Abstract
Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) combines the high sensitivity of PET with the excellent spatial resolution provided by CT, making FDG-PET/CT a potentially powerful tool for capturing and quantifying early vascular diseases. Patients with chronic inflammatory states have an increased risk of cardiovascular events; there is also increased vascular FDG uptake seen compared to healthy controls. This review examines the use of FDG PET/CT in assessing low grade vascular inflammation in chronic inflammation and then review FDG PET/CT as a tool in monitoring the efficacy of various treatments known to modulate cardiovascular disease.
Keywords: Fluorodeoxyglucose-positron emission tomography/computed tomography, inflammation, vascular disease
Introduction
Cardiovascular disease, including atherosclerosis, is the leading cause of death worldwide (1) and therefore significant efforts to capture early sub-clinical vascular disease have been made in the past decade. In this context, fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) utilizes a radiolabeled glucose analogue taken up by cells in the vessel wall in proportion to their metabolic activity. FDG PET/CT combines the high sensitivity of PET for the detection of intra-plaque inflammation using spatial resolution and morphologic information provided by CT (1). This review will cover how FDG PET/CT has been utilized to detect subclinical vascular disease in various inflammatory states.
Low grade vascular inflammation in chronic inflammatory states.
Chronic inflammatory diseases are associated with high incidence of cardiovascular events (2). Inflammation and lipid dysfunction associates with initiation and progression of atherosclerosis. Systemic inflammatory biomarkers, such as high sensitivity C-reactive protein, have been associated with atherosclerotic disease and future cardiovascular events (3). Moreover, inflammatory cells, such as circulating monocytes, have been useful in detecting plaque vulnerability (4) and distinct monocyte subpopulations are significantly associated with subclinical atherosclerosis (5). Chronic inflammatory diseases, including psoriasis, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and persons living with human immunodeficiency virus (HIV), have all been independently associated with increased risk of cardiovascular disease (6, 7). Compared to age and gender matched controls, these patients have heightened macrophage activity and inflammatory cytokines including TNF-α IL-6, IL-1 and IL-17A, which have been linked to key pathways contributing to atherogenesis. Because of this, individuals with chronic inflammatory states are at higher risk for inflammatory driven cardiovascular disease compared to their healthy counterparts (2, 6). In these patients there is an unmet need to detect subclinical vascular disease prior to CV events.
Vascular Uptake of FDG by PET/CT
Early atherosclerotic lesions have increased presence myeloid-derived immune cells; lesion progression is accelerated by secreted products and subsequent chemotaxis. First, a lesion in the blood vessel is started with a fatty streak, an accumulation of lipids and macrophages beneath the endothelium. Next asymmetric, focal thickening of the intima consists of “foam cells”, pro-inflammatory macrophages, oxidized lipoproteins, and extracellular lipid droplets surrounded by cap of smooth-muscle cells and a collagen-rich matrix. (7, 8). Atherosclerotic plaques differ in risk of rupture based on several features. High-risk plaques have a thin fibrous cap, a lipid necrotic core, microcalcifications, neovascularization and an abundance of activated immune cells (8). Plaque rupture leads to intraluminal thrombus formation via exposure of the thrombogenic core to circulating platelets and clotting factors. Thus, a potent driver for plaque destabilization and subsequent cardiovascular events is inflammation.
18F-FDG is a radiolabeled glucose analogue that is taken up by glucose transporters (GLUT) and phosphorylated by hexokinase, the enzyme responsible for the first step in glycolysis. Normally glucose’s 2-hydroxyl group is then utilized for further glycolysis, however because 18F-FDG lacks this 2-hydroxy group, it cannot be further processed nor can it leave the cell before radioactive decay. 18F-FDG accumulation at sites of acute and chronic inflammation is primarily due to overexpression of GLUT transporters as well as hexokinase in active inflammatory cells (9). Furthermore, when compared to resting macrophages, activated macrophages have a dramatically increased level of hexokinase activity (10). Vascular FDG uptake in sub-clinical atherosclerosis, a chronic low-grade inflammatory disease has long been documented through PET imaging in major vessels throughout the body, usually to aorta, carotids, and most recently the coronary arteries (11). FDG-PET uptake was incidentally noted in the vascular wall of large arteries, during an oncologic-indicated exam, and found that FDG vascular uptake correlated with cardiovascular (CV) risk factors (12). Validation of FDG-PET as a marker of the inflammation in an atherosclerotic plaque originated from in vitro histology studies including immunostaining for macrophages in endarterectomy samples from carotid plaques (13, 14). There is upregulation of CD68, a recognized marker of macrophages, in lesions with increased FDG uptake. Subsequent clinical studies of FDG-PET/CT showed that CT derived vascular calcification and PET metabolic activity in fact were detecting potentially different stages of plaque development (15). Additionally, high-risk plaque morphology by CT corresponded to the inflammation burden detected by FDG-PET (16).
Inflammatory vascular conditions also show extensive FDG-PET uptake in blood vessel walls. Most notably is central and peripheral vasculitis, which generally affects the medium to large vessels preferentially. Additionally, FDG-PET uptake occurs in vascular graft-related infection, intravascular thrombosis, vascular tumors, fistulas and aneurysms (17). Symptomatic aortic aneurysms have increased FDG uptake and this potentially may associate with rupture risk (18). FDG PET/CT also has a role in detecting and quantifying aortitis of infectious and noninfectious etiologies. Most notably it has a very high sensitivity and specificity in the diagnosis of large vessel vasculitis (LVV), such as Takayasu arteritis and giant cell arteritis, which typically affect the large arteries of the body such as the aorta and its major branches. FDG uptake in LVV is characteristically lineal diffuse uptake, differentiated from low-grade patchy uptake seen in atherosclerotic vessels, and is in increased in patients with LVV compared to controls (19). Furthermore while rare, FDG PET/CT can be useful in the evaluation of indolent primary aortic malignancies, most commonly sarcomas, and embolic spread, distant metastasis or extension into adjacent arteries (18).
FDG PET/CT and Association between Vascular Disease
The Cardiovascular Committee of the European Association of Nuclear Medicine recently recommended standardized protocols for imaging and interpretation of atherosclerosis imaging via FDG PET/CT, however, the lack of conclusive evidence limits these recommendations (20). Despite this, multiple prospective studies have shown relationships of increased aortic vascular inflammation in atherosclerosis by FDG PET/CT with higher incidence of cardiovascular disease (CVD) including myocardial infarction, heart failure and claudication. FDG PET has been used to image vascular uptake in the aorta, carotid arteries, and coronary arteries (21).
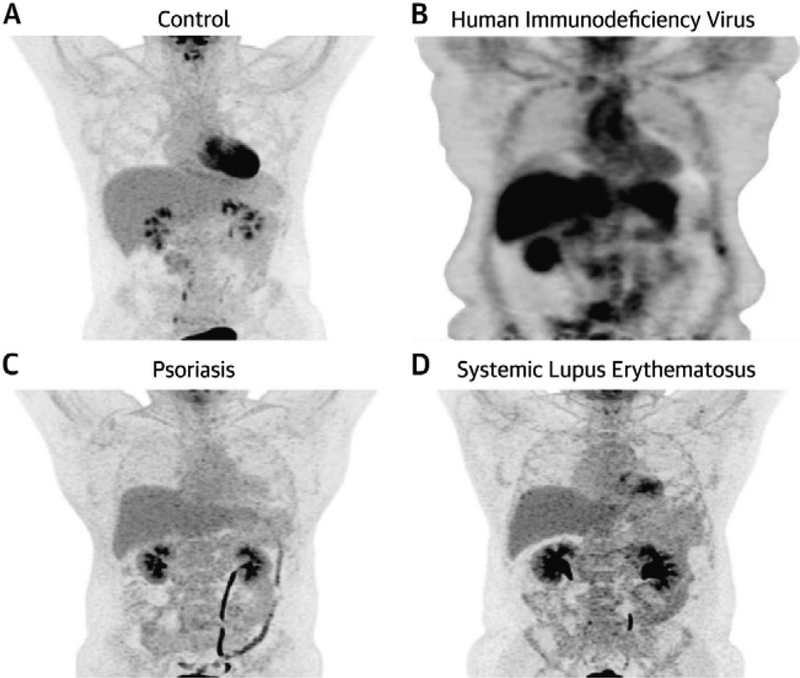
Inflammatory disease states have been associated with higher incidence of vascular disease compared to age and gender matched controls (22). In chronic low-grade inflammatory states such as psoriasis, RA, HIV, and SLE, vascular FDG uptake is higher compared to controls (Figure 1) (21, 23–26). In a case-control study, increased vascular FDG uptake consistent with early vascular disease was observed in psoriasis patients compared to controls (27). Also, psoriasis severity markers of neutrophil activation associated with vascular inflammation assessed as target-to-background ratio (TBR) in the aorta (maximal SUV in the artery/mean SUV in the vein) beyond traditional CV risk factors (28). Additionally, in ninety-one RA patients without CVD, increased FDG uptake was associated with RA disease activity (25). Finally, individuals with SLE and HIV had increased vascular inflammation compared to healthy controls who underwent FDG PET/CT (23, 26). These studies demonstrate the ability of FDG PET/CT to be used as a tool in detecting aortic vascular inflammation in inflammatory disease patients without clinical CVD.
Figure 1.
Aortic Vascular FDG uptake in various chronically inflamed human models. Representative 18F-fluorodeoxyglucose positron emission tomographic/computed imaging of the aorta in a healthy control (A), patient with human immunodeficiency virus (B), psoriasis (C), and systemic lupus erythematosus (D).
FDG PET is also used to assess carotid disease in individuals. Rudd et. all first reported that in eight patients with symptomatic plaques there was increased FDG accumulation in macrophage dense regions of carotid artery plaques compared to asymptomatic plaques, with no uptake in normal arteries (13). Later, increased ipsilateral FDG-PET uptake as measured by max standardized uptake value (SUV) ratios in atherosclerotic carotid lesions was associated to be in a close time interval to events such as stroke or transient ischemic attack when compared to those with solely chronic obstructive stenosis (29), suggesting that FDG PET/CT may be used to tailor stroke prevention strategies (30).
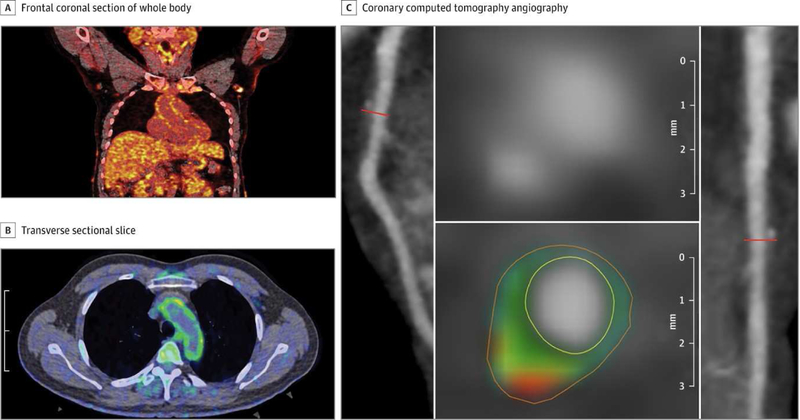
Recently, FDG uptake in the aorta has been associated with coronary plaque characteristics by coronary computed tomography angiography (CCTA), providing further evidence for FDG/PET in capturing sub-clinical atherosclerosis (31). High vascular FDG uptake was associated with high risk plaque detected by CCTA in subclinical atherosclerosis in psoriasis and HIV (Figure 2) (31, 32). Finally, increased FDG-PET uptake has been shown to be associated with culprit coronary lesions in the relatively immobile left main coronary artery in patients presenting with acute coronary syndromes (11); however spatial resolution of PET precludes future use for coronary imaging at this time.
Figure 2.
Aortic Vascular Uptake of FDG by PET/CT and Coronary Artery Disease Characterization by CCTA. Frontal coronal section of whole body FDG PET/CT scan with increased uptake of FDG throughout the body, specifically in the aortic wall, in a patient with psoriasis (A). Transverse sectional slice from FDG PET/CT that shows increased vascular FDG uptake in the aortic wall (green) in a patient with psoriasis (B). Panel of reconstructed images from CCTA. Left anterior descending coronary artery (left) depicts noncalcified burden of the coronary artery and transverse section of left anterior descending coronary artery (right). The planar reconstruction (middle) demonstrates low-attenuation lipid-rich high-risk plaque (green and red).
FDG PET/CT and response to treatment
Vascular FDG uptake detects changes in aortic vascular inflammation in response to classical coronary artery disease (CAD) interventions. Primary prevention of CAD is centered on risk factor control and improvements in lifestyle which have shown to modify vascular inflammation is detected by FDG PET/CT. In a study assessing 60 adults who underwent both atherogenic risk-factor assessment and FDG PET/CT at baseline and again after 17 months follow-up with intense lifestyle modification, changes in CV risk factors including diastolic blood pressure, total cholesterol, and low-density lipoprotein as well as increase in high-density lipoprotein associated with a decrease in vascular inflammation (33).
Currently, statins are commonly used for maintaining and preventing CVD. HMG Co-A reductase or “statins” have been shown to decrease vascular uptake of FDG and this reduction is later mirrored by a concordant reduction in CVD events. A prior study demonstrated that intense statin therapy led to a reduction in atherosclerotic inflammation as measured by FDG PET/CT. In 83 adults with known atherosclerotic disease not on high-intensity statin therapy serially imaged with FDG PET/CT at baseline, 4, and 12 weeks follow-up following randomization to low or high dose stain. Vascular inflammation as assessed by FDG PET-CT TBR was significantly reduced in the high-dose statin group (atorvastatin 80 mg) compared to the low-dose statin group (atorvastatin 10 mg) (change in TBR from baseline to 4 weeks 80 mg: 7.6 (2.6 to 12.3) versus 10 mg: 2.0 (−3.1 to 6.8) and change in TBR from baseline to 12 weeks 80 mg: 6.7 (1.8 to 11.3) vs 10 mg: −0.1 (−5.6 to 5.1) (34).
Novel emerging medications and their influence arterial inflammation may also be assessed by FDG PET/CT to support or refute clinical trials of emerging drugs. p38 map kinase inhibitors notably were not associated with decreased vascular FDG uptake. Later, a follow up events study failed to demonstrate that p38 map kinase inhibition had any impact on CVD events. Therefore, small-scale FDG PET/CT imaging trials may be useful in identifying strategies that may have promise to reduce cardiovascular events in larger endpoint trials.
The impact of primary disease therapy on vascular inflammation has been assessed by FDG PET. Notably, anti-tumor necrosis factor (TNF-a), as well as anti-Interleukin (IL)12/23, anti-IL 17, and anti-IL-1b therapies are all indicated in the treatment of inflammatory skin, and joint disease. Small observational preliminary findings implied that initiating a biologic treatment, such as anti-TNF, precedes a decrease in vascular inflammation by arterial FDG uptake (24, 35), however, randomized control trials showed no effect of anti-TNF therapy at follow-up. The Vascular Inflammation in Psoriasis (VIP) trial aimed to understand the impact of anti-cytokine treatments on vascular disease. Through a series of randomized placebo-controlled trials, anti-TNF therapy was found to have no impact on vascular inflammation by FDG-PET compared to a placebo. On the other hand, anti-IL12/23 therapy resulted in an initial transient reduction in aortic inflammation (36). Moreover, antiretroviral therapies that were studied using FDG PET have shown no effect on vascular inflammation (37).
Limitations
FDG PET/CT is useful in detecting inflammation, but it also has certain clinical and analytical limitations. Patient factors such as pre-scan glucose levels and high BMI can influence FDG uptake values. As pre-scan glucose levels increase, there is a reduction in FDG uptake due to the competition between glucose and FDG; therefore pre-scan glucose levels > 200 mg/dL should be avoided to improve tracer uptake. Those with increased BMI additionally have worse image quality compared to those with normal BMI due to scatter. These limitations can be by somewhat overcome by increasing the injected dose of radiotracer or increasing the acquisition time, however these strategies have led to little improvement (38). Additionally, imaging protocols such as patient preparation and timing of imaging are all potential patient factors that can impact the measured FDG uptake. Protocols often differ in scan acquisition times, and can vary from one to three hours; however fortunately numerous studies have shown that a circulation time greater than two hours gives a higher TBR (39). Furthermore, background variability is also a limitation. Specifically, background blood activity is often normalized when quantifying TBR, however because of fundamental differences in blood pool activity TBR may be influenced. Moreover, the choice of background tissue also affects TBR uptake values. Lastly, the spatial resolution of PET imaging can be difficult when attempting to capture uptake in small structures and change in size has shown to associate with apparent uptake due to the partial volume effect, however coupling PET to CT has substantially overcome this. In attempts to offer a higher quality of information, PET scanners have undergone notable improvements. One such advancement is the time of flight (TOF) PET scanner. The current generation TOF PET scanners offer a higher resolution and have the ability to operate in three-dimensional mode (40). Furthermore, the TOF PET scanner can be used with patients who have higher BMI’s, allowing PET CT to accommodate for many more patients (8). While there are many factors that can introduce variation in FDG uptake in the vessel wall however many can be controlled for, despite this careful analysis of results is required.
Future Directions
FDG PET/CT overall shows promise in detecting subclinical vascular inflammation and changes in vascular inflammation may indicate disease progression or improvement with interventions. Notably, FDG PET/CT has been utilized in detecting culprit coronary lesions, however its technical limitations including high myocardial FDG uptake, cardiac motion, and the small size of plaque make its application difficult. However promising methods to overcome high myocardial FDG uptake can be accomplished by have patients trial a high-fat, low-carbohydrate diet before imaging (41) and thus its role in coronary inflammation has yet to be elucidated.
Disclosures:
NNM is a full-time US government employee and has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants/other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals, Inc, and Novartis receiving grants and/or research funding and as a principal investigator for the National Institute of Health receiving grants and/or research funding.
DEU is funded by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant # 2014194), Genentech, Elsevier, and other private donors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors have no conflict of interest.
References:
- 1.Chaturvedi A, Dey AK, Joshi AA, Mehta NN. Vascular Inflammation Imaging in Psoriasis. Current Cardiovascular Imaging Reports. 2017;10(2):4. [Google Scholar]
- 2.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39(4):633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. The New England journal of medicine. 1997;336(14):973–9. [DOI] [PubMed] [Google Scholar]
- 4.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005;5(12):953–64. [DOI] [PubMed] [Google Scholar]
- 5.Rogacev KS, Ulrich C, Blomer L, Hornof F, Oster K, Ziegelin M, et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. European heart journal. 2010;31(3):369–76. [DOI] [PubMed] [Google Scholar]
- 6.Boehncke WH. Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Front Immunol. 2018;9(579):579.29675020 [Google Scholar]
- 7.Bural GG, Torigian DA, Chamroonrat W, Alkhawaldeh K, Houseni M, El-Haddad G, et al. Quantitative assessment of the atherosclerotic burden of the aorta by combined FDG-PET and CT image analysis: a new concept. Nucl Med Biol. 2006;33(8):1037–43. [DOI] [PubMed] [Google Scholar]
- 8.Joseph P, Tawakol A. Imaging atherosclerosis with positron emission tomography. European heart journal. 2016;37(39):2974–80. [DOI] [PubMed] [Google Scholar]
- 9.Chrapko BE, Chrapko M, Nocun A, Stefaniak B, Zubilewicz T, Drop A. Role of 18F-FDG PET/CT in the diagnosis of inflammatory and infectious vascular disease. Nucl Med Rev Cent East Eur. 2016;19(1):28–36. [DOI] [PubMed] [Google Scholar]
- 10.Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55(23):2527–35. [DOI] [PubMed] [Google Scholar]
- 11.Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovascular imaging. 2010;3(4):388–97. [DOI] [PubMed] [Google Scholar]
- 12.Yun M, Jang S, Cucchiara A, Newberg AB, Alavi A. 18F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors. Seminars in nuclear medicine. 2002;32(1):70–6. [DOI] [PubMed] [Google Scholar]
- 13.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11. [DOI] [PubMed] [Google Scholar]
- 14.Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2009;37(6):714–21. [DOI] [PubMed] [Google Scholar]
- 15.Menezes LJ, Kotze CW, Agu O, Richards T, Brookes J, Goh VJ, et al. Investigating vulnerable atheroma using combined (18)F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med. 2011;52(11):1698–703. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5(1):69–77. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang H, Codreanu I. Growing applications of FDG PET-CT imaging in non-oncologic conditions. Journal of biomedical research. 2015;29(3):189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Song HC. Role of PET/CT in the Evaluation of Aortic Disease. Chonnam Med J. 2018;54(3):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawal I, Sathekge M. F-18 FDG PET/CT imaging of cardiac and vascular inflammation and infection. Br Med Bull. 2016;120(1):55–74. [DOI] [PubMed] [Google Scholar]
- 20.Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. European journal of nuclear medicine and molecular imaging. 2016;43(4):780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teague HL, Ahlman MA, Alavi A, Wagner DD, Lichtman AH, Nahrendorf M, et al. Unraveling Vascular Inflammation: From Immunology to Imaging. J Am Coll Cardiol. 2017;70(11):1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noh SM, Choi WJ, Kang BT, Jeong SW, Lee DK, Schellingerhout D, et al. Complementarity between F-18-FDG PET/CT and Ultrasonography or Angiography in Carotid Plaque Characterization. Journal of Clinical Neurology. 2013;9(3):176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlucci PM, Purmalek MM, Dey AK, Temesgen-Oyelakin Y, Sakhardande S, Joshi AA, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI insight. 2018;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol. 2017;2(9):1013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geraldino-Pardilla L, Zartoshti A, Ozbek AB, Giles JT, Weinberg R, Kinkhabwala M, et al. Arterial Inflammation Detected With (18) F-Fluorodeoxyglucose-Positron Emission Tomography in Rheumatoid Arthritis. Arthritis Rheumatol. 2018;70(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarasheski KE, Laciny E, Overton ET, Reeds DN, Harrod M, Baldwin S, et al. 18FDG PET-CT imaging detects arterial inflammation and early atherosclerosis in HIV-infected adults with cardiovascular disease risk factors. J Inflamm (Lond). 2012;9(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Archives of dermatology. 2011;147(9):1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noh SM, Choi WJ, Kang BT, Jeong SW, Lee DK, Schellingerhout D, et al. Complementarity between (18)F-FDG PET/CT and Ultrasonography or Angiography in Carotid Plaque Characterization. J Clin Neurol. 2013;9(3):176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly PJ, Camps-Renom P, Giannotti N, Marti-Fabregas J, Murphy S, McNulty J, et al. Carotid Plaque Inflammation Imaged by (18)F-Fluorodeoxyglucose Positron Emission Tomography and Risk of Early Recurrent Stroke. Stroke. 2019;50(7):1766–73. [DOI] [PubMed] [Google Scholar]
- 31.Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, et al. Association Between Aortic Vascular Inflammation and Coronary Artery Plaque Characteristics in Psoriasis. JAMA Cardiol. 2018;3(10):949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh P, Emami H, Subramanian S, Maurovich-Horvat P, Marincheva-Savcheva G, Medina HM, et al. Coronary Plaque Morphology and the Anti-Inflammatory Impact of Atorvastatin: A Multicenter 18F-Fluorodeoxyglucose Positron Emission Tomographic/Computed Tomographic Study. Circ Cardiovasc Imaging. 2016;9(12):e004195–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YB, Choi KM. Diet-Modulated Lipoprotein Metabolism and Vascular Inflammation Evaluated by (18)F-fluorodeoxyglucose Positron Emission Tomography. Nutrients. 2018;10(10):1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–17. [DOI] [PubMed] [Google Scholar]
- 35.Lee JL, Sinnathurai P, Buchbinder R, Hill C, Lassere M, March L. Biologics and cardiovascular events in inflammatory arthritis: a prospective national cohort study. Arthritis Res Ther. 2018;20(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ Cardiovasc Imaging. 2018;11(6):e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinhart CR, Emons MF. Risks of cardiovascular disease in patients receiving antiretroviral therapy for HIV infection: implications for treatment. The AIDS reader. 2004;14(2):86–90, 3–5. [PubMed] [Google Scholar]
- 38.Botkin CD, Osman MM. Prevalence, challenges, and solutions for (18)F-FDG PET studies of obese patients: a technologist’s perspective. Journal of nuclear medicine technology. 2007;35(2):80–3. [DOI] [PubMed] [Google Scholar]
- 39.Bucerius J, Mani V, Moncrieff C, Machac J, Fuster V, Farkouh ME, et al. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: the impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. European journal of nuclear medicine and molecular imaging. 2014;41(2):369–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surti S Update on time-of-flight PET imaging. J Nucl Med. 2015;56(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR American journal of roentgenology. 2008;190(2):W151–6. [DOI] [PubMed] [Google Scholar]