnight. The isolated islets with similar size were manually picked under a microscope and then washed twice with Krebs buffer containing 0.1% fatty acid-free BSA and 2.8-mM glucose for 1 h, followed by stimulation with different stimulants for various time periods as specified in each figure legend. In Fig. 2H, the islets were pretreated with 0.5-µM latrunculin A (Cat. no. 76343–93-6, Cayman Chemical) or dimethyl sulfoxide (DMSO) (as a vehicle control) for 1 h. In SI Appendix, Fig. S7A, the islets were pretreated with 5-µM cytochalasin B (Cat. no. C6762, Sigma-Aldrich) or DMSO for 1.5 h. For the determination of dynamic insulin secretion, the isolated islets were incubated with Krebs buffer for 30 min and perfused with Krebs buffer containing 2.8-mM glucose for 6 min, and the perfusate was, then, switched to Krebs buffer containing 16.7-mM glucose. Eluted fractions were collected at 3-min intervals for 42 min. The first- and second-phase insulin secretions were defined as 0–9 and 10–42 min, respectively. Insulin secreted in each fraction was measured using the insulin ELISA kit and normalized with total number of islets or intracellular insulin content as indicated in the figure legend. To extract insulin from pancreatic islets, the isolated islets were incubated with acid ethanol (1.5% hydrogen chloride in 70% ethanol) at −20 °C for overnight and then sonicated. The lysate was incubated overnight at −20 °C and centrifuged at 10,000 g at 4 °C. The pancreatic extract was neutralized with equal volume of 1-M 2-amino-2-hydroxymethyl-1,3-propanediol buffer (pH 7.5). Extracted insulin content and insulin secreted in the conditional medium were measured using the insulin ELISA kit.
Real-Time Live-Cell Imaging.
F-actin fluorescent probe SPY555-actin (Cat. no. CY-SC202, Cytoskeleton) was used to stain and visualize F-actin in islets and INS-1E cells (50). Islets isolated from RIP-APPL2 KO mice and their WT controls or INS-1E cells were seeded into an 8-well Nunc Lab-Tek II chambered coverglass with a no. 1.5 borosilicate glass bottom (ThermoFisher Scientific) and cultured for overnight. Islets or INS-1E cells transfected with siRNA against APPL2 (siAPPL2), RacGAP1 (siRacGAP1), scrambled control, or plasmid vectors encoding the Rac1-Q61L mutant or empty vector for 48 h were incubated in Krebs buffer containing 2.8-mM glucose and SPY555-actin probe (1,000× dilution according to manufacturer instruction) at 37 °C for 90 min. After that, a dynamic change in F-actin signal was recorded at the basal level and then switched to glucose (16.7 mM) stimulation for 10 min using the live-cell confocal imaging systems with temperature, oxygen, and carbon dioxide control (University Life Science, PolyU, or Centre for Imaging and Flow Cytometry Core, HKU). Another batch of cells or islets with SPY555-actin labeling were subjected to recording of the F-actin signal for 10 min in the Krebs buffer containing 2.8-mM glucose, which was served as the photobleaching control. The F-actin images in INS-1E cells and islets were captured and recorded every 20 s or every 70 s using a ZEISS LSM 900 confocal microscope or a Leica TCS SP8 MP multiphoton/confocal microscope, respectively. For the analysis in islets, data were averaged from, at least, 11 islets from three animals per each genotype. F-actin fluorescence intensity in an individual islet was quantified by ImageJ and normalized with islet size. For the analysis in INS-1E cells, data were averaged from, at least, 12 cells per experiment group and, at least, eight cells from the photobleaching group from three independent experiments. Fluorescence signal intensity of F-actin in an individual cell was quantified using ZEN software. Relative intensity of the F-actin signal before and after glucose stimulation for 10 min or a dynamic change in F-actin signal was shown and specified in each figure legend.
Supplementary Material
Acknowledgments
We thank Dr. Michael Yuen from University Life Science (PolyU) and Dr. Miao Chen from Imaging and Flow Cytometry Core (HKU) for their assistance on live-cell imaging experiments. This research was funded by Hong Kong Research Grant Council General Research Grant (17101815), National Natural Science Foundation of China (NSFC) (Grants 81471015 and 91857119), National Key Research and Development Program of China (Grant 2016YFC1305003), and Hong Kong Research Grant Council Area of Excellence (AoE/M-707/18). JBK was supported by the National Research Foundation of 868 Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A3B2078617).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016997117/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Seino S., Shibasaki T., Minami K., Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J. Clin. Invest. 121, 2118–2125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z., Thurmond D. C., Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 122, 893–903 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu X., et al. , Microtubules negatively regulate insulin secretion in pancreatic β cells. Dev. Cell 34, 656–668 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C. J., et al. , Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J. Biol. Chem. 285, 339–348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orci L., Gabbay K. H., Malaisse W. J., Pancreatic beta-cell web: Its possible role in insulin secretion. Science 175, 1128–1130 (1972). [DOI] [PubMed] [Google Scholar]
- 6.Kalwat M. A., Thurmond D. C., Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp. Mol. Med. 45, e37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z., Oh E., Thurmond D. C., Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J. Biol. Chem. 282, 9536–9546 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahara S., et al. , Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. Diabetologia 56, 1088–1097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miaczynska M., et al. , APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116, 445–456 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Cheng K. K., Lam K. S., Wang B., Xu A., Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract. Res. Clin. Endocrinol. Metab. 28, 3–13 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Wang C., et al. , Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J. Biol. Chem. 284, 31608–31615 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B., Cheng K. K., Hypothalamic AMPK as a mediator of hormonal regulation of energy balance. Int. J. Mol. Sci. 19, 3552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng K. K., et al. , APPL1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc. Natl. Acad. Sci. U.S.A. 109, 8919–8924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C., et al. , Deficiency of APPL1 in mice impairs glucose-stimulated insulin secretion through inhibition of pancreatic beta cell mitochondrial function. Diabetologia 56, 1999–2009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prudente S., et al. , Loss-of-function mutations in APPL1 in familial diabetes mellitus. Am. J. Hum. Genet. 97, 177–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X., et al. , APPL1 prevents pancreatic beta cell death and inflammation by dampening NFκB activation in a mouse model of type 1 diabetes. Diabetologia 60, 464–474 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Bastos R. N., Penate X., Bates M., Hammond D., Barr F. A., CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J. Cell Biol. 198, 865–880 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquemet G., et al. , Rac1 is deactivated at integrin activation sites through an IQGAP1-filamin-A-RacGAP1 pathway. J. Cell Sci. 126, 4121–4135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B., et al. , Activation of hypothalamic RIP-Cre neurons promotes beiging of WAT via sympathetic nervous system. EMBO Rep. 19, 44977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., et al. , The MDM2-p53-pyruvate carboxylase signalling axis couples mitochondrial metabolism to glucose-stimulated insulin secretion in pancreatic β-cells. Nat. Commun. 7, 11740 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng K. K., et al. , The adaptor protein APPL2 inhibits insulin-stimulated glucose uptake by interacting with TBC1D1 in skeletal muscle. Diabetes 63, 3748–3758 (2014). [DOI] [PubMed] [Google Scholar]
- 22.King G. J., et al. , Membrane curvature protein exhibits interdomain flexibility and binds a small GTPase. J. Biol. Chem. 287, 40996–41006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qualmann B., Koch D., Kessels M. M., Let’s go bananas: Revisiting the endocytic BAR code. EMBO J. 30, 3501–3515 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurmond D. C., Gonelle-Gispert C., Furukawa M., Halban P. A., Pessin J. E., Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol. Endocrinol. 17, 732–742 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Hoboth P., et al. , Aged insulin granules display reduced microtubule-dependent mobility and are disposed within actin-positive multigranular bodies. Proc. Natl. Acad. Sci. U.S.A. 112, E667–E676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mziaut H., et al. , The F-actin modifier villin regulates insulin granule dynamics and exocytosis downstream of islet cell autoantigen 512. Mol. Metab. 5, 656–668 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomas A., Yermen B., Min L., Pessin J. E., Halban P. A., Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: Role of gelsolin and cooperation with the MAPK signalling pathway. J. Cell Sci. 119, 2156–2167 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Veluthakal R., et al. , Restoration of glucose-stimulated Cdc42-Pak1 activation and insulin secretion by a selective epac activator in type 2 diabetic human islets. Diabetes 67, 1999–2011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Luo R., Kowluru A., Li G., Novel regulation by Rac1 of glucose- and forskolin-induced insulin secretion in INS-1 beta-cells. Am. J. Physiol. Endocrinol. Metab. 286, E818–E827 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Grand R. J., Owen D., The biochemistry of ras p21. Biochem. J. 279, 609–631 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damacharla D., et al. , Quantitative proteomics reveals novel interaction partners of Rac1 in pancreatic β-cells: Evidence for increased interaction with Rac1 under hyperglycemic conditions. Mol. Cell. Endocrinol. 494, 110489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao X., et al. , APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 8, 516–523 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Cheng K. K., et al. , Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56, 1387–1394 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Zhu G., et al. , Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. EMBO J. 26, 3484–3493 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng K. K., et al. , APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 9, 417–427 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Veluthakal R., Kaur H., Goalstone M., Kowluru A., Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes 56, 204–210 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Abe K., et al. , Vav2 is an activator of Cdc42, Rac1, and RhoA. J. Biol. Chem. 275, 10141–10149 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Veluthakal R., Madathilparambil S. V., McDonald P., Olson L. K., Kowluru A., Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem. Pharmacol. 77, 101–113 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veluthakal R., et al. , VAV2, a guanine nucleotide exchange factor for Rac1, regulates glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia 58, 2573–2581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thamilselvan V., Kowluru A., Paradoxical regulation of glucose-induced Rac1 activation and insulin secretion by RhoGDIβ in pancreatic β-cells. Small GTPases, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z., Thurmond D. C., Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion. J. Biol. Chem. 285, 6186–6197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowluru A., Veluthakal R., Rho guanosine diphosphate-dissociation inhibitor plays a negative modulatory role in glucose-stimulated insulin secretion. Diabetes 54, 3523–3529 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Canman J. C., et al. , Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science 322, 1543–1546 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aspenstrom P., BAR domain proteins regulate Rho GTPase signaling. Adv. Exp. Med. Biol. 1111, 33–53 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Carman P. J., Dominguez R., BAR domain proteins-a linkage between cellular membranes, signaling pathways, and the actin cytoskeleton. Biophys. Rev. 10, 1587–1604 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jian X., et al. , Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J. Biol. Chem. 284, 1652–1663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gehart H., et al. , The BAR domain protein Arfaptin-1 controls secretory granule biogenesis at the trans-Golgi network. Dev. Cell 23, 756–768 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Cao M., et al. , PICK1 and ICA69 control insulin granule trafficking and their deficiencies lead to impaired glucose tolerance. PLoS Biol. 11, e1001541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holst B., et al. , PICK1 deficiency impairs secretory vesicle biogenesis and leads to growth retardation and decreased glucose tolerance. PLoS Biol. 11, e1001542 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukinavičius G., et al. , Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 11, 731–733 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.