Dear Editor:
In this letter, we update the significant progress made in fundamental research to identify new paradigms in head and neck cancer (HNC), including emerging issues in translating relevant findings into patient‐oriented clinical applications. This overview stems from relevant presentations by renowned leaders in this field given at the Multidisciplinary International Symposium in Translational Head and Neck Cancer Research.
It is noticeable that new cases of HNC are increasing rapidly in the past decades, especially among young adults. This is not only associated with tobacco and alcohol consumption, but due to the HPV infection that has emerged as an additional risk factor, defining a new subtype of tumor that is distinct from the “conventional” HPV‐negative ones. 1 Like other malignancies, HNC is remarkably heterogeneous comprising several subtypes in different anatomic sites of the upper aerodigestive tracts with complex pathological and molecular features. 1 However, HNC patients still receive standard treatment based on surgery, radiotherapy, chemotherapy, immunotherapy, or combinations of these modalities, independently of the molecular heterogeneity and/or HPV status. Despite of the advances in medical imaging and therapeutic approaches, the outcome of patients with advanced HNC remains poor and the 5‐years survival is stagnant at <50%. 2 Indeed, the lack of flexibility in therapeutic strategies often leads to patients suffering from inadequate or excessive treatment. The postoperative follow‐up mode of watchful waiting has also deprived most patients with recurrent HNC of early treatment opportunities.
With no doubt, the identification of reliable and unequivocal diagnostic and prognostic biomarkers is the ultimate goal of clinician and oncoscientists. Basic research in cancer biology provides preclinical data and new technology to support clinical advances. The strategic plan to promote the translational research follows a framework involving different phases (Figure 1). 3 In order to generate meaningful biological knowledge, an increasing number of investigators are conducting multi‐omics experiments, which allow a rapid and comprehensive analysis of cancer individual patients even using a small biopsy or blood circulating tumor cells. 4 The ever‐growing amount and sophistication combined with the quantity of data and technical expertise required for data collection and powerful bioinformatics analysis and tools are far from trivial. Nevertheless, the clinical integration of these high‐throughput technologies to offer precision health assessments with valuable therapeutic approaches remains a challenging task for routine implementation on a population scale, which certainly will require further improvements in data acquisition and analysis at reasonable cost effectiveness.
FIGURE 1.
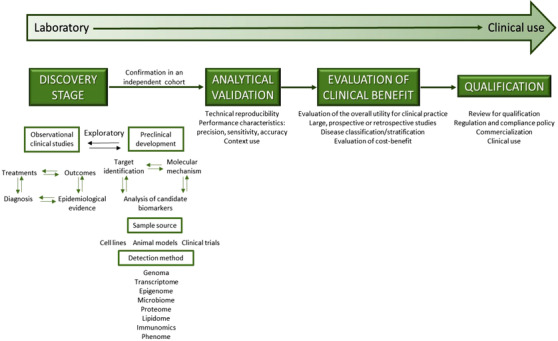
Biomarker development process from bench to bedside. Basic research in cancer biology provides preclinical data and new technology to support clinical advances in biomarkers discovery related to early detection (to determine specific healthy disorder), prognosis (to chart the likely course of the disease), prediction (to indicate drug response), and predisposition (to indicate risk of developing disease). Initially, clinical translational research had two main phases: (1) bench‐to‐bedside, in which new discoveries from the laboratory could be translated toward clinical research (proof of concept, phases I and II clinical trials) and (2) clinical use, in which these applications could be translated into the practice (phase III clinical trials, studies of clinical efficacy and development of guidelines). Recently, this process was further expanded by the addition of four more elements: discovery stage, analytical validation, evaluation of clinical benefits, and qualification
Unfortunately, HNC biomarkers studies are still hampered by several limitations due in part to the technical parameters such as sample size, suitable analysis strategy, standardized protocol for sample collection and storage, rational study design, detailed methods, as well as the complex nature of the disease itself. 5 As noted above HNC is not a uniform disease, but a heterogeneous neoplasm with an array of genetic and epigenetic modifications associated with different risk factors. 5 This certainly confers advantages for cancer cell division and survival, including growth factor‐independent proliferation, resistance to apoptosis, and an enhanced capability to overcome extracellular matrix (ECM) barriers and invade adjacent/distant tissues. 6 Indeed, one of the most difficult barriers to overcome HNC invasion is the ability of cancer cells to disrupt the basal lamina, degrade ECM, and move beyond primary sites to gain access to lymphatic/blood vessels and establish metastasis. HNC is characterized by different invasive growth patterns, frequently associated with high risk of regional metastases and significant recurrences and secondary tumors. 7
It is interesting to note that the majority of published studies in HNC focused on single signaling molecule/biomarker, often involving small number of patients with different tumor location, clinical stage, tumor grade, treatment, and no clear experimental design to address clinical questions. Besides, few investigations seem to shift their focus to multiplex gene/protein signatures, which can significantly improve diagnostic accuracy and enhance the predictive statistical power. 8 The use of some biomarkers may become a reality in routine clinical management of HNC (Table 1), though the correlations with certain histopathological and clinical features are often inconsistently. Clearly, this is an open field and additional research is necessary to lend further insights into the molecular basis of HNC including large prospective well‐designed studies as well as deep mechanistic research are essential for validation and for the development of reliable indicators and tools for assessment of clinical events such as early diagnosis, recurrences, metastasis, and eventually culminating into better patient's outcome.
TABLE 1.
Common gene/protein alterations and potential biomarkers in head and neck cancer
| Cellular process | Gene | Protein | Type of gene | Significance/association |
|---|---|---|---|---|
| Cell cycle | CDKN2A | p16INK4A | Tumor suppressor | Decreased overall survival |
| TP53 | p53 | Tumor suppressor | Decreased overall survival | |
| CCND1 | G1–S‐specific cyclin D1 | Oncogene | Nodal metastases; more rapid clinical course | |
| CDKN1B | p27 | Tumor suppressor | Poor prognosis | |
| MDM2 | Mdm2 | Oncogene | Tumorigenesis | |
| Growth signals | EGFR | EGFR | Oncogene | Nodal metastases; more rapid clinical course, consideration for targeted therapy |
| MYC | c‐Myc | Oncogene | Tumor progression | |
| RARB | Retinoic acid receptor beta | Tumor suppressor | Decreased overall survival | |
| Survival | PIK3CA | Catalytic p110α subunit of class 1 PI3Ks | Oncogene | High risk of recurrence |
| PTEN | PTEN | Tumor suppressor | High risk of recurrence | |
| WNT signaling | FAT1 | Protocadherin FAT1 | Tumor suppressor | Overall survival |
| AJUBA | LIM domain‐containing protein AJUBA | Tumor suppressor | Treatment prediction | |
| NOTCH1 | NOTCH1 | Tumor suppressor | Chemosensitivity and overall survival | |
| STAT3 | STAT3 | Oncogene | Decreased overall survival |
Moreover, immune system and tumor microenvironment (TME) are characterized by profound heterogeneity, dynamicity, and intercellular cross‐talks that add a new layer of complexity to define new paradigm in precision medicine (Figure 2). 9 Investigation of the differences in HNC‐TME composition and their impact on cancer progression may help to understand the mechanism behind different tumor response, thus define possible targets for clinical intervention involving not a single modality treatment but rather a multitarget therapy. Recently, immunotherapy has provided promises and exciting treatment options for HNC, but the translation into clinical practice is limited to recurrent or metastatic cases. Pembrolizumab and nivolumab are the two PD‐1 antibodies approved by US‐FDA for the treatment of recurrent or platinum refractory metastatic HNC. However, no conclusions can be drawn on the role of PD‐L1 in identifying patients responding to immunotherapy, given that similar studies lead to contrasting results. 10 A better understanding of the complex network between tumor, immune system, and combination of oncologic treatments will help us to refined patient selection and develop more efficient multimodality treatments.
FIGURE 2.
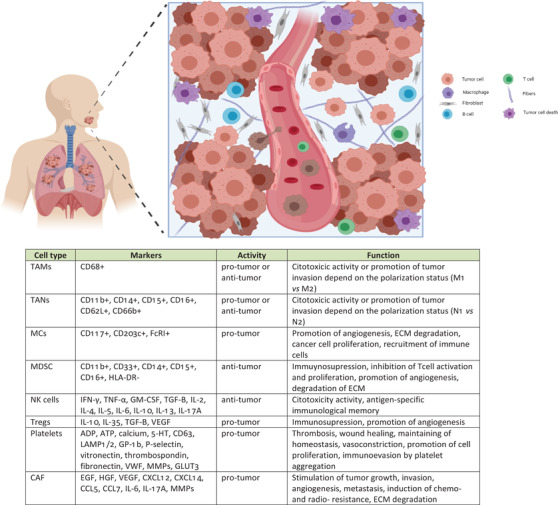
Different cell populations exhibit distinct functions within the tumor microenvironment (TME). In addition to the cancer cells, the tumor stroma is composed of many other supporting cell populations as well as the extracellular matrix, which crucially contribute to the tumor progression. The multiple different cell types include carcinoma‐associated fibroblasts (CAFs), endothelial cells, and inflammatory or immune cells (like neutrophils, macrophages, regulatory T cells, myeloid‐derived suppressor cells, natural killer cells, platelets, and mast cells). These cell subpopulations interact with each other and with the cancer cells via complex communication network through soluble factors (like growth factors, cytokines, chemokines, matrix proteins, and proteases) as well as proteins of the extracellular matrix (ECM). Abbreviations: TAM, tumor‐associated macrophage; TAN, tumor‐associated neutrophil; MCs, mast cells; MDSC, myeloid‐derived suppressor cell; NK, natural killer cell; Treg, regulatory T cell; CAF, cancer‐associated fibroblast; ECM, extracellular matrix (image created using Biorender Free Software)
In conclusion, we still do not fully understand HNC tumor behavior to manage this devastating disease. Given the deep biological heterogeneity and complex histological grade of this malignancy and its microenvironment, personalized therapeutic approaches possibly based on the use of combination of targeted therapy will provide a rational approach to improve treatments for patients with HNC. Understanding the aspects of the cell functionality and how molecular pathways interplay are the basis and the key strategy to develop new therapeutic approaches to improve patient's prognosis.
FUNDING INFORMATION
Global Affair/DFATD (#249584), Brazil‐Canada (#249569), RSBO#80596; NCOHR (New Frontier Seed Grant); FAPESP.
CONFLICT OF INTEREST
The authors declare that there is conflict of interest.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr Ulisses Ribaldo Nicolau (Brazil), Gilberto Castro (Brazil), Rossana V. Mendoza López (Peru), Daniel Lambert MSc (UK), and Clarissa Araujo Gurgel Rocha (Brazil) for their valuable lectures and discussion during the Multidisciplinary International Symposium in Translational Head and Neck Cancer Research held in Sao Paulo on November 2019 (https://www.accamargo.org.br/cursos-e-eventos/head-and-neck-cancer-research-concepts-future-clinical-management). We also acknowledged the testimonial kindly shared by a patient and the presentation of the laryngectomized choir (Coral Sua Voz) during this event.
REFERENCES
- 1. Lewis A, Kang R, Levine A, Maghami E. The new face of head and neck cancer: the HPV epidemic. Oncology. 2015;29:616‐626. [PubMed] [Google Scholar]
- 2. Kowalski LP, Carvalho AL, Martins Priante AV, Magrin J. Predictive factors for distant metastasis from oral squamous cell carcinoma. Oral Oncol. 2005;;41:534‐541. [DOI] [PubMed] [Google Scholar]
- 3. Califf RM. Biomarker definitions and their applications. Exp Biol Med. 2018;243:213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasin Y, Seldin M, Lusis A. Multi‐omics approaches to disease. Genome Biol. 2017;18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18:269‐282. [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 7. da Silva SD, Kowalski LP. Perineural invasion in oral cancer: challenges, controversies and clinical impact. Chin Clin Oncol. 2019;8:S5. [DOI] [PubMed] [Google Scholar]
- 8. Ozturk K, Dow M, Carlin DE, Bejar R, Carter H. The emerging potential for network analysis to inform precision cancer medicine. J Mol Biol. 2018;430:2875‐2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peltanova B, Raudenska M, Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mol Cancer. 2019;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauml JM, Aggarwal C, Cohen RB. Immunotherapy for head and neck cancer: where are we now and where are we going? Ann Transl Med. 2019;7:S75. [DOI] [PMC free article] [PubMed] [Google Scholar]


