Abstract
Background:
This study investigated the impact of perioperative systemic chemotherapy on the recurrence rate and pattern following resection of colorectal liver metastases.
Methods:
A retrospective cohort study was conducted in two centers. Rates and patterns of recurrence and overall survival (OS) were compared between patients treated with and without perioperative systemic chemotherapy. The clinical risk score (CRS) was used to stratify patients in low-risk (CRS 0-2) and high-risk (CRS 3-5) of recurrence.
Results:
A total of 2020 patients were included, of whom 1442 (71%) received perioperative systemic chemotherapy. The median follow-up was 88 months and 1289 patients (64%) developed a recurrence. The recurrence pattern was independent of chemotherapy in low-risk patients: intrahepatic recurrences (30% vs 30%, p=0.97) and extrahepatic recurrences (38% vs 39%, p=0.52). In high-risk patients, no difference in intrahepatic recurrences was found (48% vs 50%, p=0.59). However, a lower rate of extrahepatic recurrences (43% vs 55%, p=0.007) was observed with perioperative systemic chemotherapy, mainly due to a reduction in pulmonary recurrences (25% vs 35%, p=0.007). In competing risk analysis, the cumulative incidence of extrahepatic recurrence was significantly lower with perioperative systemic chemotherapy in high-risk patients only (5-year cumulative incidence 44% vs 59%, p<0.001). Perioperative chemotherapy was associated with improved OS in high-risk patients (adjusted HR 0.73, 95%CI 0.57-0.94, p=0.02), but not in low-risk patients (adjusted HR 0.99, 95% CI 0.82-1.19, p=0.90).
Conclusions:
Perioperative systemic chemotherapy had no association with intrahepatic recurrence, but was associated with fewer pulmonary recurrences and superior OS in high-risk patients only.
Keywords: Surgery, colorectal, chemotherapy
Introduction
After surgery for colorectal liver metastasis (CRLM), up to 70% of patients develop recurrent disease. Recurrences occur mostly within the first two years after resection.1 The 5-year survival probability is about 50% after curative-intent resection of CRLM.2
Perioperative systemic chemotherapy was found to improve progression-free survival (PFS), but not overall survival (OS) in a randomized controlled trial.2 In some countries (e.g., the United States), perioperative systemic chemotherapy is the standard of care in patients with resectable CRLM; in other countries (e.g., The Netherlands) it is not. Some studies suggested that the truth lies in the middle. They found that only patients with high-risk oncological features have superior OS with perioperative systemic chemotherapy.3-5 In the above mentioned randomized trial, mainly patients with low-risk oncological features were included.2The clinical risk score (CRS) stratifies patients in subgroups of low-risk and high-risk of recurrence and OS.6 The CRS is the sum of five poor prognostic factors, assigning one point to each factor if present: positive nodal status of primary tumor, disease-free interval between resection of primary and diagnosis of CRLM less than 1 year, more than one CRLM, size of largest CRLM exceeds 5 cm, and preoperative serum carcinoembryonic antigen (CEA) level above 200 μg/L. Patients can be stratified into low-risk (0-2 points) and high-risk (3-5 points) of recurrence.6
Perioperative systemic chemotherapy may avoid or postpone intrahepatic and/or extrahepatic recurrence after resection of CRLM. The aim of this study is to investigate the impact of perioperative systemic chemotherapy on the recurrence rate and pattern in low- and high-risk patients after resection of CRLM.
Material and Methods
Patients
Patients who underwent surgical treatment for CRLM between 1991-2012 at the Memorial Sloan Kettering Cancer Center (MSKCC, New York, United States), and between 2000-2016 at the Erasmus MC Cancer Institute (Rotterdam, The Netherlands), were evaluated for inclusion. At MSKCC, perioperative systemic chemotherapy was typically administered in the induction, neoadjuvant, and/or adjuvant setting. At Erasmus MC, patients received perioperative systemic chemotherapy almost exclusively as induction chemotherapy for initially (borderline) unresectable CRLM, according to the Dutch national guidelines. This study was conducted according the STROBE guidelines.
In- and exclusion criteria
Patients were excluded from analysis for the following reasons: administration of perioperative Hepatic Arterial Infusion Pump (HAIP) chemotherapy, extrahepatic disease (EHD) diagnosed before or at the time of CRLM resection, no complete liver resection, no resection of the primary tumor, lost to follow-up, and ablative procedures without CRLM resection. Patients treated with a combined resection and ablation (Radio Frequency Ablation (RFA) or Microwave Ablation (MWA)) were eligible. Patients that could not be classified in low-risk or high-risk due to missing values were excluded from further analyses.
Definitions
Clinicopathological data were retrieved from two prospectively maintained databases. Data on patient and tumor characteristics, surgical outcome, recurrence of disease, and survival were gathered. Only the site(s) of initial recurrence were available. Perioperative systemic chemotherapy was defined as any systemic chemotherapy within three months of resection. EHD was defined as the presence of disease outside the liver (other than the primary CRC) prior to or at surgery. Primary tumors were classified as right-sided if localized proximal to the splenic flexure, left-sided tumors if localized at or distal to the splenic flexure, or rectal tumors. The total number of CRLM was calculated by the total number of lesions at the pathology report combined with the total number of lesions ablated. The size of largest tumor was derived from the pathology report. Patients were stratified into low-risk (CRS 0-2) and high-risk (CRS 3-5).6 Recurrences were classified into intrahepatic or extrahepatic. Since patients could have an initial recurrence in more than one organ, the sum of intrahepatic and extrahepatic recurrence exceeds the total recurrence rate.
Statistical analysis
Baseline characteristic were compared using the Chi-square test for categorical variables and the Mann-Whitney U-test for continues variables. Median follow-up time was calculated using the reversed Kaplan-Meier method. OS was defined from the date of resection of CRLM until the date of death or last follow-up. The Kaplan-Meier method was used to calculate OS. Groups were compared using the log-rank test. Uni- and multivariable Cox regression analyses for OS were performed, and results were presented as hazard ratios (HR) with corresponding 95% confidence intervals (CIs). Cumulative incidence functions (CIF) for patients treated with and without perioperative systemic chemotherapy were estimated using competing risk methods and compared over the entire follow-up time using Gray’s test.7 A CIF estimates the probability of an event up to a follow-up time point t. The cumulative incidence was adjusted by the occurrence of the competing events. Patients developing a competing event (i.e. initial recurrence at a specific location other than the location of interest or dying before they have developed a recurrence) were no longer at risk for the event of interest. A p-value smaller than 0.05 was considered statistically significant. Analyses were performed using SPSS (IBM Corp, version 24, Armonk, NY) and RStudio (RStudio, version 1.0.153, Boston, MA).
Follow-up
During follow-up at MSKCC, serum CEA measurements and radiological imaging (abdominal and thoracic CT-scan) were performed every 3-6 months for the first three years, and yearly thereafter. At Erasmus MC follow-up was similar with radiological imaging every 3-6 months for the first two years, and yearly thereafter until 5 years.
Results
A total of 3470 patients were evaluated for inclusion (Figure 1). Approximately 38% (n=1334) of the patients were excluded, primarily due to perioperative HAIP chemotherapy (53.1%, n=709) and the presence of EHD (30.3%, n=404). The remaining 2020 patients were included for analysis, of whom 1442 patients (71.4%) received perioperative systemic chemotherapy. Most patients were treated at MSKCC (n=1244, n=61.6%) and the remainder at Erasmus MC (n=776, 38.4%). At MSKCC 1102 (88.6%) patients received perioperative systemic chemotherapy compared to 334 (43.0%) patients at Erasmus MC (p<0.001). Perioperative systemic chemotherapy was administered preoperatively in 568 patients (39.9%), postoperatively (i.e. adjuvant) in 404 patients (28.1%), and both pre- and postoperatively in 464 patients (32.3%). Most patients received either oxaliplatin- or irinotecan-based therapy (72.3%), and the remainder received 5-flurouracil-based monotherapy, mostly in the era prior to oxaliplatin and irinotecan.
Figure 1. Study flowchart.
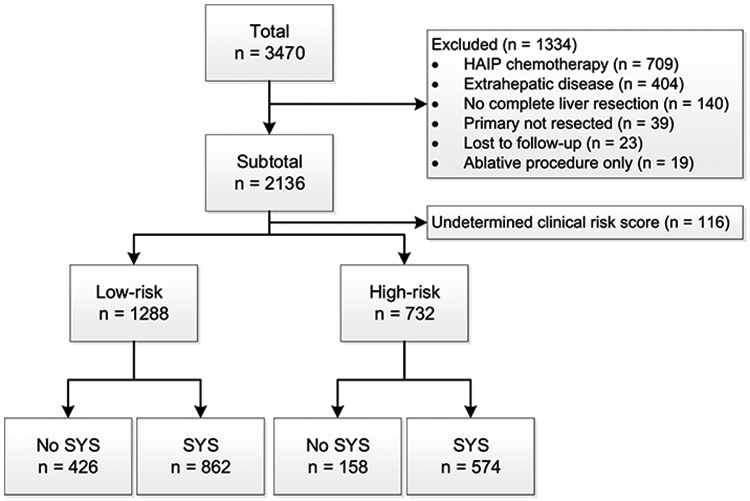
Clinical risk score
Most patients were classified according to the CRS as low-risk (n=1288, 63.7%), and about a third as high-risk (n=732, 36.3%). A complete overview of the number of patients within each CRS class can be found in Appendix Table 1. High-risk patients more often received perioperative systemic chemotherapy compared to low-risk patients (78.4% versus 67.3%, p<0.001). The baseline characteristics of low-risk and high-risk patients are stratified by whether they received perioperative systemic chemotherapy (Table 1). Low-risk patients treated with perioperative systemic chemotherapy were younger at the time of resection of the CRLM (median age 64.4 months versus 67.0 months, p<0.001), were more likely to have right-sided CRC (24.9% versus 19.2%, p=0.01), more often had a DFI of less than 12 months (50.3% versus 41.1%, p=0.002), more than 1 CRLM (33.8% versus 27.0%, p=0.01), or CRLM smaller than 5 cm (86.2% versus 81.2%, p=0.02). For high-risk patients, no statistically significant differences were found between patients treated with and without perioperative systemic chemotherapy.
Table 1.
Baseline characteristics
| Low-risk | High-risk | |||||||
|---|---|---|---|---|---|---|---|---|
| All patients | No SYS | SYS | P-value | All patients | No SYS | SYS | P-value | |
| Sample size | 1288 | 426 (33.1%) | 862 (66.9%) | - | 732 | 158 (21.7%) | 574 (78.4%) | - |
| Age (median, IQR) | 65.5 (57.0-72.3) | 67.0 (60.0-74.0) | 64.4 (55.7-71.4) | <0.001 | 62.1 (53.0-70.0) | 64.0 (58.0-72.0) | 62.0 (51.6-69.4) | 0.15 |
| Gender | 0.63 | 0.71 | ||||||
| Male | 794 (61.6%) | 267 (62.7%) | 527 (61.1%) | 448 (61.2%) | 99 (62.7%) | 349 (60.8%) | ||
| Female | 494 (38.4%) | 159 (37.3%) | 335 (38.9%) | 284 (38.8%) | 59 (37.3%) | 225 (39.2%) | ||
| Primary tumor location | 0.01 | 0.60 | ||||||
| Right-sided | 288 (23.0%) | 79 (19.2%) | 209 (24.9%) | 196 (27.3%) | 41 (26.5% | 155 (27.5%) | ||
| Left-sided | 559 (44.7%) | 180 (43.7%) | 379 (45.2%) | 327 (45.5%) | 67 (43.2%) | 260 (46.2%) | ||
| Rectum | 404 (32.3%) | 153 (37.1%) | 251 (29.9%) | 195 (27.2%) | 47 (30.3%) | 148 (26.3%) | ||
| Missing | 37 | 14 | ||||||
| Nodal status primary tumor | 0.09 | 0.84 | ||||||
| N0 | 751 (58.6%) | 262 (61.9%) | 489 (57.0%) | 77 (10.6%) | 16 (10.1%) | 61 (10.7%) | ||
| N+ | 530 (41.4%) | 161 (38.1%) | 369 (43.0%) | 652 (89.4%) | 142 (89.9%) | 510 (89.3%) | ||
| Missing | 7 | 3 | ||||||
| Disease free interval | 0.002 | 0.82 | ||||||
| ≤ 12 months | 609 (47.3%) | 175 (41.1%) | 434 (50.3%) | 684 (93.4%) | 147 (93.0%) | 147 (93.0%) | ||
| > 12 months | 679 (52.7%) | 251 (58.9%) | 428 (49.7%) | 48 (6.6%) | 11 (7.0%) | 11 (7.0%) | ||
| Number CRLM | 0.01 | 0.59 | ||||||
| ≤ 1 | 882 (68.5%) | 311 (73.0%) | 571 (66.2%) | 80 (11.0%) | 19 (12.2%) | 61 (10.6%) | ||
| > 1 | 406 (31.5%) | 115 (27.0%) | 291 (33.8%) | 649 (89.0%) | 137 (87.8%) | 512 (89.4%) | ||
| Missing | 3 | |||||||
| Size largest tumor | 0.02 | 0.33 | ||||||
| ≤ 5cm | 1079 (84.6%) | 337 (81.2%) | 742 (86.2%) | 462 (63.7%) | 104 (67.1%) | 358 (62.8%) | ||
| > 5cm | 197 (15.4%) | 78 (18.8%) | 119 (13.8%) | 263 (36.3%) | 51 (32.9%) | 212 (37.2%) | ||
| Missing | 12 | 7 | ||||||
| CEA | 0.27 | 0.78 | ||||||
| ≤ 200 μg/L | 1204 (97.3%) | 400 (96.6%) | 804 (97.7%) | 531 (78.9%) | 118 (79.7%) | 413 (78.7%) | ||
| > 200 μg/L | 33 (2.7%) | 14 (3.4%) | 19 (2.3%) | 142 (21.1%) | 30 (20.3%) | 112 (21.3%) | ||
| Missing | 51 | 59 | ||||||
| Resection margin involved | 0.66 | 0.65 | ||||||
| Yes | 109 (8.5%) | 38 (9.0%) | 71 (8.3%) | 115 (15.7%) | 23 (14.6%) | 92 (16.1%) | ||
| No | 1166 (91.5%) | 382 (91.0%) | 784 (91.7%) | 616 (83.3%) | 135 (85.4%) | 481 (83.9%) | ||
| Missing | 13 | 1 | ||||||
| Tumor ablation at time of resection | 0.09 | 0.90 | ||||||
| Yes | 78 (6.1%) | 19 (4.5%) | 59 (6.8%) | 165 (22.5%) | 35 (22.2%) | 130 (22.6%) | ||
| No | 1210 (93.9%) | 407 (95.5%) | 803 (93.2%) | 567 (77.5%) | 123 (77.8%) | 444 (77.4%) | ||
Recurrence rates
The median follow-up for survivors for all patients was 88 months (Interquartile Range (IQR) 50-129 months). In total 1154 patients (57.1%) died during follow-up. During follow-up 1289 patients (63.8%) developed a recurrence after resection of CRLM. A total of 741 low-risk patients (57.5%) developed a recurrence compared to 548 high-risk patients (74.9%, p<0.001). The overall recurrence rate with and without perioperative systemic chemotherapy was similar in both low-risk (57% versus 58%, p=0.73) and high-risk patients (74% versus 77%, p=0.44).
Recurrence pattern and OS in low-risk patients
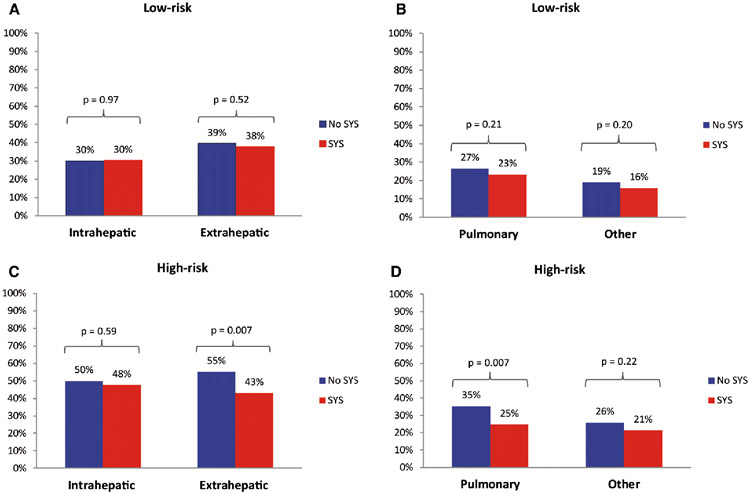
Organ-specific recurrence patterns are presented in Table 2. Among low-risk patients (Figure 2a and 2b), no difference in initial intrahepatic recurrence rate was found between both treatment groups (30% versus 30%, p=0.97). Similar, no difference was found in the rate of extrahepatic recurrence (38% versus 39%, p=0.52) and of pulmonary recurrence (23% versus 27%, p=0.21). Subdividing of low-risk patients in CRS 0, 1, and 2 did not change the results (Appendix Table 2).
Table 2.
Recurrences by location
| Low-risk | High-risk | |||||
|---|---|---|---|---|---|---|
| Location | No SYS | SYS | P-value | No SYS | SYS | P-value |
| Intrahepatic | 128 (30.0%) | 260 (30.2%) | 0.97 | 79 (50.0%) | 273 (47.6%) | 0.59 |
| Pulmonary | 113 (26.5%) | 201 (23.3%) | 0.21 | 56 (35.4%) | 142 (24.7%) | 0.007 |
| Distant lymph nodes | 28 (6.6%) | 47 (5.5%) | 0.42 | 18 (11.4%) | 49 (8.5%) | 0.27 |
| Peritoneal | 7 (1.6%) | 18 (2.1%) | 0.59 | 10 (6.3%) | 20 (3.5%) | 0.11 |
| Local recurrence | 12 (2.8%) | 35 (4.1%) | 0.26 | 5 (3.2%) | 22 (3.8%) | 0.69 |
| Bone | 6 (1.4%) | 11 (1.3%) | 0.85 | 4 (2.5%) | 15 (2.6%) | 0.95 |
| Other | 19 (4.5%) | 41 (4.8%) | 0.81 | 8 (5.1%) | 34 (5.9%) | 0.68 |
Figure 2. Recurrence patterns stratified by CRS.
Only initial recurrences are counted. Patients can have multiple initial recurrence sites, for example, intrahepatic and pulmonary.
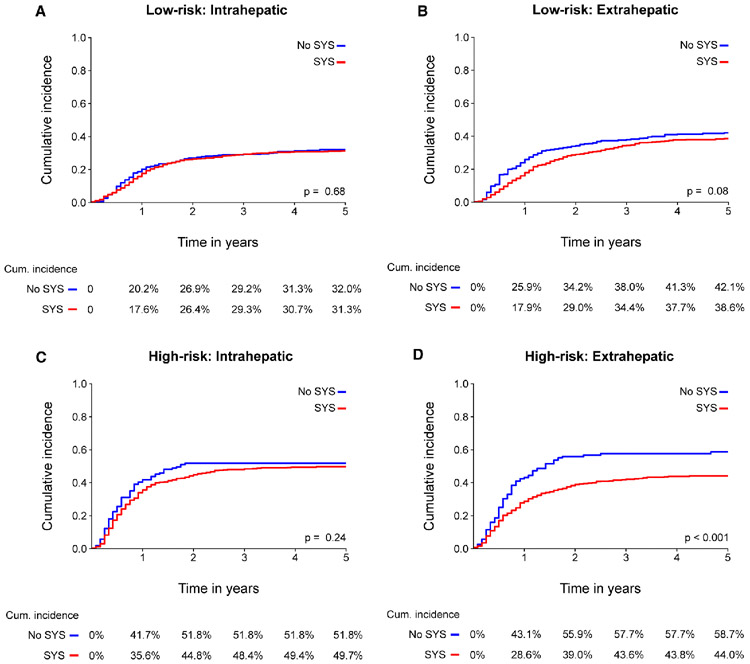
These results were confirmed in competing risk analysis (Figure 3a-b), showing no difference in the incidence of intrahepatic recurrence (p=0.68; 5-year cumulative incidence 31% versus 32%), and no difference in the incidence of extrahepatic recurrence (p=0.08; 5-year cumulative incidence 39% versus 42%). Subdividing of low-risk patients in CRS 0, 1, and 2 did not change the results (Appendix Figure 1a).
Figure 3. Cumulative incidence function for location specific recurrence stratified by CRS.
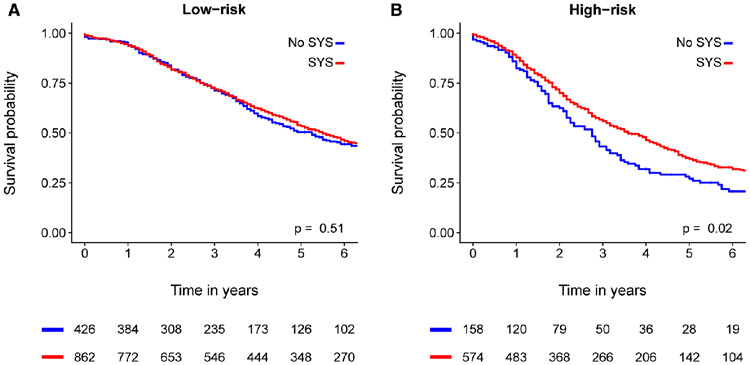
In terms of survival (Figure 4a), no benefit on median OS for low-risk patients treated with perioperative systemic chemotherapy was found (66 months versus 63 months, p=0.51). In multivariable analysis for OS in low-risk patients, perioperative systemic chemotherapy was not an independent prognostic factor (adjusted HR 0.99, 95% CI 0.82-1.19, p=0.90, Table 3a).
Figure 4. Kaplan-Meier analysis for overall survival stratified by CRS.
Table 3a.
Multivariable Cox regression analysis for overall survival of low-risk patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age at resection | 1.02 | 1.01-1.03 | <0.001 | 1.02 | 1.01-1.03 | <0.001 |
| Right-sided tumor | 1.15 | 0.94-1.41 | 0.17 | 1.10 | 0.89-1.36 | 0.40 |
| Node positive primary tumor | 1.18 | 1.02-1.38 | 0.03 | 1.40 | 1.19-1.65 | <0.001 |
| Disease-free interval (cont.) | 1.00 | 1.00-1.01 | 0.26 | 0.99 | 0.99-1.00 | 0.01 |
| Number CRLM (cont.) | 1.06 | 1.01-1.12 | 0.02 | 1.08 | 1.07-1.1 | 0.005 |
| Diameter CRLM (cont.) | 1.09 | 1.07-1.12 | <0.001 | 1.10 | 1.07-1.13 | <0.001 |
| Preoperative CEA (cont.) | 1.00 | 1.00-1.00 | 0.007 | 1.00 | 1.00-1.00 | 0.03 |
| Irradical resection (R1) | 1.67 | 1.32-2.13 | <0.001 | 1.58 | 1.22-2.03 | <0.001 |
| Additional ablation | 1.17 | 0.82-1.66 | 0.39 | 1.34 | 0.90-2.01 | 0.15 |
| Year of surgery | 0.98 | 0.97-1.00 | 0.008 | 0.99 | 0.98-1.01 | 0.22 |
| Perioperative SYS | 0.95 | 0.81-1.11 | 0.51 | 0.99 | 0.82-1.19 | 0.90 |
Recurrence pattern and OS in high-risk patients
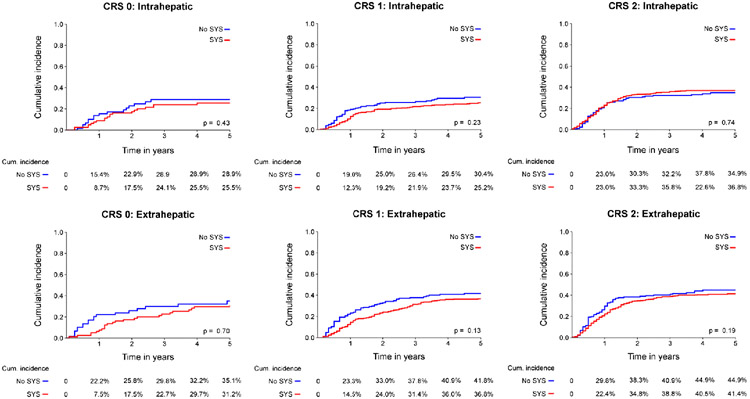
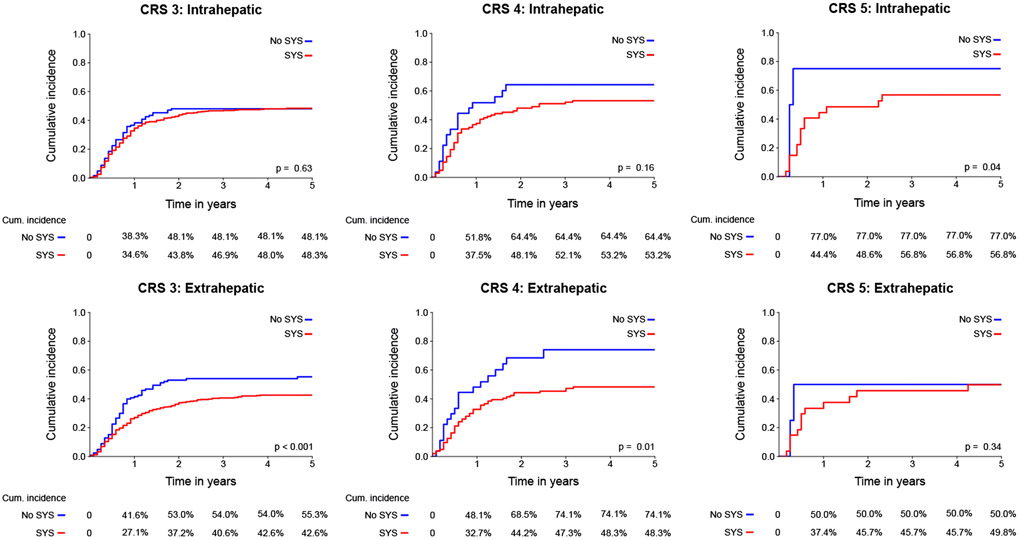
An overview of recurrence patterns in high-risk patients is presented in Table 2. Among high-risk patients (Figure 2c and 2d), no difference in initial intrahepatic recurrence rate was found between both treatment groups (48% versus 50%, p=0.59). A lower rate of extrahepatic recurrence was found after treatment with perioperative systemic chemotherapy (43% versus 55%, p=0.007). This was largely explained by a difference in pulmonary recurrence with perioperative systemic chemotherapy (25% versus 35%, p=0.007). Subdividing of low-risk patients in CRS 3, 4, and 5 demonstrated that the effect was primarily due to a difference in patients with a CRS of 3, however number of patients with a CRS of 4 or 5 is limited (Appendix Table 2).
These results were confirmed in competing risk analysis (Figure 3c-d), showing no difference in the incidence of intrahepatic recurrence (p=0.24; 5-year cumulative incidence 50% versus 52%), but a significant reduction of extrahepatic recurrence after perioperative systemic chemotherapy (p<0.001; 5-year cumulative incidence 44% versus 59%). Subdividing of low-risk patients in CRS 3, 4, and 5 demonstrated that the difference in cumulative difference was primarily due to a difference in patients with a CRS of 3 (Appendix Figure 1b).
Moreover, high-risk patients treated with perioperative systemic chemotherapy (Figure 4b) had a superior OS compared to patients that were not treated with perioperative systemic chemotherapy (median OS 43 months versus 33 months, p=0.02). Finally, perioperative systemic chemotherapy was an independent prognostic factor (adjusted HR 0.73, 95%CI 0.57-0.94, p=0.02) in multivariable for OS (Table 3b).
Table 3b.
Multivariable Cox regression analysis for overall survival of high-risk patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age at resection | 1.02 | 1.01-1.02 | 0.001 | 1.01 | 1.00-1.02 | 0.005 |
| Right-sided tumor | 1.46 | 1.13-1.87 | 0.004 | 1.32 | 1.00-1.74 | 0.05 |
| Node positive primary tumor | 1.04 | 0.77-1.39 | 0.82 | 1.36 | 0.97-1.90 | 0.08 |
| Disease-free interval (cont.) | 1.01 | 1.00-1.02 | 0.009 | 1.00 | 0.99-1.01 | 0.97 |
| Number CRLM (cont.) | 1.08 | 1.05-1.10 | <0.001 | 1.06 | 1.04-1.09 | <0.001 |
| Diameter CRLM (cont.) | 1.04 | 1.02-1.07 | <0.001 | 1.04 | 1.01-1.07 | 0.006 |
| Preoperative CEA (cont.) | 1.00 | 1.00-1.00 | 0.75 | 1.00 | 1.00-1.00 | 0.17 |
| Irradical resection (R1) | 1.38 | 1.09-1.76 | 0.008 | 1.33 | 1.02-1.75 | 0.04 |
| Additional ablation | 1.04 | 0.82-1.32 | 0.74 | 1.35 | 1.00-1.81 | 0.05 |
| Year of surgery | 0.97 | 0.96-0.98 | 0.008 | 0.97 | 0.95-0.99 | 0.001 |
| Perioperative SYS | 0.76 | 0.61-0.95 | 0.02 | 0.73 | 0.57-0.94 | 0.02 |
Discussion
We found a significant decrease in extrahepatic recurrences (43% versus 55%, p=0.007) in high-risk patients treated with perioperative systemic chemotherapy. This was confirmed in a competing risk analysis; 5-years cumulative incidence of extrahepatic recurrence was 44% with perioperative systemic chemotherapy versus 59% without (p<0.001). This decrease in extrahepatic recurrences could largely be attributed to a decrease in pulmonary recurrences (25% versus 35%, p=0.007). No difference in intrahepatic recurrence rate was found. Moreover, low-risk patients had similar recurrence rates and patterns with and without perioperative systemic chemotherapy.
In the present study, 1289 patients (64%) developed a recurrence after resection of CRLM. Approximately equal rates of recurrence were found in a previous study of 1669 patients after curative resection of CRLM. In that study, after a median follow-up of 30 months, 947 (57%) of patients developed a recurrence.8 This study reported intrahepatic recurrences in 36% of the patients, and similarly extrahepatic recurrences in 36% of the patients.
Another large study evaluating 2320 patients after resection of CRLM, reported a recurrence rate of 47% after a median follow-up of only 27 months.9 The proportion of patients with an intrahepatic recurrence was 32%, compared to 25% for extrahepatic recurrence. Both studies underestimated the recurrence rate because of a much shorter length of follow-up and a smaller proportion of high-risk patients.
Based on the results of previous studies, the role of perioperative systemic chemotherapy in patients with resectable CRLM is still debated.2, 10, 11 No significant OS benefit was found in a large randomized trial that evaluated the effectiveness of perioperative FOLFOX in patients with resectable CRLM (EORTC 40983).2 Although OS was not the primary endpoint of the study, OS curves were overlapping, even after long-term follow-up.12 Importantly, in the EORTC 40983 trial most patients had low-risk disease. Several non-randomized studies evaluated whether high-risk patients had superior OS with perioperative systemic chemotherapy.3, 4 In the first study, a superior OS was found for high-risk patients treated with neoadjuvant chemotherapy (adjusted HR 0.57, 95% CI 0.39-0.84, p=0.004).3 A second study found similar results for adjuvant systemic chemotherapy (HR 0.40, 95% CI 0.23-0.70, p=0.001).4 The superior OS of perioperative systemic chemotherapy in high-risk patients was confirmed in the present much larger study. Moreover, we found that the superior OS could be explained by a reduction in pulmonary recurrences, without an impact on intrahepatic recurrences (Figure 2c-d). Pulmonary recurrences were less common after perioperative systemic chemotherapy in high-risk patients (25% versus 35%, p=0.007). It appears that perioperative systemic chemotherapy can avoid the appearance of pulmonary recurrences with an absolute risk reduction of 10%. Moreover, competing risk analyses demonstrated that perioperative systemic chemotherapy can also avoid or postpone pulmonary recurrence in high-risk patients. This could explain the superior OS found in this subgroup. Subdividing CRS groups from 0 to 5 demonstrated that the effect found in high-risk patients is primarily a result of a difference found in patients with a CRS of 3, however number of patients with a CRS of 4 and 5 is low, limiting interpretation of the results in these specific subgroups.
No such effect of perioperative systemic chemotherapy was found in low-risk patients, or for intrahepatic recurrence. In low-risk patients, both previous studies found similar OS with and without systemic chemotherapy.3, 4 The present study confirmed these findings, and found no difference in OS when comparing low-risk patients with and without perioperative systemic chemotherapy. Moreover, we found that perioperative systemic chemotherapy did not improve OS because, possibly since no association on the recurrence rate and pattern in these low-risk patients (in contrast to high risk patients) could be demonstrated (Figure 2a-b).
The retrospective nature of this study contributed to several limitations. The administration of chemotherapy was not at random, at MSKCC most patients received perioperative chemotherapy (%) compared to a minority of patients at Erasmus MC (43.0%). The types and duration of chemotherapy regimens varied across centers and in time. However, most patients (72.3%) received oxaliplatin- or irinotecan-based regimens. Furthermore, follow-up differed between the two centers, which could have biased recurrence intervals. Moreover, baseline tumor characteristics between patients treated with and without perioperative systemic chemotherapy varied considerably in low-risk patients. Stratification of patients in low-risk and high-risk reduced bias, but residual differences in low-risk patients remained. However, for OS these differences were addressed in multivariable analysis. Secondly, the CRS does not consider new biomarkers such as the genetic alterations (e.g., in RAS and BRAF) or histopathological growth patterns.13-17 A previous study demonstrated that KRAS codon 13 mutations were associated with extrahepatic recurrence free survival (HR 2.27, 95%CI 1.29-3.97, p=0.004) and lung recurrence free survival (HR 2.32, 95%CI 1.12-4.78).16 Recently, a new clinical risk score (GAME score) was developed, which combines clinicopathological and biological indicators (such as RAS mutation status).18 A significant improvement of the Harrell’s C-index was found for the GAME score compared to the original CRS by Fong (0.65 versus 0.58, p=0.008).18 Mutational status was not available for our cohort unfortunately. Until mutational status will be generally available, the CRS will remain a practical classification method to determine the risk of recurrence.
Based on the present study and other smaller studies with similar findings, we recommend to consider perioperative systemic chemotherapy in high-risk patients in countries (such as the Netherlands) that currently do not recommend any systemic chemotherapy after resection of CRLM. Secondly, we recommend to consider withholding perioperative systemic chemotherapy in low-risk patients in countries (such as the USA) that currently recommend systemic chemotherapy after resection of CRLM for all patients.
In conclusion, we found that perioperative systemic chemotherapy had no association with intrahepatic recurrence, but was associated with fewer pulmonary recurrences and superior OS in high-risk patients only.
Acknowledgments
Funding: None
Appendix
Appendix Table 1.
Number of patients according to CRS class
| All patients | No SYS | SYS | P-value | |
|---|---|---|---|---|
| Clinical Risk Score | <0.001 | |||
| 0 | 141 (7.0%) | 60 (10.3%) | 81 (5.6%) | |
| 1 | 518 (25.6%) | 188 (32.2%) | 330 (23.0%) | |
| 2 | 629 (31.1%) | 178 (30.5%) | 451 (31.4%) | |
| 3 | 570 (28.2%) | 127 (21.7%) | 443 (30.8%) | |
| 4 | 131 (1.5%) | 27 (4.6%) | 104 (7.2%) | |
| 5 | 31 (1.5%) | 4 (0.7%) | 27 (1.9%) |
Appendix Table 2.
Recurrence rate’s according to CRS class
| CRS | Intrahepatic | Pulmonary | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No SYS | SYS | P-value | No SYS | SYS | P-value | No SYS | SYS | P-value | |
| 0 | 17 (28.3%) | 20 (24.7%) | 0.63 | 12 (20.0%) | 19 (23.5%) | 0.63 | 7 (14.3%) | 11 (13.6%) | 0.91 |
| 1 | 51 (27.1%) | 82 (24.8%) | 0.57 | 47 (25.0%) | 75 (22.7%) | 0.56 | 29 (19.2%) | 48 (14.5%) | 0.20 |
| 2 | 60 (33.7%) | 158 (35.0%) | 0.78 | 54 (30.3%) | 107 (23.7%) | 0.09 | 30 (20.3%) | 78 (17.3%) | 0.41 |
| 3 | 59 (46.5) | 203 (45.8%) | 0.92 | 43 (33.9%) | 104 (23.5%) | 0.02 | 30 (23.6%) | 78 (17.3%) | 0.43 |
| 4 | 17 (63.0%) | 55 (52.9%) | 0.35 | 11 (40.7%) | 29 (27.9%) | 0.20 | 9 (33.3%) | 28 (26.9%) | 0.51 |
| 5 | 3 (75.0%) | 15 (55.6%) | 0.46 | 2 (50.0%) | 9 (33.3%) | 0.52 | 2 (50.0%) | 4 (14.8%) | 0.10 |
Appendix Figure 1a.
Cumulative incidence function for location specific recurrence for CRS 0, 1 and 2
Appendix Figure 1b.
Cumulative incidence function for location specific recurrence for CRS 3, 4 and 5
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical requirements: The authors have no conflict of interest. No informed consent was required.
References
- 1.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure J Clin Oncol 2007: 25; 4575–4580 [DOI] [PubMed] [Google Scholar]
- 2.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial The Lancet. Oncology 2013: 14; 1208–1215 [DOI] [PubMed] [Google Scholar]
- 3.Ayez N, van der Stok EP, Grunhagen DJ, et al. The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: Clinical risk score as possible discriminator Eur J Surg Oncol 2015: 41; 859–867 [DOI] [PubMed] [Google Scholar]
- 4.Rahbari NN, Reissfelder C, Schulze-Bergkamen H, et al. Adjuvant therapy after resection of colorectal liver metastases: the predictive value of the MSKCC clinical risk score in the era of modern chemotherapy BMC Cancer 2014: 14; 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents Journal of the American College of Surgeons 2007: 204; 753–761; discussion 761–753 [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases Ann Surg 1999: 230; 309–318; discussion 318–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray RJ A class of K-sample tests for comparing the cumulative incidence of a competing risk The Annals of Statistics 1988: 16; 114–1154 [Google Scholar]
- 8.De Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients Annals of surgery 2009: 250; 440–447 [DOI] [PubMed] [Google Scholar]
- 9.Hallet J, Sa Cunha A, Adam R, et al. Factors influencing recurrence following initial hepatectomy for colorectal liver metastases Br J Surg 2016: 103; 1366–1376 [DOI] [PubMed] [Google Scholar]
- 10.Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial The Lancet. Oncology 2014: 15; 601–611 [DOI] [PubMed] [Google Scholar]
- 11.Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008: 26; 4906–4911 [DOI] [PubMed] [Google Scholar]
- 12.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial Lancet Oncol 2013: 14; 1208–1215 [DOI] [PubMed] [Google Scholar]
- 13.Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases Nat Med 2016: 22; 1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dam PJ, van der Stok EP, Teuwen LA, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis Br J Cancer 2017: 117; 1427–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margonis GA, Buettner S, Andreatos N, et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer JAMA Surg 2018: 153; e180996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margonis GA, Kim Y, Sasaki K, et al. Codon 13 KRAS mutation predicts patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases Cancer 2016: 122; 2698–2707 [DOI] [PubMed] [Google Scholar]
- 17.Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases Annals of surgery 2013: 258; 619–626; discussion 626–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margonis GA, Sasaki K, Gholami S, et al. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases Br J Surg 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]