Significance
Ecosystem services derive from ecosystem functions and rely on complex interactions among a diversity of organisms. By understanding the relationships between biodiversity, ecosystem functions, and the services humans receive from nature, we can anticipate how changes in land use will affect ecosystems and human wellbeing. We show that increasing land-use intensity homogenizes the synergies between three organizational levels of the ecosystem, namely, biodiversity, ecosystem functions, and services. Increasing land-use intensity changes keystone components, which are important for the functioning of the ecosystem, and alters the synergies and trade-offs between biodiversity, ecosystem functions, and services. Our approach provides a comprehensive view of ecosystem functioning and can identify the key ecosystem attributes to monitor in order to prevent critical shifts in ecosystems.
Keywords: BEF, ecosystem function–service relationships, land management intensification, co-occurrence network, Biodiversity Exploratories
Abstract
Land-use intensification can increase provisioning ecosystem services, such as food and timber production, but it also drives changes in ecosystem functioning and biodiversity loss, which may ultimately compromise human wellbeing. To understand how changes in land-use intensity affect the relationships between biodiversity, ecosystem functions, and services, we built networks from correlations between the species richness of 16 trophic groups, 10 ecosystem functions, and 15 ecosystem services. We evaluated how the properties of these networks varied across land-use intensity gradients for 150 forests and 150 grasslands. Land-use intensity significantly affected network structure in both habitats. Changes in connectance were larger in forests, while changes in modularity and evenness were more evident in grasslands. Our results show that increasing land-use intensity leads to more homogeneous networks with less integration within modules in both habitats, driven by the belowground compartment in grasslands, while forest responses to land management were more complex. Land-use intensity strongly altered hub identity and module composition in both habitats, showing that the positive correlations of provisioning services with biodiversity and ecosystem functions found at low land-use intensity levels, decline at higher intensity levels. Our approach provides a comprehensive view of the relationships between multiple components of biodiversity, ecosystem functions, and ecosystem services and how they respond to land use. This can be used to identify overall changes in the ecosystem, to derive mechanistic hypotheses, and it can be readily applied to further global change drivers.
Ecosystem services are crucial for human wellbeing, but global drivers of biodiversity loss and ecosystem change are threatening their supply (1). To understand these impacts, it is critical to investigate the relationships between biodiversity, ecosystem functioning, and ecosystem services (2, 3), and how global change drivers, such as land-use intensification, affect them. Here, we refer to ecosystem functions as ecological processes that indirectly benefit to people, such as enzymatic activities contributing to nutrient cycling in soils (4). Ecosystem services can be defined as direct benefits or contributions of nature to people, often grouped into provisioning services (e.g., food and timber production), regulating services (e.g., climate-change mitigation via carbon storage and temperature buffering), and cultural services (e.g., recreational and educational opportunities) (5). Evidence from experimental and observational research shows that the diversity of various groups of organisms has distinct effects on functions (6), with multiple trophic groups involved in supplying any given function (e.g., diversity of decomposers, predators, and plants determine primary productivity; ref. 7). Similarly, multiple functions are often needed to supply particular services (e.g., nutrient cycling, water infiltration, and decomposition rates all influence crop production; ref. 8). Therefore, ecosystem services are affected by changes in diversity across multiple trophic groups and changes in multiple ecosystem functions (6, 7, 9–11). Previous approaches to quantifying overall diversity and functioning have relied on integrated indices such as multidiversity and multifunctionality (6, 12, 13). As these indices average across diversities and functions, they may underestimate diversity–functioning relationships if trophic groups have opposing effects on functioning and may also miss shifts in the identity of organisms or functions that drive ecosystem service supply with changes in environmental conditions. We therefore need complementary approaches that examine the individual interactions between multiple diversities (i.e., the biodiversity of multiple trophic groups), functions, and services (3) and provide informative metrics quantifying how these interactions vary with global change drivers or other environmental changes. Network theory provides powerful tools to deal with highly complex systems (e.g., involving interactions between millions of social media users (14) or microbial species (15)) and metrics summarizing the interactions among multiple entities (16). Network metrics (Table 1) can, thus, be used to provide integrated measures of biodiversity–ecosystem function–service relationships and to determine how these relationships change between ecosystems or along gradients of global-change drivers.
Table 1.
Glossary of network metrics and definitions applied to this study
| Network metric | Definition | Ecological meaning |
| Node (aka Vertex) | Each element in the network; here: biodiversity (species richness of trophic groups), ecosystem functions, and ecosystem services. | Different components of the ecosystem that interact (e.g., depend upon each other or have an effect on each other) and eventually affect human wellbeing. |
| Link (aka Edge) | A positive (or negative) correlation between two nodes and its weight (i.e., absolute Spearman correlation coefficient). | Presence of a synergy (i.e., positive correlation) or trade-off (i.e., negative correlation) between two components of the ecosystem. |
| Degree (Li) | Number of positive (or negative) links of node i. | Strength of the synergies (or trade-offs) associated to a particular component of the ecosystem. |
| Hub | Most connected node of the network, i.e., node with largest D (a weighted degree metric, see Eq. 1). In this study, we define three hubs in a network, i.e., for biodiversity, ecosystem function, and ecosystem service nodes, separately. | Node with the largest synergies for other components of the network. It represents trophic groups or functions of high importance for the functioning of the whole system or the services that are most connected to biodiversity and ecosystem functions (i.e., the trophic group most correlated to ecosystem functions and services, the ecosystem function linked to most ecosystem services, or the ecosystem service most strongly correlated to biodiversity and ecosystem functions). |
| (Eq. 1) D = Li * LWi; where LWi is the absolute mean weight of all links of node i (see Eq. 2). | ||
| (Eq. 2) LWi = Σ pcc/(n−1); where pcc are the absolute partial correlation coefficients of node i and n is the number of nodes of the network. Note that in synergy networks, negative correlation coefficients are set to 0 and included in the count of n (vice versa for trade-off networks). | ||
| Connectance (L) (aka Weighted Density or Connectivity) | Proportion of positive (or negative) links from all possible links in the network, weighted by the strength of the links (see Eq. 3). | Importance of synergies in the ecosystem in relation to trade-offs (opposite for trade-off networks). High connectance indicates that many different trophic groups are important in driving functioning or service supply and that many different ecosystem functions are related to several services. In contrast, low connectance indicates a simpler system in which only a few trophic groups or functions are related to a function or service. |
| (Eq. 3) = Σpcc/(n*(n−1)/2); where pcc are the absolute partial correlation coefficients and n is the number of nodes of the network. | ||
| Module | Group of nodes highly connected among them and loosely connected to others, according to the cluster walktrap algorithm (44). | A group of ecosystem components with strong synergies among them. E.g., ecosystem services most linked to a particular trophic group or function, or the ecosystem functions related to a specific trophic group. |
| Modularity | Strength of the partition of a network into modules (43). | Dominance of highly synergistic groups of components in the ecosystem. In a highly modular network, different trophic groups drive distinct functions and services, while in a less modular network each trophic group affects a wide range of functions or services. |
| Evenness | Pielou’s evenness of the link strengths in the network | Homogeneity in the strength of the synergies (or trade-offs) in the ecosystem (64). Considering the same number of links, high evenness indicates that functional effects of different trophic groups, or function-service relationships, are similar in correlation strength, whereas low evenness indicates that ecosystem functioning or service supply is dominated by a few trophic groups or functions. |
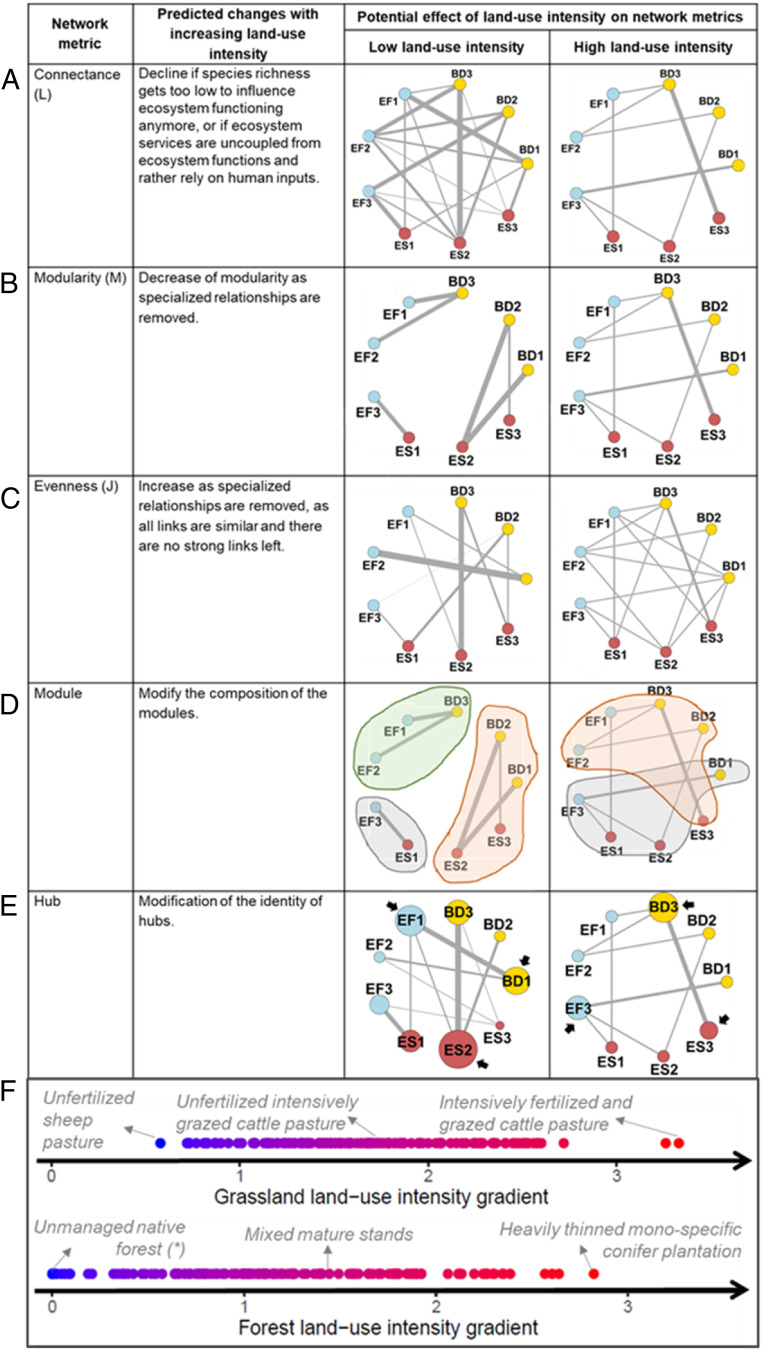
See Fig. 1 for expected changes in network metrics with land-use intensity. aka, also known as.
It is well known that land use affects biodiversity and ecosystem functioning (17–19). However, we know very little about how land-use intensity simultaneously alters the relationships between diversities, functions, and services, as very few studies have collected detailed quantitative data on multiple organism groups, functions, and services from the same sites. To address this question, we built tripartite correlation networks in which each node is a trophic group, ecosystem function, or service (i.e., three node types), and the strength of each link is the correlation coefficient between nodes of different types (i.e., between the species richness of a given trophic group, the level of a particular ecosystem function or an ecosystem service) (20) (see Fig. 1 for illustrations of network terminology). As correlations can be positive or negative, we built separate networks for the synergies (positive correlations) and for the trade-offs (negative correlations).
Fig. 1.
Predicted changes in network metrics with land–use intensity. (A–E) Simplified representation of our tripartite networks where biodiversity (BD), ecosystem functions (EF), and services (ES) are the nodes and the correlation between each pair of nodes is the link connecting them (thickness proportional to correlation strength). In E, node size is proportional to the number of positive correlations involving that node. (F) Distribution of the sampled plots along gradients of land-use intensity and management examples in grasslands (Upper) and forests (Lower). Land-use intensity is defined after ref. 37 for grasslands and ref. 38 for forests.
We focused on four key network metrics—connectance, evenness, modularity, and hubs—that are expected to change with land-use intensity (Fig. 1). First, increasing land-use intensity has been shown to reduce multitrophic diversity (18, 21), to disrupt relationships between taxa (22, 23), and to reduce ecosystem functioning (13). It is therefore plausible that land-use intensity would reduce the overall connectance (i.e., number and strength of the correlations; ref. 24 and Fig. 1A) of the biodiversity–function–service synergy network either by: (i) reducing the diversity of trophic groups until they become functionally extinct (25), (ii) disrupting ecological interactions themselves (26), or (iii) increasing trade-offs (e.g., in high land-use intensity grasslands, forage quality and biomass are strongly favored, at the expense of other functions and services and multitrophic diversity; refs. 27 and 28). Second, networks could also be homogenized if their evenness (i.e., homogeneity in correlation strength; ref. 29 and Fig. 1C) is increased because strong synergies are lost, leaving many weak links. Third, a decrease in modularity (i.e., the degree of network compartmentalization; refs. 30 and 31 and Fig. 1B) and change in module composition (Fig. 1D) is expected if functions become driven by a small number of less specialized trophic groups (32) or if ecosystem services come to depend more on external human inputs than on ecosystem functions. For example, under land-use intensification, forage quality may become less dependent on soil functions because it is determined by rates of fertilizer addition (13, 33, 34). Fourth, a shift in hub identity (i.e., the most connected nodes with the highest weighted degree; Fig. 1E) could arise when correlations among trophic groups change with land use (18, 35). We expect that cultural and regulating services are integrated into modules and are hubs at low land-use intensity, while provisioning services become hubs at high land-use intensity. Determining how these different network metrics are altered by land-use intensification can therefore provide a more integrated view of land-use effects on biodiversity, ecosystem functioning, and service supply.
Here, we use correlation networks to provide a holistic view of the effects of land-use intensity on the relationships between the biodiversity of multiple trophic groups, ecosystem functions, and ecosystem services for two major temperate ecosystems: grasslands and forests. In particular, we hypothesize that increasing land-use intensity will: (i) change network structure by reducing connectance and modularity and increasing evenness (Fig. 1 A–C); (ii) alter the composition of network modules (Fig. 1D), and (iii) shift the identity of network hubs (Fig. 1E). We use a unique dataset containing species richness of 16 trophic groups, 10 ecosystem functions, and 15 ecosystem services, assessed in 300 plots, distributed along gradients of land-use intensity in forests and grasslands (Fig. 1F). We identify the key nodes in each ecosystem and analyze how land-use intensity affects ecosystem structure by altering the linkages between biodiversity, ecosystem functions, and services. Finally, we discuss potential implications of our approach for the management of these ecosystems and show that biodiversity–function–service networks provide a complementary way to characterize land-use effects on ecosystems.
Methods
Study Area.
The study plots are part of the large-scale and long-term Biodiversity Exploratories project (http://www.biodiversity-exploratories.de/), which comprises 300 forest and grassland plots distributed across three regions in Germany; the UNESCO (United Nations Educational, Scientific and Cultural Organization) Biosphere Reserve Schwäbische Alb in the South-West, the National Park Hainich and its surroundings in the center, and the UNESCO Biosphere Reserve Schorfheide-Chorin in the North-East. The three regions differ substantially in geology, climate, and topography, covering a range of almost 3 °C in mean annual temperature and 500–1,000 mm in annual precipitation (36). In each region, 50 forest plots of 100 m × 100 m and 50 grassland plots of 50 m × 50 m were installed along gradients of land-use intensity representative for each region. The forest gradient ranges from unmanaged European beech forests (the dominant tree species in our study area) to conifer plantations of Scots pine or Norway spruce. The grassland gradient ranges from traditionally managed, extensively grazed grasslands to intensively managed, heavily fertilized and frequently grazed or mown, grasslands (36).
Land Use, Biodiversity, Ecosystem Functions, and Ecosystem Services.
Land-use intensity.
We assessed the intensity of land use in each plot using established indices for grasslands and forests. In grasslands, the index was the sum of the standardized intensities of mowing (number of cuts per year), fertilization (kg of N·ha−1·y−1), and grazing (livestock units·ha−1·y−1) (37). We calculated the index for the entire period of data collection (i.e., from 2008 to 2015) and calculated the average across years for each plot. In forests, the management intensity index was based on: (i) the ratio of harvested volume to the total wood volume (including standing, harvested, and dead wood), (ii) the proportional volume of nonnative tree species (i.e., not occurring in the stands under natural conditions), and (iii) the proportion of deadwood with saw cuts, all of which represent different aspects of forest management intensity (38).
Biodiversity.
Diversities were measured for various groups of bacteria, protists, soil fungi, plant pathogenic fungi (only for grasslands), bryophytes, lichens, vascular plants, and arthropods. We classified all species into 16 trophic groups (SI Appendix, Table S1). We used the species richness (or richness of operational taxonomic units, OTU) of each trophic group in our analyses. As different diversities were measured in different years (between 2008 and 2014), we used the year with most records per trophic group for the analyses. See SI Appendix, Supplementary Methods for detailed descriptions of the measurement procedures for each trophic group.
Ecosystem functions.
Ten different ecosystem functions (eight in grasslands and six in forests) were measured across all 300 plots. In grasslands, these comprised belowground productivity (root biomass), root decomposition, dung removal, enzymatic activities related to the carbon, nitrogen, and phosphorus cycles, and indices of nitrogen and phosphorus retention. In forests, they comprised root and dung decomposition, enzymatic activities related to the carbon and phosphorus cycles, and concentrations of available nitrogen and phosphorus. Variables were transformed if necessary (i.e., 1/x), so that high values of all variables indicate high ecosystem functioning, i.e., rapid and efficient transfer of energy and matter through the ecosystem. See SI Appendix, Supplementary Methods for details.
Ecosystem services.
Fifteen different proxies of potential ecosystem service supply were assessed (eight in grasslands and nine in forests), spanning the three main categories of provisioning, regulating, and cultural services (5). In grasslands, forage biomass and quality were the provisioning services; herbivore control, soil-carbon stocks, and infiltration rate the regulating services; and charismatic butterflies and bird-watching potential the cultural services (6). In forests, timber production was the provisioning service; biological pest control of bark beetles, temperature regulation, soil and tree carbon stocks the regulating services; edible fungi and edible plants, plants of cultural value, and bird-watching potential the cultural services (39). See SI Appendix, Supplementary Methods for details and rationale.
Statistical Analyses.
All analyses were performed using R version 3.6.2 (40). See SI Appendix, Data availability for R script.
Data preparation.
We pooled the biodiversity, ecosystem function, and ecosystem service datasets separately for forests and grasslands and rescaled all variables between 0 and 1 prior to the analyses using the formula StV = (x − xmin)/(xmax − xmin); where StV is the standardized variable, x is the target variable and xmin, xmax are the minimum and maximum value across all plots, respectively. To remove the effect of environmental variables on the relationships, we used residuals after fitting a linear model with region, elevation, topographic wetness index, soil type, soil depth, and soil pH in the grassland dataset (6); in the forest dataset we included region, elevation, soil type, soil depth, and soil pH (39). See SI Appendix, Supplementary Methods for a description of the environmental variables.
To assess the effect of increasing land-use intensity on the correlations between diversities, functions, and services, we used a moving-window approach. We sorted the plots along the land-use intensity gradient, from minimum to maximum land-use intensity, separately for forests and grasslands, and identified the minimum window size (i.e., minimum number of plots) that allowed us to compute all pairwise partial correlations. These were 60 and 50 plots per window block in grasslands and forests, respectively. This difference was due to differences in the number of nodes and missing plots in each habitat. Missing plots were dropped individually for each pairwise correlation using the “pairwise.complete.observation” mode. Therefore, we ended up with 91 windows of 60 plots in grasslands and 101 windows of 50 plots in forests. We calculated the mean land-use intensity for each window, resulting in a land-use intensity gradient ranging from 1.16 to 2.17 in grasslands (gradient across individual plots from 0.5 to 3.5) and a gradient from 0.53 to 1.97 in forests (full gradient 0–3). For each window, we calculated partial pairwise Spearman correlations between each pair of variables using an adapted version of the pcor.test function (see SI Appendix, Data availability for R script) (41). We used partial correlations to control for potential spurious correlations arising from collinearity (41) (i.e., caused by a shared driver); in this way, when correlating, e.g., a diversity variable “x” with a function value “y,” the correlation coefficient would be corrected for all other diversity, function, and service variables (“z1-n”). This is a very conservative approach because it removes all shared variance between pairs of variables. Because we were interested in tripartite relationships (i.e., across node types; see Table 1 for a definition of nodes and node types), we ignored all correlations between nodes of the same type, meaning we only allowed correlations between biodiversity and ecosystem functions, between biodiversity and ecosystem services, and between ecosystem functions and services (Fig. 1). To facilitate the interpretation of the results, we calculated separate networks for positive (synergies) and negative correlations (trade-offs), while network modules and hubs were only calculated for the synergy networks.
Network analyses.
The partial correlations from each window were converted to a network graph object and analyzed using the R package igraph (42). For each network, we calculated connectance, modularity, and evenness (Table 1) for the synergy and trade-off networks separately. For connectance, we used the function “strength” in igraph, including the correlation coefficient as a weight (42). For modularity, we used the “cluster walktrap” algorithm (43) in igraph, which separates densely connected subgraphs via random walks, and used correlation coefficients as weights. For evenness, we used the vegan package (44). We calculated all metrics for 100 randomizations of the dataset along the land-use intensity gradient to compare our results with random expectations (45) (SI Appendix, Extended Results). We fitted generalized additive models (GAMs) to analyze the effect of land-use intensity on these metrics using the mgcv package (46). We smoothed the fitted response by setting the k attribute of the GAMs as large as possible, while avoiding unexpected “wiggliness” of the curve (47) and ensuring normality of the residuals. We repeated the same procedure with subsets of the networks to test for additional potential drivers, such as differences between link types (i.e., separate networks for biodiversity-functioning, biodiversity-services, and functioning-services), and compartment (i.e., aboveground and belowground networks). We also investigated the change in correlation strength by quantiles using the package quantreg (48).
We compared the composition of the network modules at lowest and highest intensity levels in each habitat using the “cluster Louvain” algorithm in igraph, based on a hierarchical approach which assigns nodes to modules (weighted by their correlation coefficients) and identifies the assignment which maximizes modularity across the network while maintaining a balanced number of modules. Note that the existence and composition of the modules in a network is independent from its modularity value, meaning that modules can be created even if the modularity value of the network is low. Figures were depicted using the ggplot package (49).
Finally, we identified the most connected biodiversity, ecosystem function, and ecosystem service nodes (i.e., the hubs, see Table 1) and analyzed the effect of land-use intensity on nodes’ D (i.e., weighted node degree) by fitting GLMs with the interaction of land-use intensity and node identity.
Results
Effects of Land-Use Intensity on Network Structure.
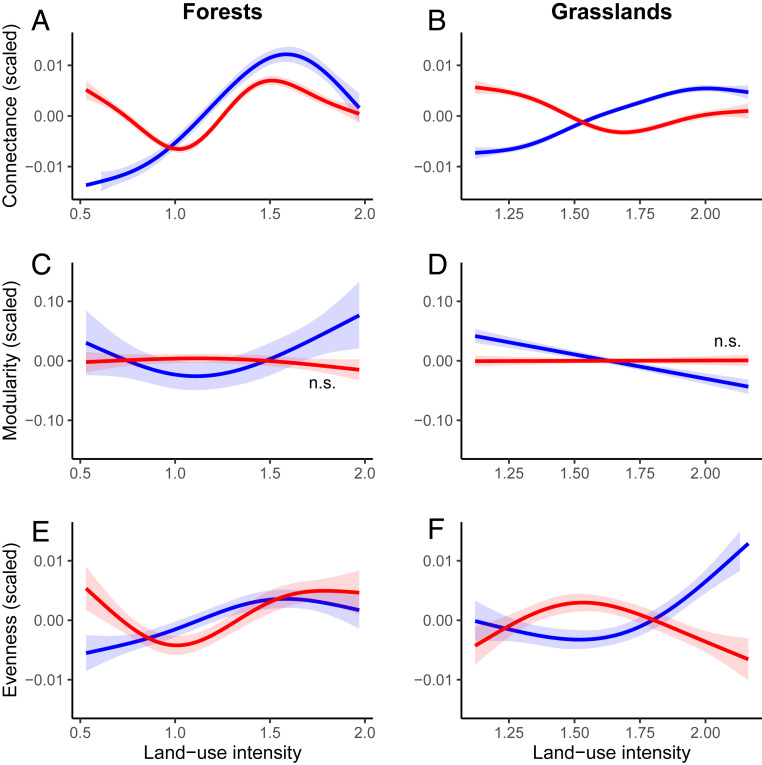
Land-use intensity significantly affected network structure in forests and grasslands (SI Appendix, Table S2). In both ecosystem types, connectance showed a nonlinear response to land-use intensity, with an overall increase in the synergy networks (i.e., number and strength of the positive correlations) and overall decrease in the trade-off networks (i.e., based on negative correlations), although forests showed more complex responses than grasslands. At high land-use intensity, connectance was similar in both synergy and trade-off networks, in both ecosystem types (Fig. 2 A and B). Changes in connectance can be driven either by changes in the number of synergies and trade-offs or by changes in the strength of individual synergies or trade-offs. With increasing land-use intensity, synergies became more numerous (SI Appendix, Fig. S2), while changes in strength were minor (SI Appendix, Fig. S3A). Interestingly, the quantile regressions showed a decrease in strong synergies at high land-use intensity in grasslands, although there were no changes in forests (SI Appendix, Fig. S3B). Changes in grassland connectance seem to be driven by biodiversity–functioning relationships (green line in SI Appendix, Fig. S4B), while in forests these were not driven by any specific linkage type, as the connectance between biodiversity–function, biodiversity–service, and function–service subnetworks responded in similar ways (SI Appendix, Fig. S4A).
Fig. 2.
Standardized effects of land-use intensity on the structure of both positive (synergies, blue lines) and negative (trade-offs, red lines) correlation networks (connectance, modularity, and evenness) for 150 forests (A, C, and E) and 150 grasslands (B, D, and F). y axes represent the scaled effects (i.e., mean 0, SD 1); note the different scales. x axes are based on different land-use intensity indices in each ecosystem type (see main text for details). Shadows represent the 95% CIs of the fitted GAMs. All effects are significant except those indicated as n.s. (not significant) (SI Appendix, Table S2). See further details in SI Appendix, Fig. S1.
Modularity in the synergy networks decreased with land-use intensity in grasslands, but in the trade-off networks, it did not change with grassland or forest land-use intensity (Fig. 2 C and D). Evenness (i.e., similarity in the strength of correlations) in the synergy networks increased with land-use intensity in both ecosystem types, while in the trade-off networks, evenness was similar at high and low land-use intensity (Fig. 2 E and F). The combined changes in modularity and evenness show that synergy networks became more homogeneous with increasing land-use intensity. In grasslands, this effect was driven by the combination of biodiversity–function and function–service relationships, as the correlations between biodiversity and ecosystem services showed the opposite trend (SI Appendix, Fig. S4 D and F). In forests, the separate relationships responded more similarly (SI Appendix, Fig. S4 C and E).
When analyzing aboveground and belowground networks separately, we found that they responded similarly (SI Appendix, Fig. S5). However, our results suggest that the belowground compartment may drive the general response to land-use intensity in grasslands, while both compartments seem similarly important in driving the responses to forest management (see SI Appendix, Extended Results, Above- and belowground comparison). Our overall results differed from random expectations (SI Appendix, Fig. S6) and were robust to the exclusion of nonsignificant correlations (SI Appendix, Fig. S7 and Extended Results).
Effects of Land-Use Intensity on Module Composition and Hub Identity.
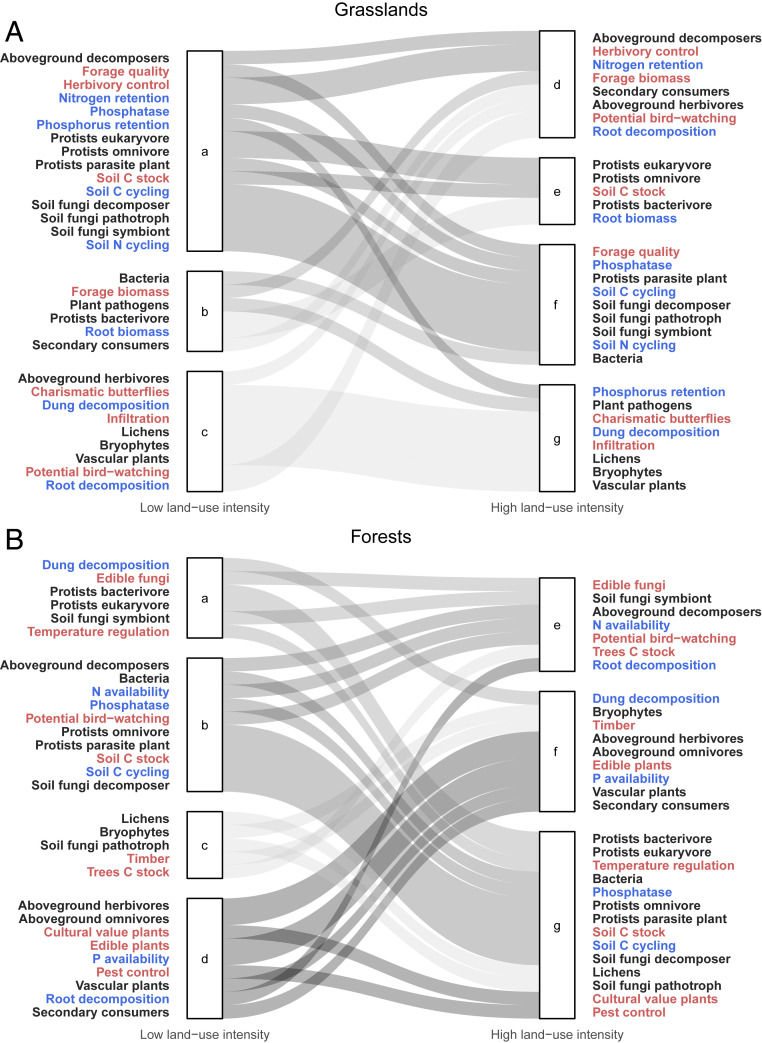
In both grasslands and forests, we observed a shift in the module composition of synergy networks with land use intensity. At low land-use intensity in the grasslands, there were three modules: one with soil microbial diversity, soil nutrient cycling, regulating services, and forage quality (Fig. 3 A, a); another with forage biomass (Fig. 3 A, b); and the third clustering aboveground diversities and cultural services, together with decomposition (Fig. 3 A, c). At high land-use intensity, these modules were disrupted (see SI Appendix, Extended Results, Changes in module composition), and there was a general decrease in modularity. In forests, at low land-use intensity we identified three clear modules, related to nutrient cycling (Fig. 3 B, b), old-growth forests (Fig. 3 B, c), and open forest glades (Fig. 3 B, d), with regulating and cultural services scattered across the modules. These modules were also rearranged with intensification of land use (SI Appendix, Extended Results).
Fig. 3.
Module composition of synergy networks and changes from the networks at the lowest land-use intensity level (left modules) to the highest land-use intensity level (right modules) in grasslands (A) and forests (B). Gray shades indicate the change in module composition from the low management intensity network: black nodes for biodiversity, blue for ecosystem functions, and red for ecosystem services.
Land-use intensification altered connections between the highly connected nodes (hubs) and the other nodes, significantly affecting the weighted node degree D (i.e., the average strength of connection to other nodes) and hub identity in both habitats (SI Appendix, Table S6). However, the mechanism behind this effect may differ in each habitat. For example, increasing land-use intensity in grasslands decreased forage biomass D, indicating a reduction in the strength of the correlations between this provisioning service and biodiversity and ecosystem functions. However, we also found that increasing management in forests also decreased D for temperature regulation, tree carbon stock, and edible fungi, which could indicate that regulating and cultural services lose more connections with increasing management in forests (SI Appendix, Table S6).
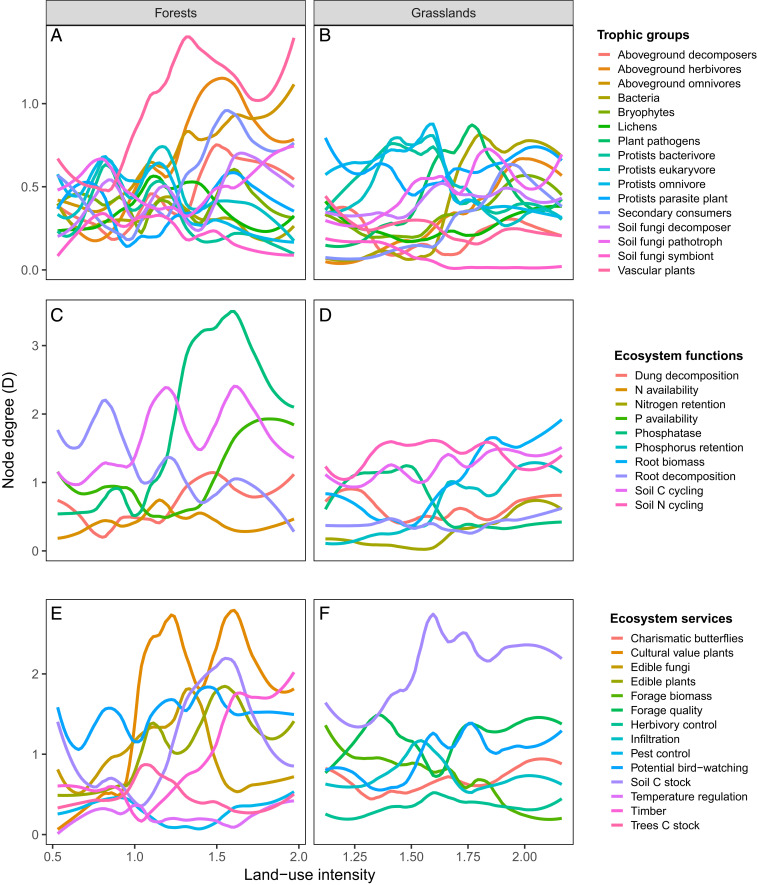
The identity of biodiversity hubs, i.e., the trophic groups most connected to functions and services, shifted from protists, at low land-use intensity in grasslands, to plant pathogens at intermediate levels and bacterial diversity at high land-use intensity, supporting the general importance of belowground diversity in grasslands (Fig. 4B). In ecosystem functions, we found a shift in grassland hubs from nitrogen cycling enzymes to root biomass at high land-use intensity (Fig. 4D). In contrast, ecosystem service hubs in grasslands were dominated by soil carbon at most intensity levels (Fig. 4F). In forests, plant diversity was the biodiversity hub at most land-use intensity levels, which could be related to an increase in understory diversity in more open managed forests (Fig. 4A). We also found a shift in forest function hubs from root decomposition at low land-use intensity to enzymatic activities related to carbon and phosphorus cycling at intermediate and high land-use intensity, respectively (Fig. 4C), which could also be linked to more conifer plantations (which are richer in phosphorus) in intensively managed forests. The shift in hubs was also evident for forest ecosystem services: from bird-watching potential at low intensity, via plants of cultural value at intermediate levels to timber production at high intensity levels (Fig. 4E).
Fig. 4.
Effect of land-use intensity on weighted node degree (D) of biodiversity (A and B), ecosystem functions (C and D), and ecosystem services nodes (E and F) for 150 forests (A, C, and E) and 150 grasslands (B, D, and F). Hubs are the nodes with largest D. Note different scales in the y axes and that only synergy networks are represented.
Discussion
Our results show that increasing land-use intensity leads to more homogeneous networks with less integration within modules in both habitats and a loss of strong synergies in grasslands. In grasslands, this effect was driven by the responses of the belowground compartment to land management, particularly by biodiversity–function and function–service relationships, while forest responses to land management were more complex. Our first hypothesis regarding changes in connectance with increasing management can only be partially supported (Fig. 1A), as we did not find an overall decrease with increasing management intensity, although at high intensity levels connectance clearly decreased in forests and strong synergies decreased in grasslands. The peak in connectance at intermediate land-use intensity in forests is intriguing and might suggest that there are different sets of diversities, functions, and services connected at low versus at high land-use intensity, and that intermediate intensity levels contain links between the two different sets. The overall decline in connectance of the trade-off networks might suggest overall increased integration of the ecosystem at high intensity levels, however, it may also reflect a loss of integration within modules (see below). It is therefore important to consider changes in strength and number of correlations, alongside overall measures of connectance.
Changes in modularity align also with our second hypothesis regarding module composition (Fig. 1D). Our findings support our expectation of changes from clearly defined modules at low land-use intensity to modules with undefined roles at high land-use intensity. The existence of clearly defined modules at low land-use intensity can be explained by the larger number of trade-offs found in these networks (SI Appendix, Fig. S2). Hence, our results suggest that at low land-use intensity levels there are unavoidable trade-offs, and maximizing belowground functions and provisioning services is not possible at the same time as maximizing aboveground diversity and cultural services. These trade-offs lead to a system which is strongly integrated within modules but with trade-offs between the modules. The rearrangement of modules at high intensity levels could be related to declines in aboveground diversities and cultural services, leading to a loss of synergies between them. Additionally, in grasslands, this could be due to the decoupling of provisioning services from soil functioning and microbial diversity (33, 34). Similar to effects of land use on the composition of trophic-group modules (16, 33), our results show how land-use intensity changes the composition of biodiversity–function–service modules. These results agree with studies indicating shifts in the bundles of functions and services (28, 50), and the trophic groups driving these functions and services (51), with land-use intensification. It also supports our third hypothesis (Fig. 1E), showing a change in the identity of the most connected ecosystem functions and services with land-use intensification (51).
The observed decrease in modularity and increase in evenness with land-use intensity in synergy networks agrees with our hypotheses (Fig. 1 B and C). Although this effect was clearer in grasslands, modularity in forests had a nonlinear response to management intensity. Our findings show that increasing land-use intensity led to a loss of strong synergies and to more homogeneous sets of interactions between biodiversity, functions, and services with less integrated modules. This is somewhat analogous to biotic homogenization, where intensively managed systems lose β-diversity (i.e., diversity among plots) and end up with very similar community compositions (23). Our results, thus, extend previous results showing a loss of correlations between local and regional diversities (i.e., α (22) and β (23), respectively) of different groups with land-use intensification.
Management Implications of Our Study.
The responses observed in both grasslands and forests suggest that although the management methods causing land-use intensification are very different in the two ecosystem types, there are common patterns in how they affect synergies and trade-offs between biodiversity, ecosystem functions, and services. So far, monitoring of environmental change effects focused on indicator taxa or particular ecosystem attributes, e.g., plant spatial patterns in drylands (52). However, our network approach can provide additional information on which are the key ecosystem attributes to monitor in order to identify important shifts in temperate forests and grasslands, complementing studies on drylands (53) and aquatic ecosystems (54). This can be done at two levels: the network level, representing ecosystem responses, and the node level, representing the responses of particular relationships in the ecosystem.
At the ecosystem level, we found that network metrics could identify thresholds of land-use intensity at which major shifts in biodiversity–function–service relationships occur, i.e., when the correlations existing at lower intensity levels are lost with increasing management intensity. For example, the shift in connectance in forests could be taken as an early warning signal of ecosystem change (53, 54). We also found that extensively managed systems had high integration within modules and also trade-offs between modules, meaning that different low intensity systems may deliver different bundles of functions and services, calling for a high heterogeneity in management across landscapes (39).
At the node level, our findings suggest that by identifying the most connected nodes (i.e., network hubs) and their relationships, we could prioritize particularly relevant groups of organisms or functions as targets for precise and efficient management and monitoring protocols (55, 56). For example, in our case, monitoring changes in plants and herbivorous arthropods in forests and soil microbes and plant pathogens in grasslands would be particularly important as changes in the species richness of these trophic groups may cascade to alter ecosystem functions and services (3, 57). Indeed, other studies found comparable weakening of ecosystem function and services hubs, suggesting a shift from biotically controlled to geochemically dependent or human coproduced ecosystem services in intensively managed systems (33, 34, 53, 57, 58). The measure of node connectance used to identify the hubs (i.e., D) can also be used as an approach to identify winners and losers of land-use intensification (Fig. 4). Nodes with increasing D had more and stronger positive correlations with other nodes as land-use intensity increased (e.g., forage quality in grasslands or aboveground herbivorous arthropods in forests), while nodes with decreasing D lost positive correlations and became less integrated or dependent of the system (e.g., aboveground herbivorous arthropods in grasslands or tree carbon stock in forests) (SI Appendix, Table S6).
In practice, these results indicate that any increment in land-use intensity will be reflected in changes in the relationships between biodiversity, ecosystem functions, and services. Particularly, we show that connectance decreases in forests and strong synergies decrease in grasslands at high intensity levels, meaning high land-use intensity is not sustainable. Managers should be aware of these changes to avoid undesired trade-offs and reduce land-use intensity levels to those that promote desirable biodiversity–function–service relationships. While we expect our results to be fairly generalizable given the extensive dataset and variety of trophic groups, functions, and services included, our results should be interpreted with caution when trying to generalize to a different set of services, functions, or organisms. Examples of common practices along the land-use intensity gradient in forests and grasslands are given in Fig. 1F; however, we acknowledge that multiple alternative management approaches can lead to the same levels of management intensity and network structure (37, 38).
Connecting Networks across Scales.
Our approach summarizes changes in the relationships between biodiversity, functions, and services across gradients of land use within a virtual landscape created by aggregating plots. It can readily be applied to understand how other global change drivers, such as climate change, alter spatial connections between ecosystem components. However, our approach does not consider the spatial position of the grasslands or forest patches within our virtual landscapes, and a further extension of the approach could consider these spatial relationships, for instance by weighting correlations by geographic distance. It would also be possible to consider different spatial scales for different relationships: For instance, plant diversity and pollination might be connected over larger spatial scales than bacterial diversity and soil nutrient cycling. In addition, our approach could be extended to consider even larger scale connections, such as telecouplings derived from trade and tourism (59, 60), and to investigate sustainability challenges in social-ecological systems (56, 61, 62).
Our study can help in developing and testing new hypotheses to investigate changes in the overall network; in subnetworks of particular linkages types, habitats, or nodes; and it can be applied to other environmental changes. Constructing networks to address these questions requires detailed information on the diversities of multiple trophic groups, measurements of multiple ecosystem functions, and proxies for key services. Only integrated research programs can deliver this type of data, and we therefore call for more projects measuring multiple groups, functions, and services in other systems.
Conclusion
Our approach of constructing biodiversity–function–service networks complements the information provided by commonly used metrics such as multidiversity and multifunctionality (3, 6). Networks of biodiversity–function–service relationships simultaneously account for all three interactions between the node types and take the original relationships into account, instead of averaging them. Metrics like connectance, evenness, modularity, and hubs, therefore, provide a complementary perspective on multitrophic diversity–function relationships and enable alternative insights into the structure and functioning of ecosystems and their connections to human wellbeing. This approach has proven useful (i) to identify key groups of synergistic ecosystem components (i.e., modules), (ii) to detect changes in module composition and key components (i.e., hubs) related to land use, and (iii) to show that land-use intensification tends to homogenize biodiversity–functioning–service relationships and lead to a system with less strong synergies and integration within modules.
Our analyses provide a comprehensive overview of ecosystem responses to land use and show that changes in connectance were larger in forests, while changes in modularity and evenness were more evident in grasslands. Land-use intensity strongly altered module composition and hub identity in both ecosystems. Our findings demonstrate that the positive correlations of provisioning services with biodiversity and ecosystem functions can be strengthened up to intermediate land-use intensity levels but decline at higher levels. These results allow us to develop mechanistic hypotheses linking land use to ecosystem functions and the services they provide to humanity.
Supplementary Material
Acknowledgments
We thank J. Bauhus, N. Blüthgen, R. Daniel, K. Jung, T. Kahl, K. Kaiser, D. Prati, S. Renner, E. Sorkau, S. Trumbore, and M. Tschapka for contributing data to the analyses. A. de Frutos provided support with graphical outputs. We acknowledge S. Kéfi and S. Pilosof for valuable discussions in the earliest stages of analyses, A. Rindisbacher for providing comments on the manuscript, and two anonymous reviewers. We thank the managers of the three Exploratories, K. Hartwich, S. Gockel, K. Wiesner, and M. Gorke and all former managers for their work in maintaining the plot and project infrastructure; C. Fischer and J. Mangels for giving support through the central office; M. Owonibi for managing the central database; and E. Linsenmair, D. Hessenmöller, E.-D. Schulze, and the late E. Kalko for their role in setting up the Biodiversity Exploratories program. Field work permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg (according to § 72 BbgNatSchG). The work has been supported by the DFG Priority Program 1374 “Infrastructure-Biodiversity-Exploratories”. S.S. was supported by the Spanish Government under Ramón y Cajal Contract RYC-2016-20604.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016210117/-/DCSupplemental.
Data Availability.
The individual datasets used in the analyses can be accessed from BEXIS (Biodiversity Exploratories Information System) via https://www.bexis.uni-jena.de/PublicData/SearchPublicData.aspx—see the IDs provided in parentheses in SI Appendix Supplementary Methods for individual datasets and SI AppendixData Availability for assembled datasets. Note that some datasets might be subject to an embargo period. In these cases, the interested user should register in BEXIS and request individual access. The R script used to analyze the data and produce all figures is available in GitHub (https://github.com/MariaFelipe-Lucia/biodiversity-function-services_networks) and Zenodo (https://zenodo.org/record/4064896#.X3jAhO2xU2x) (63).
References
- 1.IPBES , Summary for Policymakers of the Assessment Report on Land Degradation and Restoration of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Scholes R. et al., Eds (IPBES Secretariat, Bonn, Germany, 2018). [Google Scholar]
- 2.Knapp S., “The link between diversity, ecosystem functions, and ecosystem services: Drivers, risks, and societal responses” in Atlas of Ecosystem Services, Schröter M., Bonn A., Klotz S., Seppelt R., Baessler C., Eds (Springer International Publishing, 2019), pp. 13–16. [Google Scholar]
- 3.Duncan C., Thompson J. R., Pettorelli N., The quest for a mechanistic understanding of biodiversity-ecosystem services relationships. Proc. Biol. Sci. 282, 20151348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balvanera P. et al., Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Díaz S. et al., The IPBES Conceptual Framework–Connecting nature and people. Curr. Opin. Environ. Sustain. 14, 1–16 (2015). [Google Scholar]
- 6.Soliveres S. et al., Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536, 456–459 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Schuldt A. et al., Biodiversity across trophic levels drives multifunctionality in highly diverse forests. Nat. Commun. 9, 2989 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millennium Ecosystem Assessment , Ecosystems and Human Well-Being: Our Human Planet: Summary for Decision Makers, (Island Press, 2005). [Google Scholar]
- 9.Martinez‐Almoyna C. et al., Multi-trophic β-diversity mediates the effect of environmental gradients on the turnover of multiple ecosystem functions. Funct. Ecol. 33, 2053–2064 (2019). [Google Scholar]
- 10.Harrison P. A. et al., Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 9, 191–203 (2014). [Google Scholar]
- 11.Swift M. J., Izac A.-M. N., van Noordwijk M., Biodiversity and ecosystem services in agricultural landscapes–Are we asking the right questions? Agric. Ecosyst. Environ. 104, 113–134 (2004). [Google Scholar]
- 12.Byrnes J. E. K. et al., Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 5, 111–124 (2014). [Google Scholar]
- 13.Allan E. et al., Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgatti S. P., Mehra A., Brass D. J., Labianca G., Network analysis in the social sciences. Science 323, 892–895 (2009). [DOI] [PubMed] [Google Scholar]
- 15.He S. et al., Ecological diversity and co-occurrence patterns of bacterial community through soil profile in response to long-term switchgrass cultivation. Sci. Rep. 7, 3608 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pocock M. J. O. et al., “The visualisation of ecological networks, and their use as a tool for engagement, advocacy and management” in Advances in Ecological Research, Woodward G., Bohan D. A., Eds (Elsevier, 2016), pp. 41–85. [Google Scholar]
- 17.Sala O. E. et al., Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Evans D. M., Pocock M. J. O., Memmott J., The robustness of a network of ecological networks to habitat loss. Ecol. Lett. 16, 844–852 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Morrison B. M. L., Brosi B. J., Dirzo R., Agricultural intensification drives changes in hybrid network robustness by modifying network structure. Ecol. Lett. 23, 359–369 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Kleyer M. et al., Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. J. Ecol. 107, 829–842 (2019). [Google Scholar]
- 21.Allan E. et al., Interannual variation in land-use intensity enhances grassland multidiversity. Proc. Natl. Acad. Sci. U.S.A. 111, 308–313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning P. et al., Grassland management intensification weakens the associations among the diversities of multiple plant and animal taxa. Ecology 96, 1492–1501 (2015). [Google Scholar]
- 23.Gossner M. M. et al., Land-use intensification causes multitrophic homogenization of grassland communities. Nature 540, 266–269 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Dunne J. A., Williams R. J., Martinez N. D., Network structure and biodiversity loss in food webs: Robustness increases with connectance. Ecol. Lett. 5, 558–567 (2002). [Google Scholar]
- 25.Valiente‐Banuet A. et al., Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307 (2015). [Google Scholar]
- 26.Ochoa-Hueso R., Nonlinear disruption of ecological interactions in response to nitrogen deposition. Ecology 97, 2802–2814 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Foley J. A. et al., Global consequences of land use. Science 309, 570–574 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Raudsepp-Hearne C., Peterson G. D., Bennett E. M., Ecosystem service bundles for analyzing tradeoffs in diverse landscapes. Proc. Natl. Acad. Sci. U.S.A. 107, 5242–5247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez A., Rayfield B., Lindo Z., The disentangled bank: How loss of habitat fragments and disassembles ecological networks. Am. J. Bot. 98, 503–516 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Thébault E., Identifying compartments in presence–Absence matrices and bipartite networks: Insights into modularity measures. J. Biogeogr. 40, 759–768 (2018). [Google Scholar]
- 31.Olesen J. M., Bascompte J., Dupont Y. L., Jordano P., The modularity of pollination networks. Proc. Natl. Acad. Sci. U.S.A. 104, 19891–19896 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank K. et al., Global dung webs: High trophic generalism of dung beetles along the latitudinal diversity gradient. Ecol. Lett. 21, 1229–1236 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Schöps R. et al., Land-use intensity rather than plant functional identity shapes bacterial and fungal rhizosphere communities. Front. Microbiol. 9, 2711 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries F. T., Bardgett R. D., Plant–microbial linkages and ecosystem nitrogen retention: Lessons for sustainable agriculture. Front. Ecol. Environ. 10, 425–432 (2012). [Google Scholar]
- 35.Creamer R. E. et al., Ecological network analysis reveals the inter-connection between soil biodiversity and ecosystem function as affected by land use across Europe. Appl. Soil Ecol. 97, 112–124 (2016). [Google Scholar]
- 36.Fischer M. et al., Implementing large-scale and long-term functional biodiversity research: The Biodiversity Exploratories. Basic Appl. Ecol. 11, 473–485 (2010). [Google Scholar]
- 37.Blüthgen N. et al., A quantitative index of land-use intensity in grasslands: Integrating mowing, grazing and fertilization. Basic Appl. Ecol. 13, 207–220 (2012). [Google Scholar]
- 38.Kahl T., Bauhus J., An index of forest management intensity based on assessment of harvested tree volume, tree species composition and dead wood origin. Nat. Conserv. 7, 15–27 (2014). [Google Scholar]
- 39.Felipe-Lucia M. R. et al., Multiple forest attributes underpin the supply of multiple ecosystem services. Nat. Commun. 9, 4839 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Development Core Team , R: A Language and Environment for Statistical Computing (Version 3.6.2, R Foundation for Statistical Computing, 2016).
- 41.Kim S., ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Commun. Stat. Appl. Methods 22, 665–674 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csardi G., Nepusz T., The igraph software package for complex network research. InterJ. Complex Syst. 1695, 1–9 (2006). [Google Scholar]
- 43.Pons P., Latapy M., “Computing communities in large networks using random walks” in Computer and Information Sciences–ISCIS 2005, Yolum p., Güngör T., Gürgen F., Özturan C., Eds. (Lecture Notes in Computer Science, Springer, 2005), pp. 284–293. [Google Scholar]
- 44.Oksanen J., Blanchet G., Friendly M., Kindt R., vegan: Community Ecology Package (Version 2.4-5, R package, 2017).
- 45.Dormann C. F., Fründ J., Blüthgen N., Gruber B., Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24 (2009). [Google Scholar]
- 46.Wood S. N., Generalized Additive Models: An Introduction with R, (Chapman and Hall/CRC, ed. 2, 2017). [Google Scholar]
- 47.Wood S. N., Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 99, 673–686 (2004). [Google Scholar]
- 48.Koenker R., et al. , quantreg: Quantile Regression (R Software, Version 5.73, 2020).
- 49.Wickham H., ggplot2: Elegant Graphics for Data Analyses, (Springer-Verlag, New York, 2016). [Google Scholar]
- 50.Mouchet M. A. et al., Bundles of ecosystem (dis)services and multifunctionality across European landscapes. Ecol. Indic. 73, 23–28 (2017). [Google Scholar]
- 51.Soliveres S. et al., Locally rare species influence grassland ecosystem multifunctionality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berdugo M., Kéfi S., Soliveres S., Maestre F. T., Plant spatial patterns identify alternative ecosystem multifunctionality states in global drylands. Nat. Ecol. Evol. 1, 0003 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Berdugo M. et al., Global ecosystem thresholds driven by aridity. Science 367, 787–790 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Carpenter S. R. et al., Early warnings of regime shifts: A whole-ecosystem experiment. Science 332, 1079–1082 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Tylianakis J. M., Laliberté E., Nielsen A., Bascompte J., Conservation of species interaction networks. Biol. Conserv. 143, 2270–2279 (2010). [Google Scholar]
- 56.Dee L. E. et al., Operationalizing network theory for ecosystem service assessments. Trends Ecol. Evol. (Amst.) 32, 118–130 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Albrecht J. et al., Correlated loss of ecosystem services in coupled mutualistic networks. Nat. Commun. 5, 3810 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Palomo I., Felipe-Lucia M. R., Bennett E. M., Martín-López B., Pascual U., “Disentangling the pathways and effects of ecosystem service co-production” in Ecosystem Services: From Biodiversity to Society, Part 2, Woodward G., Bohan D. A., Eds. (Advances in Ecological Research, Academic Press, 2016), pp. 245–283. [Google Scholar]
- 59.Schröter M. et al., Interregional flows of ecosystem services: Concepts, typology and four cases. Ecosyst. Serv. 31, 231–241 (2018). [Google Scholar]
- 60.Liu J. et al., Multiple telecouplings and their complex interrelationships. Ecol. Soc. 20, 44 (2015). [Google Scholar]
- 61.Sayles J. S. et al., Social-ecological network analysis for sustainability sciences: A systematic review and innovative research agenda for the future. Environ. Res. Lett. 14, 093003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodin Ö. et al., Improving network approaches to the study of complex social–ecological interdependencies. Nat. Sustain. 2, 551–559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Felipe-Lucia M., R script used to run the analyses of "Land-use intensity alters networks between biodiversity, ecosystem functions and services." Zenodo. https://zenodo.org/record/4064896#.X3jAhO2xU2x. Deposited 3 October 2020. [DOI] [PMC free article] [PubMed]
- 64.Rodewald A. D., Rohr R. P., Fortuna M. A., Bascompte J., Community-level demographic consequences of urbanization: an ecological network approach. J. Anim. Ecol. 83, 1409–1417 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual datasets used in the analyses can be accessed from BEXIS (Biodiversity Exploratories Information System) via https://www.bexis.uni-jena.de/PublicData/SearchPublicData.aspx—see the IDs provided in parentheses in SI Appendix Supplementary Methods for individual datasets and SI AppendixData Availability for assembled datasets. Note that some datasets might be subject to an embargo period. In these cases, the interested user should register in BEXIS and request individual access. The R script used to analyze the data and produce all figures is available in GitHub (https://github.com/MariaFelipe-Lucia/biodiversity-function-services_networks) and Zenodo (https://zenodo.org/record/4064896#.X3jAhO2xU2x) (63).