Abstract
Objective
To assess the efficacy and safety of favipiravir in adults with mild-to-moderate coronavirus disease 2019 (COVID-19).
Methods
In this randomized, open-label, parallel-arm, multicenter, phase 3 trial, adults (18–75 years) with RT-PCR confirmed COVID-19 and mild-to-moderate symptoms (including asymptomatic) were randomized 1:1 to oral favipiravir (day 1: 1800 mg BID and days 2−14: 800 mg BID) plus standard supportive care versus supportive care alone. The primary endpoint was time to the cessation of viral shedding; time to clinical cure was also measured.
Results
From May 14 to July 3, 2020, 150 patients were randomized to favipiravir (n = 75) or control (n = 75). Median time to the cessation of viral shedding was 5 days (95% CI: 4 days, 7 days) versus 7 days (95% CI: 5 days, 8 days), P = 0.129, and median time to clinical cure was 3 days (95% CI: 3 days, 4 days) versus 5 days (95% CI: 4 days, 6 days), P = 0.030, for favipiravir and control, respectively. Adverse events were observed in 36% of favipiravir and 8% of control patients. One control patient died due to worsening disease.
Conclusion
The lack of statistical significance on the primary endpoint was confounded by limitations of the RT-PCR assay. Significant improvement in time to clinical cure suggests favipiravir may be beneficial in mild-to-moderate COVID-19.
Keywords: Favipiravir, SARS-CoV-2, COVID-19, Coronavirus, Antiviral, Randomized clinical trial
Introduction
A novel coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2), emerged in late December 2019, which resulted in the ongoing worldwide coronavirus disease 2019 (COVID-19) pandemic (World Health Organization, 2020, Zhou et al., 2020 Zhou et al., 2020). As of September 25, 2020, the Johns Hopkins University COVID-19 global dashboard reports 32,390,204 confirmed cases and 985,302 deaths worldwide attributed to SARS-CoV-2 (The Center for Systems Science and Engineering at Johns Hopkins, 2020). Numerous antivirals, immunotherapies, and vaccines are being investigated to combat this global health crisis; however, to date, few have demonstrated efficacy in randomized, controlled clinical trials for the treatment or prevention of COVID-19 (World Health Organization, 2020, Dong et al., 2020, Song et al., 2020).
Favipiravir is an RNA-dependent RNA polymerase (RdRp) inhibitor approved for the treatment of novel influenza viruses in Japan and China and exhibits antiviral activity across a wide range of RNA viruses (Dong et al., 2020, Delang et al., 2018, Furuta et al., 2017, Furuta et al., 2013). As SARS-CoV-2 is a positive-sense, single-stranded RNA virus, RdRp represents a relevant target for the known mechanism of action of favipiravir (Buonaguro et al., 2020). Only a few favipiravir efficacy trials in COVID-19 have been reported in the literature to date, including two comparative trials in which favipiravir showed an advantage over other antivirals (Cai et al., 2020, Chen et al., 2020, Doi et al., 2020, Sanders et al., 2020, Ivashchenko et al., 2020). Numerous other favipiravir COVID-19 trials are ongoing or as yet unreported (Agrawal et al., 2020).
Initial reports from China suggested that >80% of those infected with SARS-CoV-2 will experience mild or moderate disease (Wu and McGoogan, 2020), yet few studies to date have investigated therapeutic interventions in this population. Here, we present results of a randomized, comparative, open-label, parallel group clinical trial to evaluate the efficacy and safety of oral favipiravir monotherapy in addition to standard supportive care as compared to standard supportive care alone in patients with mild-to-moderate COVID-19.
Methods
Study design
This was a randomized, open-label, parallel-arm, multicenter, phase 3 study to evaluate the efficacy and safety of oral favipiravir combined with standard supportive care in adults with mild-to-moderate COVID-19 (CTRI/2020/05/025114; 7 sites in India). The protocol (appendix) and informed consent form were approved by institutional/independent ethics committees and the Drugs Controller General of India (April 26, 2020). The trial was undertaken in accordance with current guidelines of the Central Drugs Standard Control Organization, which is the National Regulatory Authority in India (Gogtay et al., 2017), the International Conference on Harmonization Good Clinical Practice, and the Declaration of Helsinki. Written informed consent followed study explanation to patients before screening procedures or assessments.
The study consisted of screening (day -3 to day 0), baseline/randomization (day 1) at which standard supportive care was provided to all patients, and a treatment period (day 1 up to a maximum of 14 days). Study participation was a maximum of 28 days from the day of randomization. Patient care beyond day 14 was provided as per investigator judgment.
Randomization
Patients were randomized in a 1:1 ratio to oral favipiravir (1800 mg BID loading dose on day 1; 800 mg BID maintenance dose thereafter) plus standard supportive care for up to a maximum of 14 days or standard supportive care alone (the control arm) that included antipyretics, cough suppressants, antibiotics, and vitamins. Drugs thought to have antiviral activity against SARS-CoV-2 (including hydroxychloroquine) were prohibited. The randomization was stratified based on baseline disease severity into mild and moderate COVID-19. Patients were assigned to stratified randomized treatments based on a central, computer-generated randomization scheme coordinated by an independent third party. All subjects were hospitalized per prevailing treatment guidelines to allow daily RT-PCR testing and were discharged only after 2 consecutive negative SARS-CoV-2 tests, and clinical cure was achieved.
Study population
The study population comprised patients admitted to the hospital with mild (including asymptomatic) to moderate COVID-19. Key inclusion criteria were age 18–75 years, infection with SARS-CoV-2 virus confirmed by RT-PCR within 48 h prior to randomization, no participation in any other interventional clinical study, agreement to use effective contraception during the study and for ≥7 days following the last treatment, and for female patients of child-bearing potential, a negative pretreatment pregnancy test.
The time from symptom onset to randomization was to be no more than 7 days for mild disease and 10 days for moderate disease. Additional criteria to guide investigator assessment of mild disease severity included pyrexia (temperature <102.2°F), respiratory rate 12 to ≤20 breaths/min, and ≤4 of the following symptoms of mild severity (defined as symptoms not requiring any or minimal therapeutic intervention) and ≤2 of the following symptoms of moderate severity (defined as symptoms which produce small impairment of function and require some form of therapeutic intervention): cough, sore throat, headache, nasal congestion, body aches and pains, and fatigue. Additional criteria for moderate cases included pneumonia documented by chest imaging (CT as first option or x-ray if CT not possible), pyrexia (axillary ≥98.6°F or oral ≥99.5°F), and respiratory rate >20 to <30 breaths/min.
Key exclusion criteria were severe infection (defined as need for invasive or noninvasive ventilator support, extracorporeal membrane oxygenation [ECMO] or shock requiring vasopressor support), oxygen saturation ≤93% or arterial oxygen partial pressure or fraction of inspired oxygen of ≤300 mmHg, current ICU care for the management of ongoing clinical status, inability to take or tolerate oral medications, allergy or hypersensitivity to favipiravir, asthma or chronic obstructive lung disease, severe liver disease (underlying liver cirrhosis or alanine aminotransferase/aspartate aminotransferase elevated over 5 times the upper limit of normal [ULN]), history of gout or hyperuricemia (above the ULN), prolonged QT (defined as QTcF ≥450 msec for men and as QTcF ≥470 msec for women), severely reduced left ventricular function (ejection fraction <30%), or severe renal impairment (creatinine clearance <30 mL/min), or having received continuous renal replacement therapy, hemodialysis or peritoneal dialysis. Prohibited concomitant medications included hydroxychloroquine or chloroquine, pyrazinamide, repaglinide, theophylline, and famciclovir or sulindac.
Efficacy and safety assessments
The prespecified primary endpoint was time from randomization to the cessation of oral shedding of the SARS-CoV-2 virus (28-days maximum; specified as a negative RT-PCR result for both oropharyngeal and nasopharyngeal swabs). SARS-CoV-2 RT-PCR on both oropharyngeal and nasopharyngeal swabs was performed at screening, daily during days 2–28 until 2 consecutive negative results were achieved, and at hospital discharge.
Prespecified secondary endpoints included time to clinical cure for patients who presented with clinical signs and symptoms at baseline. Clinical cure was based on clinician assessment and defined as the recovery of fever (axillary temperature ≤97.8°F), respiratory rate of ≤20 breaths/minute, oxygen saturation ≥98% without oxygen supplementation (which was later revised to align with the discharge criterion of ≥95% oxygen saturation issued by the Indian Ministry of Health prior to the start of the study), and cough relief (mild or no cough) maintained for ≥72 h.
Additional secondary time-to-event endpoints were time to the first use of high flow supplemental oxygen, ventilation (noninvasive or mechanical), or ECMO, and time to hospital discharge. Hospital discharge was dependent on achieving both RT-PCR negativity on 2 consecutive tests and maintaining clinical cure for ≥72 h. Rates of clinical cure and SARS-CoV-2 RT-PCR negativity at days 4, 7, 10, and 14 were also assessed.
Clinical symptoms and vital sign parameters were assessed twice daily on days 1−28. Laboratory assessments (including hematology, chemistry, and urinalysis) were performed at screening, throughout the study as warranted per standard management for COVID-19, and on the day of hospital discharge. Safety was assessed by the frequency of serious adverse events, and treatment emergent adverse events (TEAEs) were recorded.
Statistical analysis
Based on the hazard ratio of 3.434 reported in a favipiravir COVID-19 trial (Cai et al., 2020), a total of 28 viral clearance events assessed by RT-PCR negativity would need to be observed to obtain 90% power; therefore, a sample size of 40–100 patients would be required in each of the mild and moderate populations. In accordance with regulatory recommendations, 90 subjects were to be enrolled in the mild subgroup and 60 subjects were to be enrolled in the moderate subgroup. Statistical analyses were performed with SAS 9.4 version or the latest available (SAS Institute Inc., Cary, North Carolina).
Efficacy analyses used the intent-to-treat (ITT) population, defined as all randomized patients who received ≥1 treatment and had ≥1 postbaseline efficacy assessment. Safety analyses used the safety population, defined as all randomized patients who received any amount of study treatment (favipiravir or standard supportive care alone).
The primary endpoint and secondary endpoints assessing time to event were analyzed using the Kaplan–Meier method and log-rank test as well as by Cox regression analysis. In the Cox model, time to event was set as the time variable, censoring was set as the status, and the variables, including age, treatment, and baseline comorbidity were set as independent variables. Secondary endpoints assessing categorical variables were analyzed using the chi–square or Fisher’s exact test. A priori defined subgroup analyses for primary and secondary endpoints were done based on COVID-19 disease severity using the Kaplan–Meier method, log-rank test, and Cox regression analysis. Censoring was used to handle missing data in which time to event was not observed. Patients terminating the study without a documented event were censored at day 28 to avoid bias in favor of either treatment group. Patients who died without a documented event were censored at day 28 or date of death, whichever was later.
Safety data were summarized descriptively.
Results
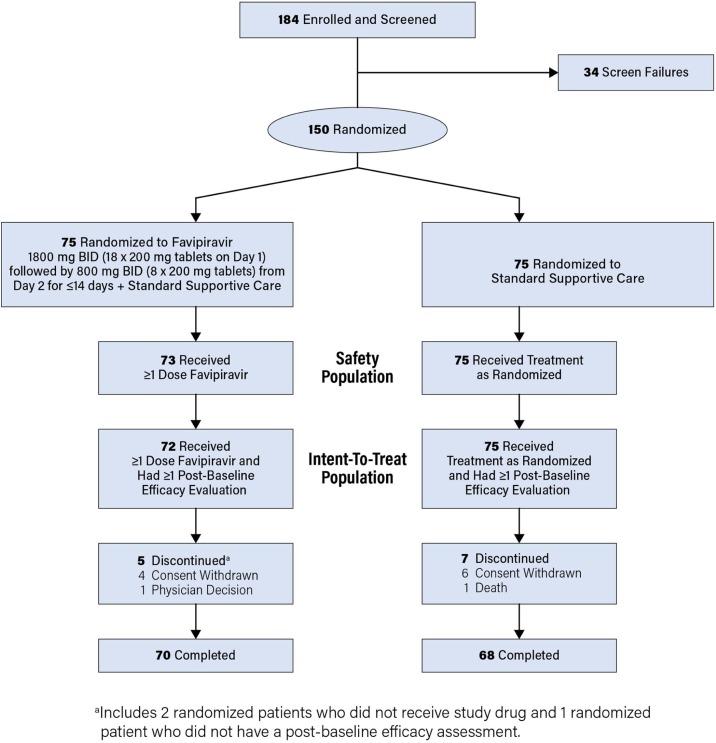
The study was conducted between May 14 and July 3, 2020; 150 patients were enrolled and randomized to favipiravir in addition to standard supportive care (n = 75) or a control group with standard supportive care alone (n = 75), Figure 1 . Two patients randomized to favipiravir did not receive the study drug and were excluded from the safety population (n = 148). An additional patient randomized to favipiravir did not have a postbaseline efficacy assessment and was excluded from the ITT population (n = 147). Among patients in the ITT population, 70/72 randomized to favipiravir and 68/75 randomized to control completed the study. The most common reason for study discontinuation was the withdrawal of consent (n = 10) (Figure 1).
Figure 1.
CONSORT Diagram of Patient Disposition.
Baseline demographic and clinical characteristics were generally similar between favipiravir and control groups (Table 1 ). All enrolled patients were Asian; most were male (108/147, 73.5%), 30-60 years of age (114/147, 77.6%), and symptomatic (102/147, 69.4%) at baseline. Approximately 60% were diagnosed with mild disease, while approximately 26% had medical comorbidities thought to be associated with more rapid and severe progression of COVID-19.
Table 1.
Baseline Demographics and Clinical Characteristics, ITT Population.
| Parameter | Favipiravir (N = 72) | Control (N = 75) | Total (N = 147) |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 43.6 ± 12.2 | 43.0 ± 11.2 | 43.3 ± 11.7 |
| Median (min, max) | 44.5 (19, 67) | 42.0 (20, 67) | 43.0 (19, 67) |
| Age subgroup, years, n (%) | |||
| 18−30 | 14 (19.4) | 7 (9.3) | 21 (14.3) |
| >30−60 | 53 (73.6) | 61 (81.3) | 114 (77.6) |
| >60 | 5 (6.9) | 7 (9.3) | 12 (8.2) |
| Weight (kg) | |||
| Mean ± SD | 64.40 ± 11.84 | 64.84 ± 9.53 | 64.62 ± 10.69 |
| Median (min, max) | 64.0 (42.0, 126.3) | 65.0 (47.0, 93.1) | 65.0 (42.0, 126.3) |
| Severity of COVID-19, n (%) | |||
| Mild | 44 (61.1) | 45 (60.0) | 89 (60.5) |
| Moderate | 28 (38.9) | 30 (40.0) | 58 (39.5) |
| Gender, n (%) | |||
| Female | 21 (29.2) | 18 (24.0) | 39 (26.5) |
| Male | 51 (70.8) | 57 (76.0) | 108 (73.5) |
| Comorbidity (Diabetes Mellitus, Hypertension, and/or Obesity), n (%) | 19 (26.4) | 19 (25.3) | 38 (25.9) |
| Baseline symptoms (Cough, Fever, Respiratory Rate, SpO2), n (%) | 53 (73.6) | 49 (65.3) | 102 (69.4) |
ITT, intent-to-treat; kg, kilogram; and SpO2, peripheral capillary oxygen saturation.
The ITT population excluded 2 subjects with no drug intake (favipiravir arm) and 1 subject with no post baseline efficacy assessment (favipiravir arm).
Percentages are based on the total number of randomized subjects in each treatment.
Patients with >1 comorbidity were counted once.
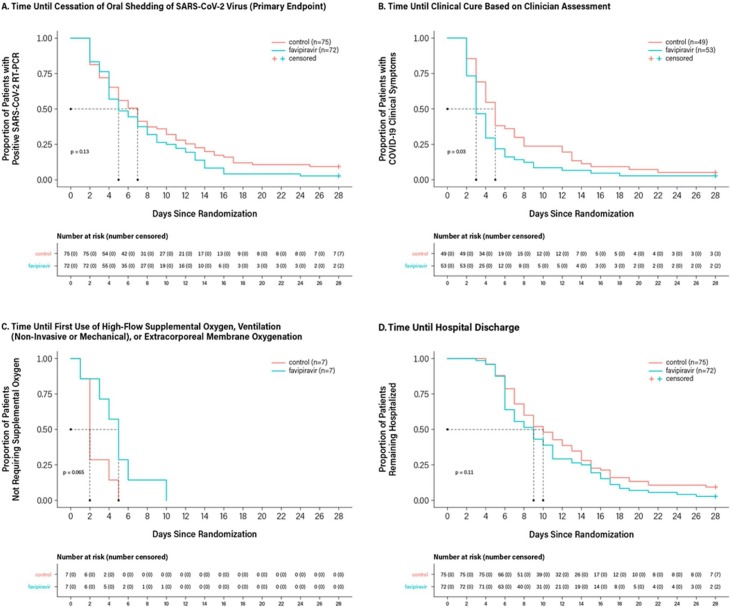
Kaplan–Meier analysis of time to the cessation of oral shedding of the SARS-CoV-2 virus, the primary endpoint, is shown in Figure 2A. The median time to the cessation of oral shedding of SARS-CoV-2 virus was 5 days (95% CI: 4 days, 7 days) in the favipiravir group compared with 7 days (95% CI: 5 days, 8 days) in the control group (P = 0.129; Table 2). The Cox proportional hazard ratio for time to the cessation of oral shedding of SARS-CoV-2 virus of 1.367 numerically favored favipiravir over control (95% CI: 0.944, 1.979; P = 0.098).
Figure 2.
Kaplan–Meier Survival Curves for Primary and Secondary Endpoints, ITT Population.
ITT, intent-to-treat.
The ITT population excluded 2 subjects with no drug intake (favipiravir arm) and 1 subject with no post baseline efficacy assessment (favipiravir arm).
The favipiravir arm received treatment with favipiravir + standard supportive care; the control arm received standard supportive care alone.
Kaplan–Meier was used to estimate the median duration of time-to-event and 95% confidence intervals. The two treatment groups were compared using a log-rank test to estimate the P value. The hazard ratio of favipiravir/control and the corresponding P value were computed based on the Cox regression model with covariates of age, treatment, and baseline comorbidity. Subjects who terminated the study without documented event were censored at day 28. Subjects who died without the documented event were censored at day 28 or the date of death, whichever was later. Analyses of time to clinical cure and time to the first use of supplemental oxygen included, respectively, only patients who were symptomatic at baseline and patients who required the use of supplemental oxygen.
Panel A: median of the time to events in days for favipiravir and control (95% CI): 5.0 (4.0, 7.0), 7.0 (5.0, 8.0); P value for favipiravir vs control: 0.1290; and hazard ratio (95% CI): 1.367 (0.944, 1.979), P = 0.098.
Panel B: median of the time to events in days for favipiravir and control (95% CI): 3.0 (3.0, 4.0), 5.0 (4.0, 6.0); P value for favipiravir vs control: 0.0297; and hazard ratio (95% CI): 1.749 (1.096, 2.792), P = 0.019.
Panel C: Median of the time to events in days for favipiravir and control (95% CI): 5.0 (1.0, 6.0), 2.0 (1.0, 4.0); P value for favipiravir vs control: 0.0653; and hazard ratio (95% CI): 0.065 (0.005, 0.809), P = 0.034; subjects who terminated the study without a documented event were excluded. Because most of the subjects did not require oxygen support, the censoring rate was too high for Kaplan–Meier analysis to estimate the median time-to-event; therefore, only results from noncensored data are presented for this endpoint.
Panel D: Median of the time to events in days for favipiravir and control (95% CI): 9.0 (7.0, 10.0), 10.0 (8.0, 12.0); P value for favipiravir vs control: 0.1079; and hazard ratio (95% CI): 1.406 (0.974, 2.030), P = 0.069.
Table 2.
Analysis of Time to Event Endpoints, ITT Population.
| Favipiravir | Control | |
|---|---|---|
| Time to Cessation of SARS-CoV-2 Oral Shedding (Primary Endpoint) | ||
| No. of patients | N = 72 | N = 75 |
| No. of events (%) | 70 (97.2) | 68 (90.7) |
| Time to event, median days (95%CI) | 5.0 (4.0, 7.0) | 7.0 (5.0, 8.0) |
| Log-rank P value | 0.1290 | |
| Hazard Ratio (95% CI) | 1.367 (0.944, 1.979) | |
| Hazard Ratio P value | 0.098 | |
| Time to Clinical Cure | ||
| No. of patientsa | N = 53 | N = 49 |
| No. of events (%) | 51 (96.2) | 46 (93.9) |
| Time to event, median days (95% CI) | 3.0 (3.0, 4.0) | 5.0 (4.0, 6.0) |
| Log-rank P value | 0.0297 | |
| Hazard Ratio (95% CI) | 1.749 (1.096, 2.792) | |
| Hazard Ratio P value | 0.019 | |
| Time to First Use of High-Flow Supplemental Oxygen, Ventilation (Non-Invasive or Mechanical), or Extracorporeal Membrane Oxygenation | ||
| No. of patientsb | N = 7 | N = 7 |
| No. of events (%) | 7 (100) | 7 (100) |
| Time to event, median days (95% CI) | 5.0 (1.0, 6.0) | 2.0 (1.0, 4.0) |
| Log-rank P value | 0.0653 | |
| Hazard Ratio (95% CI) | 0.065 (0.005, 0.809) | |
| Hazard Ratio P value | 0.034 | |
| Time to Hospital Discharge | ||
| No. of patients | N = 72 | N = 75 |
| No. of events (%) | 70 (97.2) | 68 (90.7) |
| Time to event, median days (95% CI) | 9.0 (7.0, 10.0) | 10.0 (8.0, 12.0) |
| Log-rank P value | 0.1079 | |
| Hazard Ratio (95% CI) | 1.406 (0.974, 2.030) | |
| Hazard Ratio P value | 0.069 | |
ITT, intent-to-treat.
The ITT population excluded 2 subjects with no drug intake (favipiravir arm) and 1 subject with no post baseline efficacy assessment (favipiravir arm).
The favipiravir arm received treatment with favipiravir + standard supportive care; the control arm received standard supportive care alone.
Kaplan–Meier was used to estimate the median duration of time-to-event and 95% confidence intervals. The two treatment groups were compared using a log-rank test to estimate the P value. The hazard ratio of favipiravir/control and the corresponding P value were computed based on the Cox regression model with covariates of age, treatment, and baseline comorbidity. Subjects who terminated the study without the documented event were censored at day 28. Subjects who died without documented event were censored at day 28 or the date of death, whichever was later.
Only patients with clinical signs and symptoms at baseline were included in this analysis.
Because most of the subjects did not require oxygen support, the censoring rate is too high to run the Kaplan–Meier analysis to estimate the median time-to-event; therefore, only results from noncensored data were presented for this endpoint.
Kaplan–Meier analyses of time to clinical cure, time to first use of high flow supplemental oxygen, ventilation, or ECMO, and time to hospital discharge are shown in Figure 2B–D. Median time to clinical cure among patients who were symptomatic at baseline was significantly faster with favipiravir compared with control (3 days [95% CI: 3 days, 4 days] vs. 5 days [95% CI: 4 days, 6 days], P = 0.030). A statistical advantage in favor of favipiravir also was observed using the Cox proportional hazard ratio (1.749 [95% CI: 1.096, 2.792]; P = 0.019; Table 2).
Among patients requiring supportive oxygen therapy, median time to first use was 5 days (95% CI: 1 day, 6 days) in the favipiravir group (n = 7) when compared with 2 days (95% CI: 1 day, 4 days) in the control group (n = 7), P = 0.065; the Cox proportional hazard ratio of 0.065 (95% CI: 0.005, 0.809) was statistically significant (P = 0.034; Table 2). A total of 97.2% (70/72) and 90.7% (68/75) of patients in the favipiravir and control groups, respectively, met criteria for hospital discharge within 28 days. Median time to hospital discharge was 9 days (95% CI: 7 days, 10 days) for favipiravir when compared with 10 days (95% CI: 8 days, 12 days) for control (P = 0.108; Table 2).
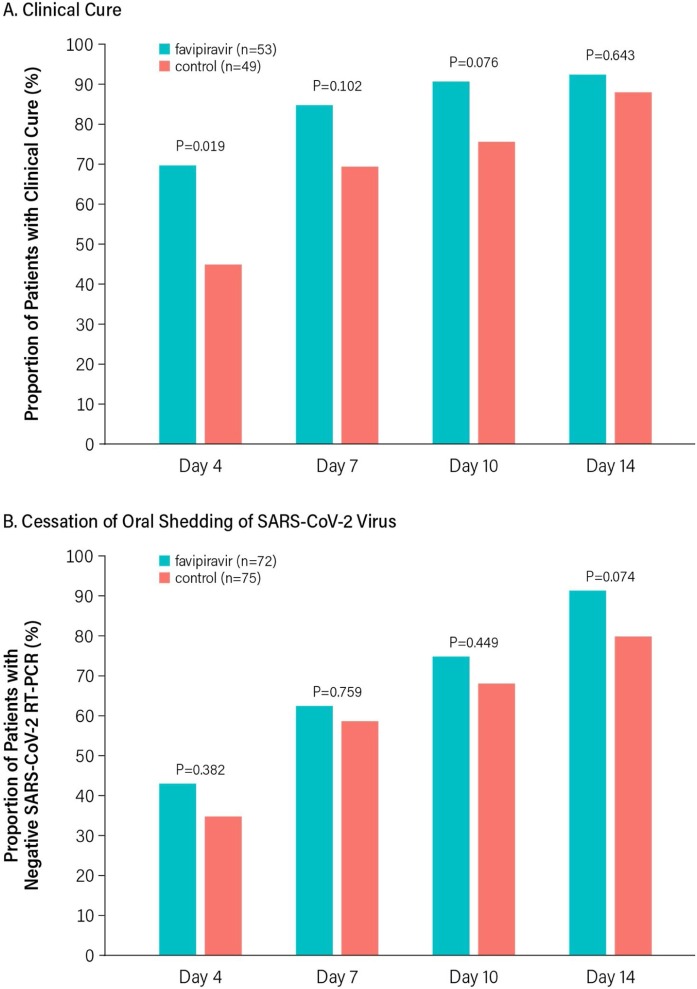
Among those in the ITT population with COVID-19 clinical symptoms at baseline, the proportion of patients in the favipiravir treatment group who achieved clinical cure at days 4, 7, 10, and 14 was numerically higher at all timepoints when compared with the control group, but the difference was statistically significant only at day 4 (Figure 3 A). Similarly, the proportion of patients in the favipiravir treatment group who achieved the cessation of oral shedding of the SARS-CoV-2 virus as measured by negative RT-PCR in both oropharyngeal and nasopharyngeal swab at days 4, 7, 10, and 14 was numerically higher at all timepoints when compared with the control group but did not reach statistical significance at any timepoint (Figure 3B).
Figure 3.
Rate of Events at Days 4, 7, 10, and 14.
Results of exploratory subgroup analyses by disease severity are shown in Table 3 . Briefly, analyses of time to the cessation of viral shedding, time to clinical cure, and time to hospital discharge numerically favored favipiravir when compared with control in all cases, regardless of baseline disease severity; however, only the analysis of time to clinical cure in patients with moderate disease at baseline achieved statistical significance (Table 3). Median time to clinical cure among moderate patients was 3.5 days (95% CI: 3 days, 4 days) for favipiravir compared with 6 days (95% CI: 4 days, 12 days) for control (P = 0.030). Median time to hospital discharge was 4 days earlier in the moderate subgroup treated with favipiravir (median: 9 days, 95% CI: 6 days, 11 days) when compared with control (median: 13 days, 95% CI: 8 days, 17 days, P = 0.067) (Table 3).
Table 3.
Analysis of Time to Event Endpoints by COVID-19 Severity, ITT Population.
| Mild COVID-19 |
Moderate COVID-19 |
|||
|---|---|---|---|---|
| Favipiravir | Control | Favipiravir | Control | |
| Time to Cessation of SARS-CoV-2 Oral Shedding | ||||
| No. of patients | N = 44 | N = 45 | N = 28 | N = 30 |
| No. of events (%) | 43 (97.7) | 44 (97.8) | 27 (96.4) | 24 (80.0) |
| Time to event, median days (95% CI) | 6.0 (4.0, 8.0) | 7.0 (5.0, 7.0) | 4.5 (3.0, 7.0) | 6.5 (3.0, 14.0) |
| Log-rank P value | 0.8529 | 0.0672 | ||
| Hazard Ratio (95% CI) | 1.145 (0.716, 1.833) | 1.855 (0.991, 3.471) | ||
| Time to Clinical Cure | ||||
| No. of patientsa | N = 29 | N = 25 | N = 24 | N = 24 |
| No. of events (%) | 28 (96.6) | 25 (100) | 23 (95.8) | 21 (87.5) |
| Time to event, median days (95% CI) | 3.0 (2.0, 4.0) | 4.0 (3.0, 5.0) | 3.5 (3.0, 4.0) | 6.0 (4.0, 12.0) |
| Log-rank P value | 0.5087 | 0.0296 | ||
| Hazard Ratio (95% CI) | 1.466 (0.766, 2.807) | 2.094 (1.057, 4.147) | ||
| Time to Hospital Discharge | ||||
| No. of patients | N = 44 | N = 45 | N = 28 | N = 30 |
| No. of events (%) | 43 (97.7) | 44 (97.8) | 27 (96.4) | 24 (80.0) |
| Time to event, median days (95% CI) | 8.5 (6.0, 11.0) | 9.0 (7.0, 11.0) | 9.0 (6.0, 11.0) | 13.0 (8.0, 17.0) |
| Log-rank P value | 0.7455 | 0.0668 | ||
| Hazard Ratio (95% CI) | 1.181 (0.735, 1.897) | 1.776 (0.973, 3.241) | ||
ITT, intent-to-treat.
The ITT population excluded 2 subjects with no drug intake (favipiravir arm) and 1 subject with no post baseline efficacy assessment (favipiravir arm).
The favipiravir arm received treatment with favipiravir + standard supportive care; the control arm received standard supportive care alone.
Kaplan–Meier was used to estimate the median duration of time-to-event and 95% confidence intervals. The two treatment groups were compared using a log-rank test to estimate the P value. The hazard ratio of favipiravir/control was computed based on the Cox regression model with covariates of age, treatment, and baseline comorbidity. Subjects who terminated the study without documented event were censored at day 28. Subjects who died without documented event were censored at day 28 or the date of death, whichever was later.
Only patients with clinical signs and symptoms at baseline were included in this analysis.
TEAEs were experienced by 32/148 (21.6%) patients—26/73 (36%) favipiravir and 6/75 (8%) control (Table 4). The most common TEAEs that occurred in a higher proportion of patients treated with favipiravir when compared with control were increased blood uric acid in 12/73 (16.4%), abnormal liver function tests in 5/73 (6.8%), and viral pneumonia in 2/73 (2.7%); all were considered mild (20/26) or moderate (6/26) in severity. Most (21/26) were considered related to treatment, while viral pneumonia (2/26) was related to the underlying infection. The most commonly reported TEAEs in the control group were abnormal liver function tests and gastrointestinal disorders, which occurred in 2 patients each (2.7%) and were considered mild in severity. One patient in the control group experienced a serious adverse event, acute respiratory distress syndrome, which led to death and was not considered related to treatment but rather to worsening of COVID-19.
Table 4.
Treatment Emergent Adverse Events, Safety Population.
| Favipiravir (N = 73) n (%) | Control (N = 75) n (%) | |
|---|---|---|
| TEAE Incidence | ||
| Overall TEAEs | 26 (35.6) | 6 (8.0) |
| Common TEAEs (>2% in either group) | ||
| Blood uric acid increased | 12 (16.4) | 0 |
| Liver function test abnormal | 5 (6.8) | 2 (2.7) |
| Viral pneumonia | 2 (2.7) | 0 |
| Gastrointestinal disorder | 1 (1.4) | 2 (2.7) |
| Summary of Safety | ||
| AE leading to death | 0 | 1 (1.3)a |
| AE leading to treatment discontinuation | 0 | 0 |
| AE leading to early termination | 0 | 0 |
| AE by relationship to treatment | ||
| Yes | 21 (28.8) | 0 |
| No | 5 (6.8) | 6 (8.0) |
| AE by severity | ||
| Mild | 20 (27.4) | 5 (6.7) |
| Moderate | 6 (8.2) | 0 |
| Severe | 0 | 0 |
| Life threatening | 0 | 0 |
| Death | 0 | 1 (1.3)a |
| SAE | 0 | 1 (1.3)a |
| SAE leading to death | 0 | 1 (1.3)a |
| SAE leading to early termination | 0 | 0 |
| SAE by relationship to treatment | ||
| Yes | 0 | 0 |
| No | 0 | 1 (1.3) |
AE, adverse event; SAE, serious adverse event; and TEAE, treatment emergent adverse event.
The safety population excluded 2 subjects with no drug intake (favipiravir arm).
The favipiravir arm received treatment with favipiravir + standard supportive care; the control arm received standard supportive care alone.
An SAE (acute respiratory distress syndrome) was reported in 1 subject in the control group, and death due to SAE was reported in this same subject, which was considered to be not related to treatment.
Discussion
The present study evaluted the potential clinical benefit of favipiravir in patients with mild-to-moderate COVID-19 based on its oral administration, established use for novel influenza viruses, well-characterized safety profile, activity against the RdRp of SARS-CoV-2, and promising efficacy in treating COVID-19 as reported from other countries, including China, Russia, and Japan (Cai et al., 2020, Chen et al., 2020, Doi et al., 2020, Ivashchenko et al., 2020, Sanders et al., 2020, Anon, 2020a). In this study, the primary endpoint of median time to RT-PCR negativity was 28.7% earlier for favipiravir plus standard supportive care when compared with supportive care alone (5 days vs 7 days), consistent with median time to viral clearance of 4 days reported in an earlier favipiravir study in moderate COVID-19 patients also receiving interferon-α (Cai et al., 2020), and with findings from a recent study in patients with mild COVID-19 suggesting that early treatment with favipiravir may be associated with more rapid viral clearance (Doi et al., 2020). However, the median time to viral clearance in the control group of the present study was shorter (7 days) than in the study by Cai et al., (11 days) (Cai et al., 2020) and also shorter than that reported more widely for COVID-19 patients (from 12 to 20 days) (Widders et al., 2020).
While a statistically significant difference in the primary endpoint was not achieved, statistically significant results were observed on the clinically meaningful secondary endpoint of time to clinical cure. Moreover, there was a statistically significant difference in time to the first use of oxygen, although this observation must be interpreted with utmost caution because of the small number of patients (7 in each treatment group) who required supplemental oxygen suppport in this population. In addition, when evaluated by COVID-19 severity, patients with moderate COVID-19 showed a statistically significant benefit with respect to time to clinical cure.
Faster viral clearance would be expected to reduce the duration of infectivity during the early course of disease, which would yield critical reductions in the secondary attack rate, duration of quarantine, and burden on healthcare providers and facilities (Schiffer et al., 2020). For the individual, faster viral clearance would be expected to translate into earlier clinical cure (Schiffer et al., 2020, Goyal et al., 2020); however, the relationship between time to the cessation of viral shedding and time to clinical cure in COVID-19 has yet to be fully characterized. A recent study by Fu et al. (2020) showed that RT-PCR negativity lags a clinical cure in COVID-19 and the relationship between RT-PCR and clinical cure may be affected by external or intrinsic patient factors (Fu et al., 2020, Xu et al., 2020, Qi et al., 2020). The possibility of the detection of replication incompetent virus confounds the interpretation of qualitative RT-PCR positivity as does the lack of quantitative viral load data, limiting the ability to establish the true cessation of viral activity (Carmo et al., 2020, Anon, 2020b).
The median time to clinical cure of 3 days in the favipiravir group was 40% faster than in the control arm (5 days) and within the range considered clinically relevant for antiviral therapy. Put in perspective, a Cochrane review of 20 trials of oseltamivir in influenza showed this widely used drug reduced the time to the clinical alleviation of symptoms by 16.8 h (Heneghan et al., 2016). Direct comparisons to other trials in COVID-19 patients are limited by different study designs, patient severity, inclusion criteria, and endpoints assessed. A previously reported study in hospitalized patients with moderate COVID-19 treated with a 5-day course of remdesivir showed a favorable significant difference in clinical status as compared to the standard of care, while those treated with a 10-day course of remdesivir did not show a significant difference (Spinner et al., 2020). Remdesivir is intravenously administered and at this time reserved for more severely ill, hospitalized patients. Patients enrolled in the current study were hospitalized both per prevailing treatment guidelines and to enable daily RT-PCR testing to evaluate time to cessation of oral shedding of the SARS-CoV-2 virus. However, the relatively rapid time to RT-PCR negativity and clinical cure observed in both the favipiravir and control groups suggest that the population we studied–those who are mildly to moderately ill–could well be treated as outpatients. The availability of an effective oral antiviral like favipiravir would allow for earlier administration in patients who are mildly to moderately symptomatic but not severely ill requiring hospitalization. The availability of an oral medication is also expected to improve patient compliance and reduce burden on already stressed healthcare systems.
Favipiravir was found to be safe and well tolerated in this study, despite the potential bias for overreporting of TEAEs in open-label trials. There were no new safety signals, and no adverse events that led to drug discontinuation or change in the dosing regimen; a single patient in the control group experienced a serious adverse event, acute respiratory distress syndrome, which led to death. The majority of adverse events were mild to moderate, and the most commonly observed events were asymptomatic transient increases in uric acid and liver enzymes. Gastrointestinal disturbance was minimal and seen across both the groups. The safety results are in line with what is known of the safety profile of favipiravir from studies in influenza (Pilkington et al., 2020). However, its use in women of childbearing potential is contraindicated unless adequate contraceptive methods are employed. Other contraindications to favipiravir use are breastfeeding, severe hepatic impairment, and severe renal impairment.
There are limitations to this study. As previously discussed, the primary endpoint was confounded by interpretation issues with RT-PCR positivity and its lack of correlation with clinical cure (Carmo et al., 2020, Anon, 2020b). Furthermore, the impact of RT-PCR assay variables such as cycle time was not evaluated in the current study, and it is not known how such factors may have influenced the results. Additionally, the hazard ratios observed in this trial were much smaller than reported by Cai et al. (2020) which had been used for sample size estimation. Thus, a larger sample size may have been required to have sufficient power to detect statistically significant differences between treatment groups. The open-label design of the current study is another limitation, particularly as some of the secondary endpoints rely on clinician assessment and are therefore subject to potential bias. Although the trial was conducted open-label, rather than double-blind and placebo-controlled, precautions were taken to reduce bias associated with the design. The primary endpoint, for example, was an objective measure (RT-PCR negativity), and allocation to treatment groups was randomized and conducted centrally by an independent third party to avoid selection bias.
In conclusion, the results of this study suggest that despite failure to achieve statistical significance on the primary endpoint of time to RT-PCR negativity, early administration of oral favipiravir may reduce the duration of clinical signs and symptoms in patients with mild-to-moderate COVID-19, as demonstrated by the significantly decreased time to clinical cure. The adverse events reported for favipiravir were mild to moderate in severity and transient in nature, consistent with previous experience with the drug. There were no new safety signals. In view of the urgent clinical need for safe and effective treatments for mild-to-moderate COVID-19, orally administered favipiravir appears to be a promising drug candidate. Ongoing studies, including randomized, double-blind, placebo-controlled trials and those in combination with other antiviral therapies, will further clarify the role of favipiravir in COVID-19 patient management.
Conflict of interest
Monika Tandon, Pawan Singh, Hanmant Barkate, Saiprasad Patil, Shabbir Rangwala, Amol Pendse, and Jatin Kadam are full-time employees of Glenmark Pharmaceuticals Limited, India. Wen Wu is a full-time employee of Glenmark Pharmaceuticals Ltd, United Kingdom. Cynthia Caracta is a full-time employee of Glenmark Pharmaceuticals Inc., USA. Zarir F Udwadia received an investigator grant from Glenmark Pharmaceuticals Limited, India, as a site principal investigator for this study.
Funding source
This study was sponsored and funded by Glenmark Pharmaceuticals Limited, India.
Ethical approval
The protocol and informed consent form were approved by institutional/independent ethics committees and the Drugs Controller General of India (April 26, 2020). The trial was undertaken in accordance with current guidelines of the Central Drugs Standard Control Organization, which is the National Regulatory Authority in India, the International Conference on Harmonization Good Clinical Practice, and the Declaration of Helsinki. All patients provided written informed consent.
Contributions
Author contributions: All authors contributed equally to this report. Zarir F Udwadia, Monika Tandon, Pawan Singh, Hanmant Barkate, Shabbir Rangwala, Amol Pendse, and Wen Wu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Monika Tandon, Pawan Singh, Hanmant Barkate, Saiprasad Patil, and Wen Wu.
Acquisition, analysis, or interpretation of data: Jatin Kadam, Amol Pendse, Wen Wu, Shabbir Rangwala, Monika Tandon, Cynthia F Caracta, Pawan Singh, Hanmant Barkate, and Zarir F Udwadia.
Critical revision of the manuscript and important intellectual content: Zarir F Udwadia, Monika Tandon, Cynthia F Caracta, Pawan Singh, Hanmant Barkate, Saiprasad Patil, Shabbir Rangwala, Amol Pendse, and Wen Wu.
Statistical analysis: Wen Wu.
Study supervision: Shabbir Rangwala, Amol Pendse, Jatin Kadam, and Pawan Singh.
Role of thefunder/sponsor: The role of the sponsor’s employees in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication is disclosed in the Acknowledgments.
Datasharing: The study protocol is available in the appendix. Individual participant data that underlie the reported results are not available.
Additionalcontributions: Dr Rajesh Gosavi, Dr Meenakshi Bhattacharya, Dr Atul Jindal, Dr Urman Dhruv, Dr Chirag Rathod, Dr Chandrakant Pawar, Dr Joanne Mascarenhas, and Dr Aruna Pujari were study investigators. Dr. Hrishikesh Kulkarni and Dr. Vinayak Sapakal provided study documentation. Dr. Rahul Bhagat oversaw the safety review. Utkarsh Gandhi provided data analytics. Prafulla Shetty and Reshma More were responsible for data management. Yogesh Gadage and Krishna Gupta provided the statistical programming. Dr. Sharon Lobo, Dr. Urmila Gupta, and Sagar Borle wrote the protocol and clinical study report. Yashodhan Warke was responsible for the management of the investigational product. Umendra Harode, Sunil Jagdale, and Mahendra Patwal served as clinical research associates. Dr. Rahul Kodgule provided critical review of the manuscript. Medical writing support for the manuscript was provided by Mary Susan Prescott, MS, Jennifer Hepker, Ph.D., and Merrilee Johnstone, Ph.D., of Prescott Medical Communications Group (Chicago, IL), with financial support from Glenmark Pharmaceuticals Limited, India.
Declaration of Competing Interest
The authors report no declarations of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.11.142.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020 doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anti-influenza drug Avigan® Tablet Meets Primary Endpoint in Phase III Clinical Trial in Japan for COVID-19 patients. Press Release. September 23, 2020. https://www.fujifilm.com/jp/en/news/hq/5451.
- Duration of Isolation and Precautions for Adults with COVID-19. July 22, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html [Accessed 30 July 2020].
- Buonaguro L., Tagliamonte M., Tornesello M.L., Buonaguro F.M. SARS-CoV-2 RNA polymerase as target for antiviral therapy. J Transl Med. 2020;18(1):185. doi: 10.1186/s12967-020-02355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020;10:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo A., Pereira-Vaz J., Mota V., et al. Clearance and persistence of SARS-CoV-2 RNA in patients with COVID-19. J Med Virol. 2020;10:2227–2231. doi: 10.1002/jmv.26103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Huang J., Cheng Z., et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020;(March) doi: 10.1101/2020.03.17.20037432. Preprint posted. [DOI] [Google Scholar]
- Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Doi Y., Hibino M., Hase R., et al. A prospective, randomized, open-label trial of early versus late favipiravir in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64(12):e01897–20. doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Fu Y., Han P., Zhu R., et al. Risk factors for viral RNA shedding in COVID-19 patients. Eur Respir J. 2020;56(1):2001190. doi: 10.1183/13993003.01190-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N.J., Ravi R., Thatte U.M. Regulatory requirements for clinical trials in India: what academicians need to know. Indian J Anaesth. 2017;61(3):192–199. doi: 10.4103/ija.IJA_143_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A., Cardozo-Ojeda Fabian E., Schiffer J.T. Potency and timing of antiviral therapy as determinants of duration of SARS CoV-2 shedding and intensity of inflammatory response. Sci Adv. 2020;6(47):eabc7112. doi: 10.1126/sciadv.abc7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneghan C.J., Onakpoya I., Jones M.A., et al. Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Health Technol Assess. 2016;20(42):1–242. doi: 10.3310/hta20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., et al. AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6(2):45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L., Yang Y., Jiang D., et al. Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020;96:531–537. doi: 10.1016/j.ijid.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Schiffer J.T., Johnston C., Wald A., Corey L. An early test-and-treat strategy for severe acute respiratory syndrome coronavirus 2. Open Forum Infect Dis. 2020;7(7) doi: 10.1093/ofid/ofaa232. ofaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhang M., Yin L., et al. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus. Int J Antimicrob Agents. 2020;56(2):106080. doi: 10.1016/j.ijantimicag.2020.106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Center for Systems Science and Engineering at Johns Hopkins . 2020. COVID-19 Global Case Dashboard.https://coronavirus.jhu.edu/map.html [Google Scholar]
- Widders A., Broom A., Broom J. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect Dis Health. 2020;25(3):210–215. doi: 10.1016/j.idh.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Timeline of WHO’s Response to COVID-19.https://www.who.int/news-room/detail/29-06-2020-covidtimeline [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xu K., Chen Y., Yuan J., et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.