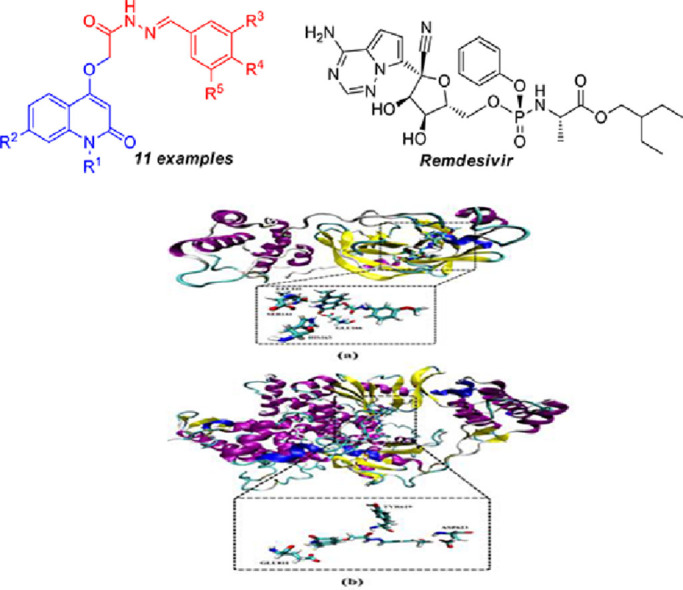
Abstract
We report herein a new series of synthesized N-substituted-2-quinolonylacetohydrazides aiming to evaluate their activity towards SARS-CoV-2. The structures of the obtained products were fully confirmed by NMR, mass, IR spectra and elemental analysis as well. Molecular docking calculations showed that most of the tested compounds possessed good binding affinity to the SARS-CoV-2 main protease (Mpro) comparable toRemdesivir.
Keywords: Quinolone, N-substituted-2-quinolonylacetohydrazides, Nmr, Remdesivir, Molecular docking
Graphical abstract
1. Introduction
Recently, human coronaviruses have attracted much interest. A new strain for Corona viruses (CoVs) identified in late December 2019 named SARS-CoV-2 resulted in a massive outbreak initially in Wuhan, China and propagated to different nations around the globe. The World Health Organization (WHO) declared the resulting disease named COVID-19 as a pandemic [1,2]. It is safe to say that a sufficient understanding of SARS-CoV-2, and the full clinical picture of the resulting COVID-19 disease will take some time [3], [4], [5], [6], [7], [8].
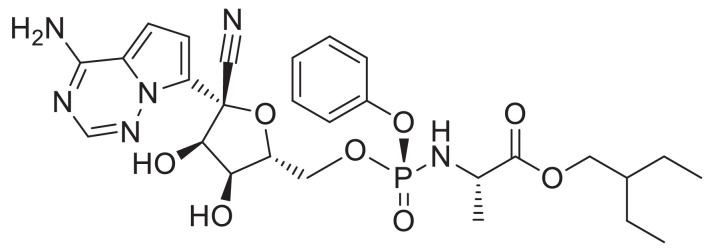
Remdesivir (Fig. 1 ) an adenosine analogue, has been recently recognized as a promising antiviral drug against a wide array of RNA viruses (including SARS/MERS-CoV) [9,10] infection in cultured cells, mice and nonhuman primate (NHP) models. It is currently under clinical development for the treatment of the Ebola virus infection [11]. Remdesivir binds to ribonucleic acid (RNA)-dependent RNA polymerase and acts as an RNA-chain terminator. It displays potent in vitro activity against SARS-CoV-2 with an EC50 at 48 h of 0.77 µM in Vero E6 cells. Remdesivir is highly selective for viral polymerases and is therefore expected to have a low propensity to cause human toxicity [12].
Fig. 1.
Structure of Remdesivir.
During last few decades, 4‑hydroxy-2-quinolones as privileged structures in drug discovery are beyond doubt, one of the major areas in medicinal chemistry [13], [14], [15]. The 4‑hydroxy-2-quinolinones scaffold is widely found in alkaloids [16] and they are important as a characteristic building block for a series of significant biologically active compounds. Many efforts have been done on antiviral properties of quinolines and quinolones and their structural analogues against the human immunodeficiency virus (HIV), but their antiviral activity was also demonstrated against the human cytomegalovirus (HCMV), SARS corona virus, Zika virus, Chikungunya virus, hepatitis C virus (HCV), and Ebola virus [17], [18], [19], [20], [21], [22]. The mechanism of action of antiviral quinolone remains unclear. Specific studies aimed at understanding the nature of drug's targets at the molecular level indicated that quinolones inhibit viral transcription [23].
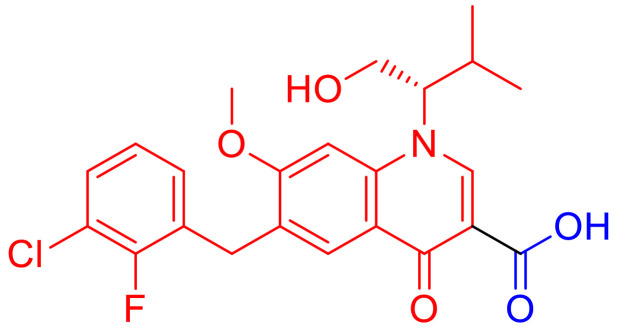
Elvitegravir, (Fig. 2) is the first quinolone-based anti-HIV drug, exhibiting potent inhibitory activity against integrase-catalyzed DNA strand transfer [24,25]. Another series of quinolone-3-carboxylic acids have been synthesized by introducing different hydrophobic groups at the N(1), C(2), C(7), and C(8) positions [26]. Most of the compounds of this group showed anti-HIV activity without cytotoxicity at a concentration of 100 μM.
Fig. 2.
Chemical structure of Elvitegravir.
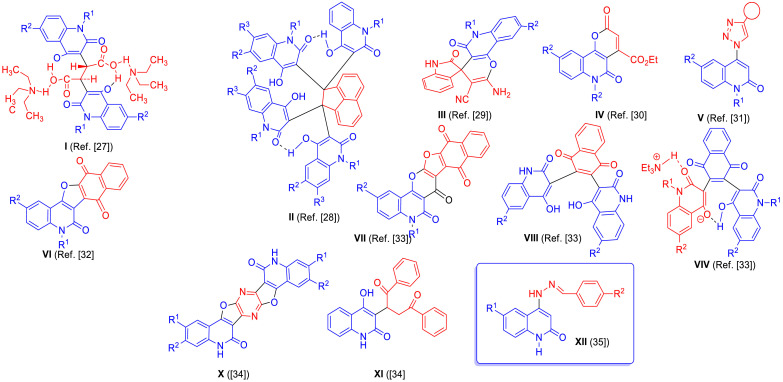
Fig. 3 summarizes the work of Aly et al. in the synthesis of 4‑hydroxy-2-quinolones of potential biologically active compounds. As an example, 2,3-bis-(4‑hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinic acid derivative I, was obtained by a one-pot reaction of one equivalent of aromatic amines with two equivalents of diethyl malonate in diphenyl ether and catalyzed with triethylamine [27]. A reaction of four equivalents of 4-hydroxyquinolin-2(1H)-one with one equivalent of acenaphthoquinone gave acenaphthylene-1,1,2,2-tetrayl-tetrakis(4-hydroxyquinolin-2(1H)-one) (II, [28]. We also reported that quinoline-2,4‑dione reacted with 2-(2-oxo-1,2-dihydroindol-3-ylidene)malononitrile in pyridine to give spiro(indoline-3,4′-pyrano[3,2-c]quinoline)−3′-carbonitrile (III, [29], whereas 2-quinolone reacted with diethyl acetylenedicarboxylate to give pyrano[3,2-c]quinoline-4-carboxylate (IV, [30]. Also a class of 1,2,3-triazoles derived by 2-quinolone (V, [31]) was synthesized, via Cu-catalyzed [3 + 2]cycloadditions (Meldal-Sharpless ‘click’-reactions) [31]. We also synthesized naphthofuro[3,2-c]quinoline-6,7,12-trione VI, and pyrano[3,2-c]quinoline-6,7,8,13-tetraone, VII that have shown potential as ERK inhibitors [32], whereas synthesis of bis(6-substituted-4‑hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)naphtha-ene-1,4‑dione VIII and (substituted N-(alkyl)bis-quinolinone)-triethylammonium salt VIV [33], were explored as candidates for extracellular signal-regulated kinases 1/2 (ERK1/2) having antineoplastic activity [33]. Recently, we have reported the synthesis of 5,12-dihydro-pyrazino[2,3-c:5,6-c']difuro[2,3-c:4,5-c']-diquinoline-6,14(5H,12H)‑dione X [34] and 2-(4‑hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)−1,4-diphenyl-butane-1,4‑dione XI [34]. Most indicative is our recent synthesis of 6-substiuted-4-(2-(4-substituted-benzylidene)hydrazinyl)quinolin-2(1H)-one derivative XII [35] which was evaluated for their in vitro cytotoxic activity against 60 cancer cell lines according to NCI protocol [35].
Fig. 3.
Structures of compounds that previously reported 2-quinolones I-XII.
On the other hand, Schiff bases have large importance in medicinal and pharmaceutical fields due to a broad spectrum of biological activities like analgesic [36], [37], [38], [39], anti-inflammatory [36,38,40], anti-tubercular [41], anti-cancer [42,43], and so forth. So, from the highly biological and pharmaceutical activities of 4‑hydroxy-2-quinolinones and Schiff bases, we focused in our paper to merging the activity of these compounds and compare them with Remdesivir (as one of the prospective drugs) against COVID-19.
2. Results and discussion
2.1. Chemistry section
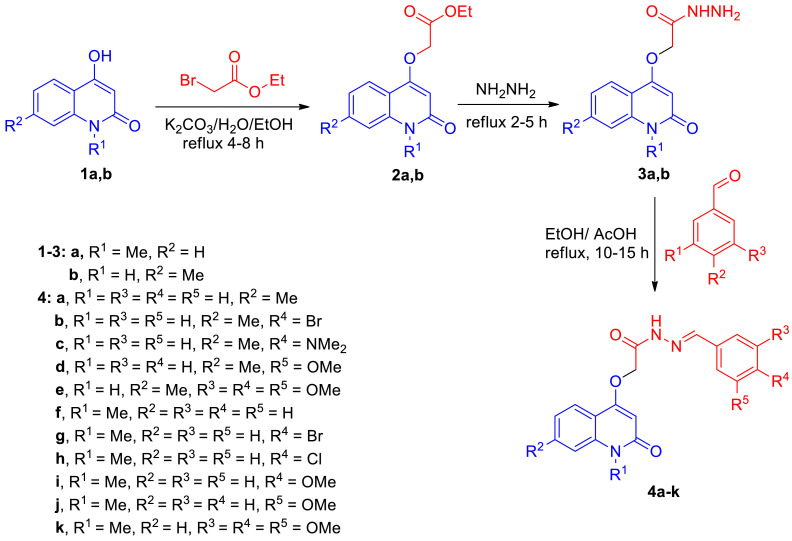
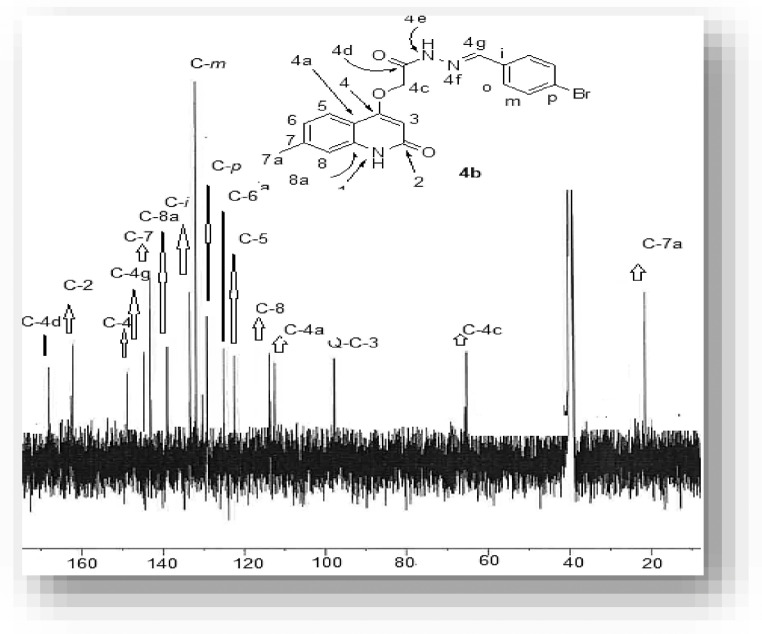
Our research plane started by preparation of compounds 2a,b during reaction between quinolones 1a,b and ethyl bromoacetate. Compounds 2a,b reacted with hydrazine in EtOH and gave the corresponding 2-((2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide 3a,b in good yields (Scheme 1 ) [44,45]. By refluxing equimolar amounts of compounds 3a,b with an aldehydes in absolute ethanol with few drops of acetic acid gave our target new Schiff bases 4a-k in 75–90% yields (Scheme 1). The structure assignments of compounds 4a-k were established using different spectroscopic tools like IR, NMR (1H, 13C, 15N, 2D), elemental analyses and mass spectrometry. The elemental analysis showed that the corresponding molecular formula for all new compounds 4a-k are formed form one molecule of compound 3a,b and one molecule of the entered aldehyde with elimination for a H2O molecule. To illustrate the structure for the obtained compounds 4a-k, we choose compound 4b as an example which was assigned as N'-(4-bromobenzylidene)−2-((7-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide (4b) with a molecular formula C19H16BrN3O3 (m/z = 414). Its IR spectrum did not show any absorption band corresponding to hydrazine-NH2, thus indicated that condensation has occurred (Fig. 4 ).
Scheme 1.
Preparation of new Schiff bases 4a-k.
Fig. 4.
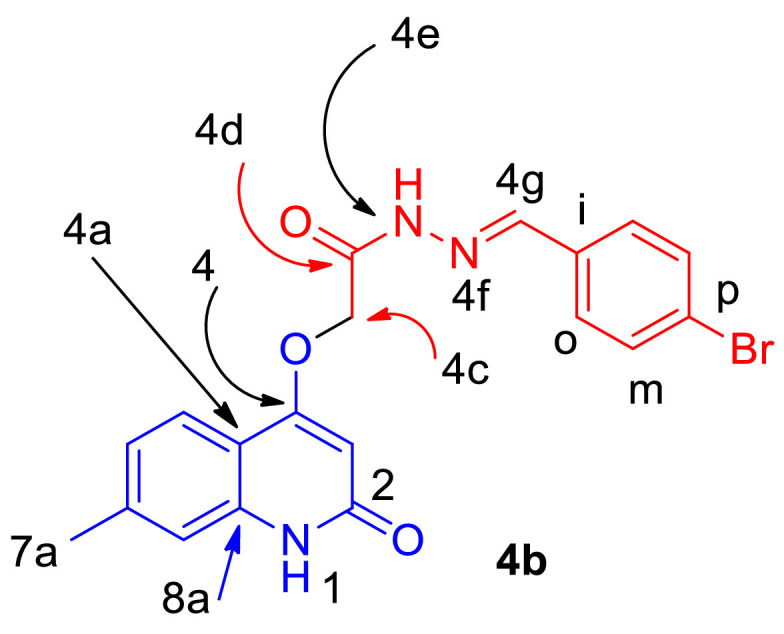
(E)-N'-(4-Bromobenzylidene)−2-((7-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)-acetohydrazide (4b).
This was fully supported by its 1H NMR spectrum, which displayed a characteristic singlet at δH = 11.80, 11.34 ppm, integrating for two D2O exchangeable protons which were assigned as NH-4e and NH-1, respectively. Also, a singlet at δH = 5.33 ppm (2H) corresponding to –OCH2- (H-4c) was further confirmed from 13C NMR (Fig. 5 ) with a characteristic singlet at δC = 65.15 ppm (Table 1 ).
Fig. 5.
13C NMR spectrum of4b.
Table 1.
1H NMR for compound4b.
| 1H NMR | 1H–1H COSY | Assignment: |
|---|---|---|
| 11.80 (s, 1H) | NH-4e | |
| 11.34 (s, 1H) | 5.73 | NH-1 |
| 8.01 (s, 1H) | H-4 g | |
| 7.76 (d, J = 8.2 Hz, 1H) | 7.03 | H-5 |
| 7.72 (d, J = 8.5 Hz, 2H) | 7.65 | H-o |
| 7.65 (d, J = 8.5 Hz, 2H) | 7.72 | H-m |
| 7.09 (s; 1H) | 2.38 | H-8 |
| 7.03 (d, J = 8.4 Hz; 1H) | 7.81, 7.76 | H-6 |
| 5.74 (s, 1H) | 11.34 | H-3 |
| 5.33 (s, 2H) | H-4c | |
| 2.38 (s; 3H) | 7.09 | H-7a |
The protons of the phenyl group exhibit a 1,4-disubstituted system and was observed as a double-doublet at δH = 7.72 (d, J = 8.5 Hz; 2H, H-o) and δH = 7.65 (d, J = 8.5 Hz; 2H, H-m) and both of them give a 1H–1H-COXY with each other (Table 1). The 13C NMR spectrum for compound 4b showed characteristic singlets at δC = 167.78, 163.25, 162.05, 147 and 21.28 which were assigned as C-4d, quinolone-C-2, C-4, C = N (C-4 g), and methyl group (C-7a), respectively (Table 2 ). In 15N-NMR, the signal at δN = 317.2 ppm, indicated as N-4f gave HMBC correlation with proton at δH = 8.01 ppm which was assigned as H-4 g and didn't have any HSQC correlation, and this proton give HSQC correlation with carbon at δC = 142.93 ppm, which was assigned as C-4 g that indicates the absence of hydrazine-NH2 and condensation takes place on it, with other signals at δN = 177.9 and 144.4 ppm, which were assigned as N-4e and N-1, respectively, and these nitrogen gave an HSQC correlation which indicates that these nitrogen atoms are carrying protons (Table 2). The former correlation indicates the E-form of the azomethine structure.
Table 2.
13C and 15N NMR spectral data for compound 4b.
| 13C NMR | HSQC | HMBC | Assignment: |
|---|---|---|---|
| 167.78 | 11.80, 11.79, 5.33, 5.30 | C-4d | |
| 163.25 | 11.78, 5.33, 4.81, 2.89, 2.735 | C-2 | |
| 162.05 | 11.78, 7.76, 5.74, 5.33, 4.85, 2.89, 2.735 | C-4 | |
| 142.93 | 8.02, 8.01, 7.96 | 11.80, 11.79, 7.72 | C-4 g |
| 141.07 | 7.81, 7.76, 7.72 | C-7 | |
| 140.15 | 7.81, 7.76, 7.36 | C-8a | |
| 133.20 | 8.29, 8.25, 8.02, 8.01, 7.65 | C-i | |
| 131.74 | 7.67 | 7.67 | C-m |
| 129.02 | 7.72 | 7.65 | C-o |
| 123.20 | 7.03 | 7.72, 7.65, 7.09 | C-p |
| 122.76 | 7.81 | 7.09, 2.38 | C-6 |
| 122.35 | 7.76 | 7.09, 2.38 | C-5 |
| 114.88 | 7.09 | 11.34, 7.04, 6.95, 2.38 | C-8 |
| 112.38 | 7.09, 5.74 | C-4a | |
| 96.68 | 5.77, 5.74 | 11.34 | C-3 |
| 65.15 | 5.33, 5.30, 4.85, 4.81 | 11.79 | C-4c |
| 21.28 | 2.38 | 7.09, 7.03 | C-7a |
| 15N NMR | HSQC | HMBC | Assignment: |
| 317.2 | 8.02, 8.01 | N-4f | |
| 177.9 | 11.80, 11.79 | 8.02, 8.01 | N-4e |
| 144.4 | 11.39, 11.34 | N-1 | |
2.2. Molecular docking calculations
To reveal the binding modes and affinities of the synthesized compounds 4a-k with SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp), molecular docking calculations were performed using Autodock4.2.6 software. The predicted docking scores and binding features of compounds 4a-k with Mpro and RdRp receptors are listed in Table 3 .
Table 3.
Calculated docking scores (in kcal/mol) and predicted binding features for compounds 4a-k with SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA Polymerase (RdRp).
| Compound | Main Protease (Mpro) |
RNA-dependent RNA Polymerase (RdRp) |
||
|---|---|---|---|---|
| Docking Score (kcal/mol) | Binding Features (Hydrogen bond length in Å) | Docking Score (kcal/mol) | Binding Features (Hydrogen bond length in Å) | |
| 4a | −9.1 | GLU166 (1.89 Å), LEU141 (1.97 Å), HIS163 (1.97), SER144 (2.31 Å) | −7.3 | TYR619 (2.15 Å), GLU811 (2.15 Å) |
| 4b | −9.0 | GLU166 (2.15 Å), SER144 (2.24 Å), LEU141 (1.99 Å) | −7.4 | GLU811 (1.92 Å), TYR619 (2.07 Å) |
| 4c | −7.7 | ARG188 (1.82 Å), GLU166 (2.13 Å), MET165 (2.52 Å) | −7.5 | TRP800 (1.91 Å), SER814 (1.92 Å) |
| 4d | −9.7 | SER144 (2.22 Å), GLU166 (2.07 Å), LEU141 (2.02 Å), HIS163 (1.83 Å) | −7.7 | ASP623 (2.12 Å), TYR619 (2.19 Å), GLU811 (2.24 Å) |
| 4e | −7.5 | GLU166 (2.13 Å) | −7.3 | TYR619 (2.08 Å), ASP623 (2.28 Å) |
| 4f | −7.9 | GLN189 (2.23 Å), GLY143 (1.75 Å) | −6.5 | TYR619 (1.96 Å), TRP800 (1.96 Å) |
| 4g | −8.2 | GLU166 (2.32 Å), GLN192 (1.92 Å) | −7.6 | ASP761 (1.69 Å), TRP800 (1.81 Å), SER814 (1.91 Å) |
| 4h | −8.5 | GLU166 (1.86 Å), GLN192 (1.99 Å) | −7.5 | SER814 (1.97 Å), ASP761 (1.62 Å), TRP800 (1.96 Å) |
| 4i | −8.6 | GLU166 (1.86 Å), GLN192 (2.24 Å), SER144 (2.21 Å), CYS145 (2.37 Å) | −7.2 | ASP761 (1.68 Å), TRP800 (1.78 Å), SER814 (1.86 Å) |
| 4j | −8.7 | GLU166 (2.21 Å), GLN192 (2.19 Å) | −7.5 | TRP800 (1.90 Å), ASP760 (2.06 Å), LYS621(1.94 Å) |
| 4k | −8.8 | GLU166 (2.02 Å), HIS163 (1.93 Å), GLN192 (2.22 Å) | −7.6 | TYR619 (2.78 Å), TRP800 (1.78 Å), ASP623 (2.02 Å), LYS621 (1.88 Å) |
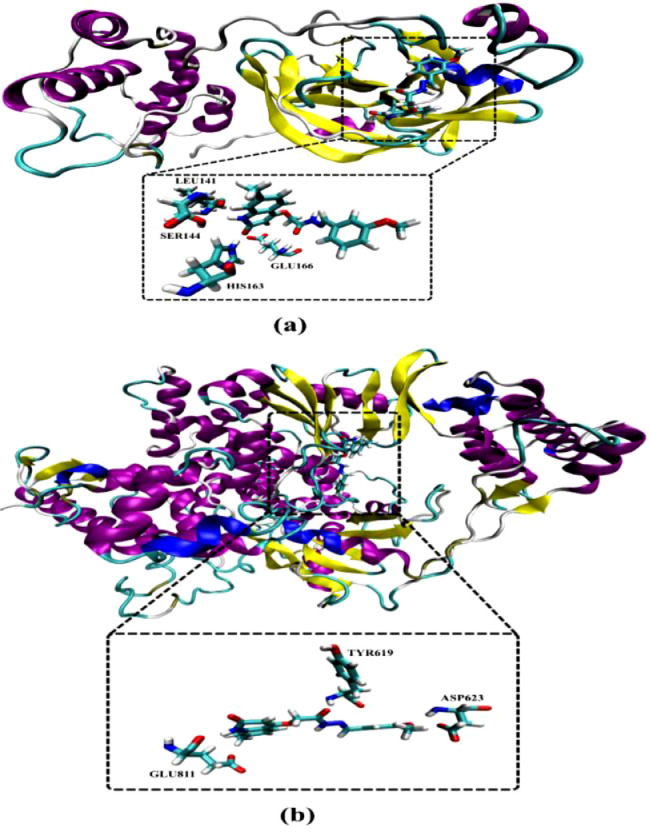
According to the calculated docking scores (Table 3), compounds 4a-k showed good binding affinities towards Mpro with values in range −7.5 to −9.7 kcal/mol. Compared to main protease (Mpro), the synthesized compounds showed lower binding affinities towards RdRP with docking scores in range −6.5 to −7.7 kcal/mol. However, molecular docking of Remdesivir gave binding affinities of −8.5 and −5.6 kcal/mol with Mpro and RdRp, respectively (Table 3). Comparison of the binding affinities revealed that compound 4d exhibited the largest binding affinities towards both of Mpro and RdRp with values of −9.7 and −7.7 kcal/mol, respectively. The high binding affinity of compound 4d towards Mpro may be attributed to its potentiality to form four essential hydrogen bonds with lengths of 2.02, 2.22, 1.83 and 2.07 Å with LEU141, SER144, HIS163 and GLU166 amino acids, respectively (Fig. 6).
Fig. 6.
Cartoon backbone representation of predicted binding modes of compound 4d with SARS-CoV-2 (a) main protease (Mpro) and (b) RNA-dependent RNA Polymerase (RdRp). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
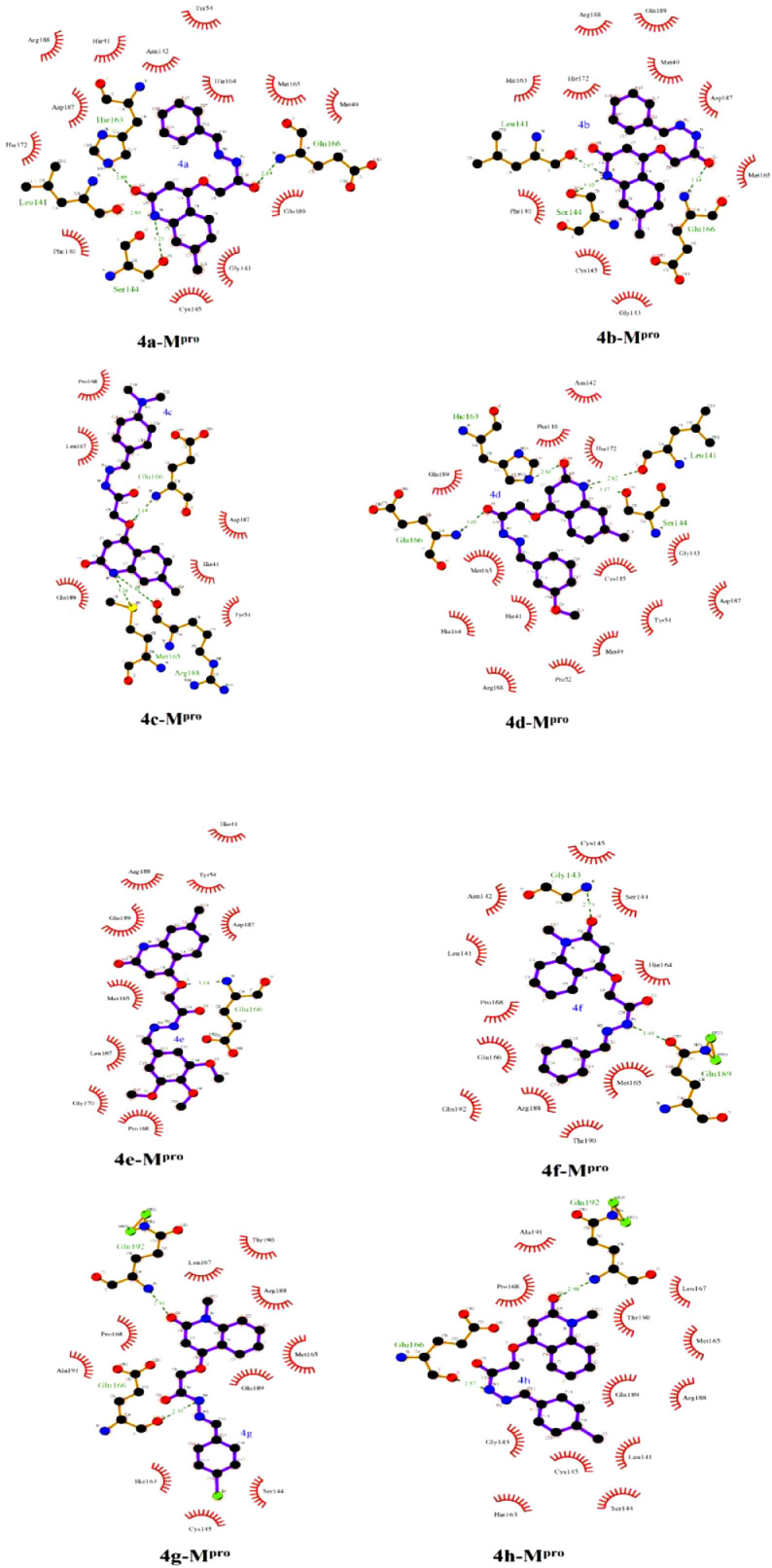
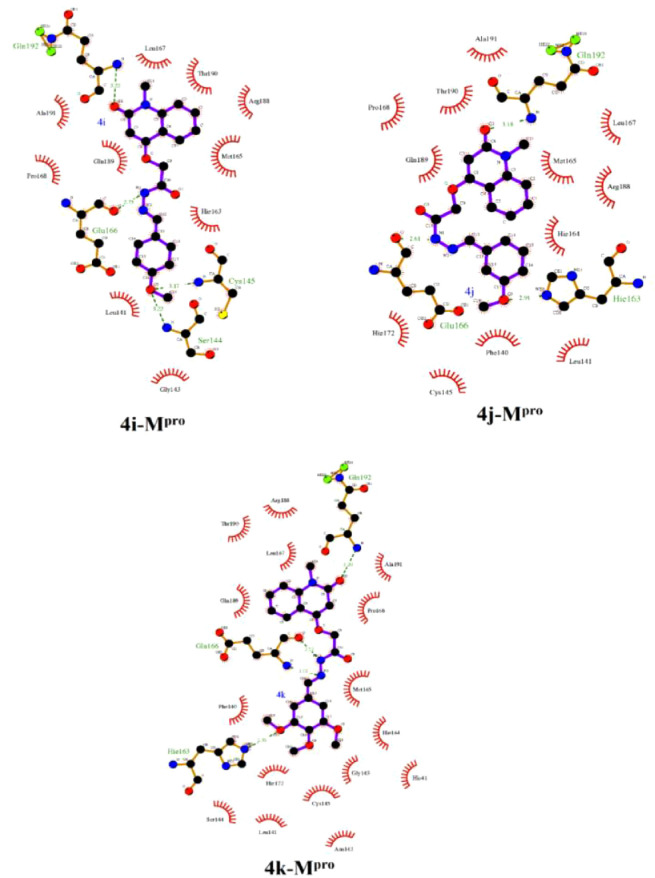
Analysis of the docked 4d-RdRp complex, compound 4d forms three essential hydrogen bonds with TYR619, ASP623 and GLU811 amino acids with average bond lengths of 2.19, 2.12 and 2.24 Å, respectively (Fig. 6 ). 2D LigPlus representations of interactions of compounds 4a-k with important amino acid residues of SARS-CoV-2 Mpro are depicted in Fig. 7 . Overall, the molecular docking results could support the postulation that the synthesized compounds may act as potent SARS-CoV-2 Mpro inhibitors.
Fig. 7.
2D LigPlus representation of interactions of compounds 4a-k with important amino acid residues of SARS-CoV-2 main protease (Mpro).
3. Conclusion
In conclusion, a new series of N-substituted-2-quinolonylacetohydrazides was here synthesized in order to evaluate their activity towards SARS-CoV-2 Mpro and RdRp. The NMR spectra (1H, 13C, 15N, 2D) were used to prove the structure of the isolated compounds. Molecular docking calculations showed that most of the tested compounds possessed good binding affinity to the main protease (Mpro) comparable to Remdesivir. Analysis of the docked (E)-N'-(3-methoxybenzylidene)−2-((7-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide-RdRp complex shows formation of three essential hydrogen bonds with TYR619, ASP623 and GLU811 amino acids with average bond lengths of 2.19, 2.12 and 2.24 Å, respectively. Much work has be done to evaluate more quinolones compounds, especially in the direction to find out how drugs capable to treat infections caused by the SARS-CoV-2 virus.
4. Experimental
Melting points were determined on Stuart electrothermal melting point apparatus and were uncorrected. TLC analysis was performed on analytical Merck 9385 silica aluminum sheets (Kiselgel 60) with PF254 indicator. Spectra were measured in DMSO‑d6 on a Bruker AV-400 spectrometer (400 MHz for 1H, 100 MHz for 13C, and 40.54 MHz for 15N), purchased with assistance from the National Science Foundation (CHE 03–42,251) at the Florida Institute of Technology, 150 W University Blvd, Melbourne, FL 32,901, USA. Chemical shifts are reported vs TMS = 0 for 1H and 13C, and vs NH3 = 0 for 15N. 15N signals were detected indirectly, via HSQC and HMBC experiments. The samples were dissolved in DMSO‑d6, s = singlet, d = doublet, dd = doublet of doublet and t = triplet. Mass spectrometry were recorded on a Varian MAT 312 instrument in EI mode (70 eV), at the Karlsruhe Institut für Technologie (KIT), Institute of Organic Chemistry, Karlsruhe, Germany.
Synthesis of substituted (E)-N'-(substituted benzylidene)−2-((7-substituted-2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide 4a-k.
A mixture of 3a,b (1 mmol), aldehydes (1 mmol) and a few drops of acetic acid in 20 mL of absolute ethanol which was stirred and refluxed for 6–8 h (the reaction was followed by TLC analysis). After the reaction's completion, the solid was filtered off and washed with a hot ethanol to give pure compounds 4a-k.
(E)-N'-Benzylidene-2-((7-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide (4a).
This compound was obtained as a colorless compound, yield 0.28 g (83%); Rf = 0.4 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.75 (s; 1H, NH, H-4e), 11.34 (s; 1H, NH-1), 8.04 (s; 1H, H-4 g), 7.76 (m; 3H, H-5,o), 7.45 (m; 3H, H-m,p), 7.09 (s; 1H, H-8), 7.04 (d; J = 8.2, 1H, H-6), 5.73 (s; 1H, H-3), 5.34 (s; 2H, H-4c), 2.38 (s; 3H, H-7a); 13C NMR (DMSO‑d6): δc = 167.78 (C-4d), 163.25 (C-2), 162.08 (C-4), 144.13 (C-4 g), 141.07 (C-7), 140.15 (C-8a), 133.89 (C-i), 129.98 (C-p), 127.75 (C-m), 126.99 (C-o), 122.76 (C-6), 122.35 (C-5), 114.87 (C-8), 112.38 (C-4a), 96.64 (C-3), 65.14 (C-4c), 21.28 (C-7a); 15N NMR (DMSO‑d6): δN = 315.1 (N-4f), 177.4 (N-4e), 144.1 (N-1a). MS (70 eV): m/z (%) = 335 (M+, 60). Anal. Calcd for C19H17N3O3 (335.36): C 68.05; H 5.11; N 12.53. Found: C 68.1;, H 4.99; N 12.66.
(E)-N'-(4-Bromobenzylidene)−2-((7-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)-acetohydrazide (4b).
This compound was obtained as a colorless compound, yield 0.300 g (72%); Rf = 0.3 (Toluene: Ethyl acetate; 10:1); NMR (DMSO‑d6): δ (See Tables 1 and 2). MS (70 eV): m/z (%) = 415 (M + 1, 36), 414 (M+, 32). Anal. Calcd for C19H16BrN3O3 (414.25): C 55.09; H 3.89; N 10.14. Found: C 55.10; H 3.77; N 10.11.
(E)-N'-(4-(Dimethylamino)benzylidene)−2-((7-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)-acetohydrazide (4c).
This compound was obtained as a colorless compound, yield 0.300 g (79%); Rf = 0.6 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.45 (s; 1H, NH, H-4e), 11.33 (s; 1H, NH-1), 7.90 (s; 1H, H-4 g), 7.76 (d, J = 8.2 Hz; 1H, H-5), 7.54 (d, J = 8.86 Hz; 2H, H-o), 7.09 (s; 1H, H-8), 7.03 (d, J = 8.3 Hz; 1H, H-6), 6.75 (d, J = 8.8 Hz; 2H, H-m), 5.68 (s; 1H, H-3), 5.28 (s; 2H, H-4c), 2.98 (s; 6H, NMe2), 2.38 (s; 3H, H-7a); 13C NMR (DMSO‑d6): δc = 167.12 (C-4d), 163.25 (C-2), 162.14 (C-4), 151.45 (C-p), 144.95 (C-4 g), 141.07 (C-7), 140.15 (C-8a), 128.51, 128.25 (C-o,i), 122.75 (C-6), 122.36 (C-5), 114.87 (C-8), 112.40 (C-4a), 111.74 (C-m), 96.56 (C-3), 65.13 (C-4c), 39.50 (NMe2), 21.28 (C-7a); 15N NMR (DMSO‑d6): δN = 303.6 (N-4f), 176.4 (N-4e), 144.0 (N-1a), 52.8 (NMe2). MS (70 eV): m/z (%) = 378 (M+, 60). Anal. Calcd for C21H22N4O3 (378.42): C 66.65; H 5.86; N 14.81. Found: C 66.77; H 5.79; N 14.66.
(E)-N'-(3-Methoxybenzylidene)−2-((7-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide (4d).
This compound was obtained as a colourless compound, yield 0.280 g (77%); Rf = 0.65 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.76 (s; 1H, NH, H-4e), 11.34 (s; 1H, NH-1), 8.00 (s; 1H, H-4 g), 7.76 (d; J = 8.2 Hz, 1H, H-5), 7.37 (m; 1H, H-5′), 7.30, (m; 2H, H-2′,6′), 7.09 (s; 1H, H-8), 7.02 (m; 2H, H-4′,6′), 5.72 (s; 1H, H-3), 5.35 (s; 2H, H-4c), 3.80 (s; 3H, H-3a), 2.38 (s; 3H, H-7a); 13C NMR (DMSO‑d6): δc = 167.83 (C-4d), 163.24 (C-2), 162.09 (C-4), 159.51 (C-3′), 143.94 (C-4 g), 141.21 (C-7), 140.15 (C-8a), 135.31 (C-1′), 129.86 (C-5′), 122.76 (C-6), 122.33 (C-5), 119.68 (C-6′), 115.94 (C-5′), 114.88 (C-8), 112.39 (C-4a), 111.64 (C-2′), 96.66 (C-3), 65.19 (C-4c), 55.15 (C-3a'), 21.27 (C-7a); 15N NMR (DMSO‑d6): δN = 316.1 (N-4f), 177.5 (N-4e), 144.3 (N-1a). MS (70 eV): m/z (%) = 365 (M +, 27). Anal. Calcd for C20H19N3O4 (365.38): C 65.74; H 5.24; N 11.50. Found: C 65.66; H 5.09; N 11.44.
(E)−2-((7-Methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)-N'-(3,4,5-trimethoxybenzylidene)aceto-hydrazide (4e).
This compound was obtained as a colorless compound, yield 0.38 g (90%); Rf = 0.45 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.78 (s; 1H, NH, H-4e), 11.34 (s; 1H, NH-1), 7.95 (s; 1H, H-4 g), 7.76 (d; J = 8.2 Hz, 1H, H-5), 7.09 (s; 1H, H-8), 7.05 (m; 3H, H-2′,6), 5.70 (s; 1H, H-3), 5.37 (s; 2H, H-4c), 3.81 (s; 3H, H-3a',5a'), 2.89 (s; 3H, H-4a'), 2.38 (s; 3H, H-7a); 13C NMR (DMSO‑d6): δc = 167.81 (C-4d), 163.24 (C-2), 162.13 (C-4), 153.12 (C-3′), 143.92 (C-4 g), 141.06 (C-7), 140.15 (C-8a), 138.80 (C-4′), 129.40 (C-1′), 122.77 (C-6), 122.31 (C-5), 114.88 (C-8), 112.38 (C-4a), 104.34 (C-2′), 96.64 (C-3), 60.08 (C-4c), 55.92 (C-4a'), 35.73 (C-3a'), 21.26 (C-7a); 15N NMR (DMSO‑d6): δN = 313.2 (N-4f), 177.5 (N-4e), 143.9 (N-1a). MS (70 eV): m/z (%) = 425 (M+, 55). Anal. Calcd for C22H23N3O6 (425.43): C 62.11; H 5.45; N 9.88. Found: C 62.28; H 5.59; N 10.01.
(E)-N'-Benzylidene-2-((1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide (4f).
This compound was obtained as a colorless compound, yield 0.275 g (82%); Rf = 0.5 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.75 (s; 1H, NH, H-4e), 8.04 (s; 1H, H-4 g), 8.01 (d, J = 7.8 Hz; 1H, H-8), 7.76 (dd, J = 7.8, 2.2 Hz; 2H, H-o), 7.69 (m; 1H, H-7), 7.54 (d, J = 8.5 Hz; 1H, H-5), 7.46 (m; 3H, H-m,p), 7.31 (m; 1H, H-6), 5.98 (s; 1H, H-3), 5.38 (s; 2H, H-4c), 3.58 (s; 3H, H-1a); 13C NMR (DMSO‑d6): δc = 167.71 (C-4d), 162.17 (C-2), 160.65 (C-4), 144.16 (C-4 g), 139.47 (C-8a), 133.89 (C-i), 131.48 (C-7), 129.98 (C-p), 128.75 (C-m), 127.01 (C-o), 122.88 (C-8), 121.55 (C-6), 115.56 (C-4), 114.64 (C-5), 97.21 (C-3), 65.28 (C-4c), 28.62 (C-1a); 15N NMR (DMSO‑d6): δN = 315.1 (N-4f), 177.8 (N-4e), 137.4 (N-1a). MS (70 eV): m/z (%) = 335 (M+, 45). Anal. Calcd for C19H17N3O3 (335.36): C 68.05; H 5.11; N 12.53. Found: C 68.16; H 4.99; N 12.39.
(E)-N'-(4-Bromobenzylidene)−2-((1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)acetohydrazide (4g).
This compound was obtained as a colorless compound, yield 0.290 g (70%); Rf = 0.3 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.81 (s; 1H, NH, H-4e), 8.01 (s; 2H, H-4 g,8), 7.72 (d, J = 8.5 Hz; 2H, H-o), 7.64 (m; 3H, H-7,m), 7.54 (d, J = 8.5 Hz; 1H, H-5), 7.31 ("t", J = 7.5 Hz; 1H, H-6), 5.99 (s; 1H, H-3), 5.37 (s; 2H, H-4c), 3.57 (s; 3H, H-1a); 13C NMR (DMSO‑d6): δc = 167.80 (C-4d), 162.17 (C-2), 160.61 (C-4), 142.96 (C-4 g), 139.46 (C-8a), 133.20 (C-i), 131.73 (C-m), 131.48 (C-7), 128.91 (C-o), 123.21 (C-p), 122.86 (C-8), 121.55 (C-6), 114.64 (C-5), 97.23 (C-3), 65.27 (C-4c), 28.62 (C-1a); 15N NMR (DMSO‑d6): δN = 316.9 (N-4f), 178.1 (N-4e), 137.4 (N-1a). MS (70 eV): m/z (%) = 415 (M + 1, 28), 414 (M+, 33). Anal. Calcd for C19H16BrN3O3 (414.25): C 55.09; H 3.89; Br 19.29; N 10.14. Found: C 55.17; H 3.99; Br 19.33; N 10.20.
(E)-N'-(4-Chlorobenzylidene)−2-((1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)-acetohydrazide (4h).
This compound was obtained as a colorless compound, yield 0.288 g (78%); Rf = 0.35 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.81 (s; 1H, NH, H-4e), 8.02 (m; 2H, H-4 g,8), 7.79 (d, J = 8.4 Hz; 2H, H-o), 7.75 (d, J = 8.4 Hz; 1H, H-5), 7.68 ("t", J = 7.7 Hz; 1H, H-7), 7.31 ("t", J = 7.5 Hz; 1H, H-6), 5.99 (s; 1H, H-3), 5.37 (s; 2H, H-4c), 3.57 (s; 3H, H-1a); 13C NMR (DMSO‑d 6): δc = 167.79 (C-4d), 162.18 (C-2), 160.62 (C-4), 142.86 (C-4 g), 139.46 (C-8a), 134.43 (C-p), 132.86 (C-i), 131.47 (C-7), 128.81, 128.68 (C-o,5), 122.86 (C-8), 121.53 (C-6), 115.55 (C-4a), 97.22 (C-3), 65.28 (C-4c), 28.62 (C-1a); 15N NMR (DMSO‑d6): δN = 316.9 (N-4f), 177.9 (N-4e), 137.2 (N-1a). MS (70 eV): m/z (%) = 371 (M + 1, 27), 370 (M +, 30). Anal. Calcd for C19H16ClN3O3 (369.80): C 61.71; H 4.36; Cl 9.59; N 11.36. Found: C 61.77; H 4.44; Cl 9.65; N 11.45.
(E)-N'-(4-Methoxybenzylidene)−2-((1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)-oxy)acetohydrazide (4i).
This compound was obtained as a colorless compound, yield 0.260 g (71%); Rf = 0.6 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.62 (s; 1H, NH, H-4e), 8.01 (dd, J = 8.0, 1.3 Hz; 1H, H-8), 7.98 (s; 1H, H-4 g), 7.70 (d, J = 8.7 Hz; 2H, H-o), 7.67 (m; 1H, H-7), 7.54 (d, J = 8.5 Hz; 1H, H-5), 7.32 (m; 1H, H-6), 7.01 (d, J = 8.8 Hz; 2H, H-m), 5.96 (s; 1H, H-3), 5.35 (s; 2H, H-4c), 3.81 (s; 3H, OMe), 3.58 (s; 3H, H-1a); 13C NMR (DMSO‑d6): δC = 167.44 (C-4d), 162.17 (C-2), 160.76, 160.67 (C-4p), 144.03 (C-4 g), 139.47 (C-8a), 131.48 (C-7), 128.60 (C-o), 126.51 (C-i), 122.88 (C-8), 121.56 (C-6), 115.56 (C-4a), 114.64 (C-5), 114.26 (C-m), 97.18 (C-3), 65.27 (C-4c), 55.28 (OMe), 28.62 (C-1a); 15N NMR (DMSO‑d6): δN = 308.6 (N-4f), 176.7 (N-4e), 137.3 (N-1a). MS (70 eV): m/z (%) = 365 (M+, 30). Anal. Calcd for C20H19N3O4 (365.38): C 65.74; H 5.24; N 11.50. Found: C 65.77; H 5.33; N 11.44.
(E)-N'-(3-Methoxybenzylidene)−2-((1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)-oxy)acetohydrazide (4j).
This compound was obtained as a colorless compound, yield 0.277 g (76%); Rf = 0.65 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d 6): δH = 11.77 (s; 1H, NH, H-4e), 8.00 (m; 2H, H-4 g,8), 7.69 (ddd, J = 8.5, 7.1, 1.4 Hz; 1H, H-7), 7.54 (d, J = 8.5 Hz; 1H, H-5), 7.37 ("t", J = 7.9 Hz; 1H, H-5′), 7.31 (m; 3H, H-6,2′,6′), 7.00 (m; 1H, H-4′), 5.97 (s; 1H, H-3), 5.39 (s; 2H, H-4c), 3.80 (s; 3H, OMe), 3.58 (s; 3H, H-1a); 13C NMR (DMSO‑d 6): δC = 167.77 (C-4d), 162.16 (C-2), 160.66 (C-4), 159.51 (C-3′), 143.98 (C-4 g), 139.46 (C-8a), 135.31 (C-1′), 131.48 (C-7), 129.85 (C-5′), 122.86 (C-8), 121.55 (C-6), 119.68 (C-6′), 115.91 (C-4′), 115.55 (C-4a), 114.64 (C-5), 111.70 (C-2′), 97.22 (C-3), 65.33 (C-4c), 55.15 (C-3a'), 28.62 (C-1a); 15N NMR (DMSO‑d6): δN = 315.8 (N-4f), 177.7 (N-4e), 137.4 (N-1a). MS (70 eV): m/z (%) = 365 (M+, 65). Anal. Calcd for C20H19N3O4 (365.38): C 65.74; H 5.24; N 11.50. Found: C 65.70; H 5.33; N 11.40.
(E)−2-((1-Methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)-N'-(3,4,5-trimethoxy-benzylidene)aceto-hydrazide (4k).
This compound was obtained as a colorless compound, yield 0.325 g (76%); Rf = 0.35 (Toluene: Ethyl acetate; 10:1); 1H NMR (DMSO‑d6): δH = 11.78 (s; 1H, NH, H-4e), 8.00 (d, J = 7.9 Hz; 1H, H-8), 7.95 (s; 1H, H-4 g), 7.69 ("t", J = 7.8 Hz; 1H, H-7), 7.54 (d, J = 8.6 Hz; 1H, H-5), 7.37 ("t", J = 7.9 Hz; 1H, H-5′), 7.32 (m; 1H, H-6), 7.05 (s; 2H, H-2′), 5.95 (s; 1H, H-3), 5.41 (s; 2H, H-4c), 3.83 (s; 6H, H-3a'), 3.70 (s; 3H, H-4a'), 3.58 (s; 3H, H-1a'); 13C NMR (DMSO‑d 6): δC = 167.75 (C-4d), 162.15 (C-2), 160.71 (C-4), 153.13 (C-3′), 143.96 (C-4 g), 139.47 (C-8a,4′), 131.48 (C-7), 129.40 (C-1′), 122.85 (C-8), 121.55 (C-6), 115.56 (C-4a), 114.64 (C-5), 97.21 (C-3), 65.40 (C-4c), 60.09 (C-4a'), 55.95 (C-3a'), 28.62 (C-1a); 15N NMR (DMSO‑d6): δN = 313.2 (N-4f), 177.2 (N-4e), 137.2 (N-1a). MS (70 eV): m/z (%) = 425 (M+, 45). Anal. Calcd for C22H23N3O6 (425.43): C 62.11; H 5.45; N 9.88. Found: C 62.20; H 5.39; N 9.76.
5. Molecular docking calculations
The crystal structures of SARS-CoV-2 main protease (Mpro; PDB code: 6LU7 [46]) and RNA-dependent RNA polymerase (RdRp; PDB code: 6M71 [47]) were taken as templates for all molecular docking. Receptors were cleaned of water molecules, ions and the ligands. The protonation state of Mpro and RdRp was investigated using an H ++ server, and all missing hydrogen atoms were added [48]. All molecular docking calculations were carried out using Autodock4.2.6 software [49]. All docking parameters were kept to default values, except the number of genetic algorithm (GA) runs and the maximum number of energy evaluations (eval) which were set to 250 and 25,000,000, respectively. The docking grid was set to 60Å x 60Å x 60Å with a grid spacing value of 0.375 Å, and the grid center was placed at the center of the active site. The geometrical structures of all synthesized compounds were minimized with a MMFF94s force field using SZYBKI software [50] and the partial atomic charges were assigned using the Gasteiger method [51].
CRediT author statement and authorship
I would like to confirm and certify the authors’ contributions as indicated in the followings:
Mohammed B Alshammari (Writing-review & editing, Funding acquisition)
Mohamed Ramadan (Supervision, Writing-review & editing)
Ashraf A Aly (Conceptualization, Superivsion, Writing-original draft, Writing-review & editing)
Essmat M. El-Sheref (Methodology, Writing-review & editing)
Md Afroz Bakht (Visualization)
Mahmoud A. A. Ibrahim (Methodology, Writing-review & editing)
Ahmed M. Shawky (Validation_Visualization)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University under the research project No10302/1/2019.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2020.129649.
Appendix. Supplementary materials
References
- 1.Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., Tiwari R., Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet. Q. 2020;40(1):68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee P.I., Hsueh P.R. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.02.00. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV-a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony S.J., Johnson C.K., Greig D.J., Kramer S., Che X., Wells H., Hicks A.L., Joly D.O., Wolfe N.D., Daszak P., Karesh W., Lipkin W.I., Morse S.S., Consortium P., Mazet J.A.K., Goldstein T. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1) doi: 10.1093/ve/vex012. vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 8.Wang F., Chen C., Tan W., Yang K., Yang H. Structure of Main Protease from Human Coronavirus NL63: insights for Wide Spectrum Anti-Coronavirus Drug Design. Sci. Rep. 2016;6:22677. doi: 10.1038/srep22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y.X., Chen X.-.P., Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clin Pharmacol Ther 2006 (in press) 10.1002/cpt.1844. [DOI] [PubMed]
- 10.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396):l3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulangu S., Dodd L.E., Davey R.T., Mbaya O.T., Proschan M., Mukadi D., Manzo M.L., Nzolo D., Oloma A.T., Ibanda A., Ali R., Coulibaly S. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV. in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessonova I.A. Components of Haplophyllum bucharicum. Chem. Nat. Compds. 2000;36:323–324. [Google Scholar]
- 14.Detsi A., Bardakaos V., Markopoulos J., Igglessi-Markopoulou O. Reactions of 2-methyl-3,1-benzoxazin-4-one with active methylene compounds: a new route to 3-substituted 4-hydroxyquinolin-2(1H)-ones. J. Chem. Sec. Perkin. Trans. 1996;1:2909–2913. doi: 10.1039/P19960002909. [DOI] [Google Scholar]
- 15.Ukrainets V.I., Bereznyakova N.L., Mospanova E.V. 4-Hydroxy-2-quinolones 121. Synthesis and biological properties of 1-hydroxy-3-oxo-5,6-dihydro-3H-pyrrolo[3,2,1-ij]quinoline-2 carboxylic acid alkyl amides. Chem. Heterocycl. Compds. 2007;43:856–862. doi: 10.1007/s10593-007-0136-4. [DOI] [Google Scholar]
- 16.McCormick J.L., McKee T.C., Cardinella J.H., Boyd M.R. HIV Inhibitory Natural Products. Quinoline Alkaloids from Euodia roxburghiana. J. Nat. Prod. 1996;59(5):469–471. doi: 10.1021/np960250m. [DOI] [PubMed] [Google Scholar]
- 17.Luthra P., Liang J., Pietzsch C.A., Khadka S., Edwards M.R., Wei S., De S., Posner B., Bukreyev A., Ready J.M., Basler C.F. A high throughput screen identifies benzoquinoline compounds as inhibitors of Ebola virus replication. Antivir. Res. 2018;150:193–201. doi: 10.1016/j.antiviral.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loregian A., Mercorelli B., Muratore G., Sinigalia E., Pagni S., Massari S., Gribaudo G., Gatto B., Palumbo M., Tabarrini O., Cecchetti V., Palu G. The 6-aminoquinolone WC5 inhibits human cytomegalovirus replication at an early stage by interfering with the transactivating activity of viral immediate-early protein. Antimicrob. Agents Chemother. 2010;54(5):1930–1940. doi: 10.1128/AAC.01730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plantone D., Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin. Drug Investig. 2018;38(8):653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa-Lima G., Moraes A.M., Araujo A.D.S., da Silva E.T., de Freitas C.S., Vieira Y.R., Marttorelli A., Neto J.C., Bozza P.T., de Souza M.V.N., Souza T.M.L. 2,8-bis(trifluoromethyl)quinoline analogs show improved anti-Zika virus activity, compared to mefloquine. Eur. J. Med. Chem. 2017;127:334–340. doi: 10.1016/j.ejmech.2016.12.058. [DOI] [PubMed] [Google Scholar]
- 21.Al-Bari M.A. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015;70(6):1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delvecchio R., Higa, Pezzuto L.M., Valadao P., Garcez A.L., Monteiro P.P., Loiola F.L., Dias E.C., Silva A.A., Aliota F.J., Caine M.T., Osorio E.A., Bellio J.E., O’Connor M., Rehen D.H., de Aguiar S., Savarino R.S., Campanati A., Tanuri L., Chloroquine A. an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses. 2016;8(12):322. doi: 10.3390/v8120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter S., Parolin C., Palumbo M., Palù G. Antiviral Properties of Quinolone-based Drugs. Curr Drug Target – Infect Disord. 2004;4:111–116. doi: 10.2174/1568005043340920. [DOI] [PubMed] [Google Scholar]
- 24.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato M., Motomura T., Aramaki H., Matsuda T., Yamashita M., Ito Y., Kawakami H., Matsuzaki Y., Watanabe W., Yamataka K., Ikeda S., Kodama E., Matsuoka M., Shinkai H. Novel HIV-1 integrase inhibitors derived from quinolone antibiotics. J. Med. Chem. 2006;49(5):1506–1508. doi: 10.1021/jm0600139. [DOI] [PubMed] [Google Scholar]
- 26.Hajimahdi Z., Zabihollahi R., Aghasadeghi M.R., Hosseini-Ashtiani S., Zargh A. Novel quinolone-3-carboxylic acid derivatives as anti-HIV-1 agents: design, synthesis, and biological activities. Med. Chem. Res. 2016;25:1861–1876. doi: 10.1007/s00044-016-1631-x. [DOI] [Google Scholar]
- 27.Aly A.A., El-Sheref E.M., Mourad A.F.E., Bakheet M.E.M., Bräse S., Nieger M. One-pot synthesis of 2,3-bis-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinates and arylmethylene-bis-3,3′-quinoline-2-ones. Chem Pap. 2019;73:27–37. [Google Scholar]
- 28.Aly A.A., Ramadan M., El-Reedy A.A.M. Reactions of 4-hydroxyquinolin-2(1H)-ones with acenaphthoquinone: synthesis of new 1,2-dihydroacenaphthylene-spiro-tetrakis(4-hydroxy-quinolin-2(1H)-ones) J. Heterocycl. Chem. 2019;56:642–645. [Google Scholar]
- 29.Aly A.A., El-Sheref E.M., Mourad A.-F.E., Brown A.B., Bräse S., Bakheet M.E.M., Nieger M. Synthesis of spiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitriles. Monatsh. Chem. 2018;149:635–644. [Google Scholar]
- 30.El-Sheref E.M., Aly A.A., Mourad A.-F.E., Brown A.B., Bräse S., Bakheet M.E.M. Synthesis of pyrano[3,2-c]quinoline-4-carboxylates and 2-(4-oxo-1,4-dihydroquinolin-3-yl)fumarates. Chem. Pap. 2018;72:181–190. [Google Scholar]
- 31.El-Sheref E.M., Aly A.A., Ameen M.A., Brown A.B. New 4-(1,2,3-triazolo)quinolin-2(1H)-ones via Cu-catalyzed [3+2] cycloaddition. Monatsh. Chem. 2019;150:747–756. [Google Scholar]
- 32.Aly A.A., El-Sheref E.M., Bakheet M.E.M., Mourad M.A.E., Bräse S., Ibrahim M.A.A., Nieger M., Garvalov B.K., Dalby K.N., Kaoud T.S. Design, synthesis and biological evaluation of fused naphthofuro[3,2-c]quinoline-6,7,12-triones and pyrano[3,2-c]quinoline-6,7,8,13-tetraones derivatives as ERK inhibitors with efficacy in BRAF-mutant melanoma. Bioorg. Chem. 2019;82:290–305. doi: 10.1016/j.bioorg.2018.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aly A.A., El-Sheref E.M., Bakheet M.E.M., Mourad M.A.E., Brown A.B., Bräse S., Nieger M., Ibrahim M.A.A. Synthesis of novel 1,2-bis-quinolinyl-1,4-naphthoquinones: ERK2 inhibition, cytotoxicity and molecular docking studies. Bioorg. Chem. 2018;81:700–712. doi: 10.1016/j.bioorg.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Aly A.A., Hassan A.A., Mohamed N.K., Bräse S., Abd El-Haleem L.E., Polamo M., Nieger M., Brown A.B. Synthesis of new fused heterocyclic 2-quinolones and 3-alkanonyl-4-hydroxy-2-quinolones. Molecules. 2020;24:3782–3795. doi: 10.3390/molecules24203782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbastawesy M.A.I., M.El-Shaier Ramadan, Y.A.A.M. Aly A.A., El-Din A., Abuo-Rahma G. Arylidenes of Quinolin-2-one scaffold as Erlotinib analogues with activities against leukemia through inhibition of EGFR TK/ STAT-3 pathways. Biorg. Chem. 2020;96 doi: 10.1016/j.bioorg.2020.103628. [DOI] [PubMed] [Google Scholar]
- 36.Sondhi S.M., Singh N., Kumar A., Lozach O., Meijer L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1,CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff's bases. Bioorg. Med. Chem. 2006;14(11):3758–3765. doi: 10.1016/j.bmc.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 37.Pandey A., Dewangan D., Verma S., Mishra A., Dubey R.D. Synthesis of Schiff bases of 2-amino-5-aryl-1,3,4-thiadiazole And its Analgesic, AntiInflammatory, Anti-Bacterial and Antitubercular Activity. Inter. J. Chem. Tech. Res. 2011;3(1):178–184. [Google Scholar]
- 38.Chandramouli S.M.R., Nayanbhai T.B., Bheemachari Udupi R.H. Synthesis and biological screening of certain new triazole schiff bases and their derivatives bearing substituted benzothiazole moiety. J. Chem. Pharm. Res. 2012;4(2):1151–1159. [Google Scholar]
- 39.Chinnasamy R.P., Sundararajan R., Govindaraj S. Synthesis, characterization, and analgesic activity of novel Schiff base of isatin derivatives. J. Adv. Pharm. Tech. Res. 2010;1(3):342–347. doi: 10.4103/0110-5558.72428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sathe B.S., Jaychandran E., Jagtap V.A., Sreenivasa G.M. Synthesis characterization and anti-inflammatory evaluation of new fluorobenzothiazole schiff's bases. Inter. J. Pharm. Res. Develop. 2011;3(3):164–169. [Google Scholar]
- 41.Aboul-Fadl T., Mohammed F.A., Hassan E.A. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH) Arch. Pharm. Res. 2003;26(10):778–784. doi: 10.1007/BF02980020. [DOI] [PubMed] [Google Scholar]
- 42.Miri R., Razzaghi-Asl N., Mohammadi M.K. QM study and conformational analysis of an isatin Schiff base as a potential cytotoxic agent. J. Molecul. Mod. 2013;19(2):727–735. doi: 10.1007/s00894-012-1586-x. [DOI] [PubMed] [Google Scholar]
- 43.Ali S.M.M., Azad M.A.K., Jesmin M., Ahsan S., Rahman M.M., Khanam J.A., Islam M.N., Shahria S.M.S. In vivo anticancer activity of vanillin semicarbazone. Asian Pacific J. Trop. BioMed. 2012;2(6):438–442. doi: 10.1016/S2221-1691(12)60072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panda S.S., Malik R., Chand M., Jain S.C. Synthesis and antimicrobial activity of some new 4-triazolylmethoxy-2H- chromen-2-one derivatives. Med. Chem. Res. 2012;21(11):3750–3756. doi: 10.1007/s00044-011-9881-0. [DOI] [Google Scholar]
- 45.Patel R.V., Patel J.K., Kumari P., Chikhali K.H. Synthesis of Novel Quinolone and Coumarin Based 1,3,4-Thiadiazolyl and 1,3,4-Oxadiazolyl -N-Mannich Bases as Potential Antimicrobials. Lett. Org. Chem. 2012;9(7):478–486. doi: 10.2174/157017812802139681. [DOI] [Google Scholar]
- 46.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon J.C., Myers J.B., Folta T., Shoja V., Heath L.S., Onufriev A. a server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005;33(Web Server issue):W368–W371. doi: 10.1093/nar/gki464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comp. Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SZYBKI OpenEye Scientific Software: Santa Fe, 2009 NM, USA.
- 51.Gasteiger J.M., Marsili M. Iterative partial equalization of orbital electronegativity A rapid access to atomic charges. Tetrahedron. 1980;36(22):3219–3228. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.