Abstract
Previous position papers have confirmed to varying degrees associations between periodontal microbes and respiratory tract infections such as nosocomial or hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), and chronic obstructive pulmonary diseases (COPD). Causal relationships have not been confirmed and have been the source of much confusion for the medical and oral health professions.
Aim
To investigate whether sufficient evidence exists for a causal relationship between periodontal microbes and respiratory diseases, with a focus on HAP and VAP.
Methods
The PICO question was “For patients in hospitals, nursing homes or long-term care facilities who are at high risk for respiratory infections, will an oral care intervention such as toothbrushing, administration of antimicrobial agents, and/or professional care, as compared to no oral care intervention (or usual oral care) reduce the risk for respiratory infections?” Only systematic reviews (SRs) with or without a meta-analysis (MA) of randomized controlled trials published in the English language between 2007 and 2019 were included. Databases searched included PubMed, MEDLINE, EbscoHost, CINAHL, Scopus, Cochrane Registry of Systematic reviews, and Clinical Trials Registry. Quality assessments were conducted by both authors using the PRISMA checklist. The Bradford Hill criteria were used to determine evidence for causality.
Results
Of 47 respiratory studies retrieved, after elimination of duplicates and studies not meeting inclusion criteria, 10 SRs were selected, 9 of which included MAs. Although there was evidence that administration of chlorhexidine gluconate (CHX) reduced the risk for VAP, none existed for HAP. Limitations included inconsistencies among studies in population groups, CHX concentration, frequency of administration, number of applications, and insufficient evidence for use of povidone iodine or toothbrushing in ventilated patients. While some studies reported other patient-centred outcomes (i.e., ICU mortality, length of ICU stay or duration of mechanical ventilation), findings were positive only for cardiac surgery ventilated patients, who did not meet the inclusion criteria.
Conclusions
Bradford Hill criteria analysis failed to support a causal relationship between periodontal microbes/oral health care and respiratory diseases such as pneumonia.
Keywords: antiseptics, periodontal disease, COPD--oral care, meta-analysis, oral health, periodontal treatment, periodontitis, pneumonia, respiratory diseases, systematic reviews, VAP, VAP prevention
Abstract
Les exposés de position précédents ont confirmé à des degrés différents les associations entre les microbes parodontaux et les infections des voies respiratoires telles que la pneumonie nosocomiale ou de contamination hospitalière (PCH), la pneumonie sous ventilation assistée (PVA) et les maladies pulmonaires obstructives chroniques (MPOC). Les relations de cause à effet n’ont pas été confirmées et ont été la source de beaucoup de confusion pour les professions médicales et de santé buccodentaire.
Objectif
Déterminer s’il existe suffisamment de preuves qu’une relation de cause à effet existe entre les microbes parodontaux et les maladies respiratoires, en mettant l’accent sur la PCH et la PVA.
Méthodologie
La question de PICO était : « Chez les patients hospitalisés, en maisons de soins infirmiers ou en établissement de soins de longue durée qui sont à risque élevé de subir des infections respiratoires, le fait d’obtenir une intervention de soins buccodentaires telle que le brossage dentaire, l’administration d’agents antimicrobiens ou de soins professionnels, par rapport à ne pas obtenir une intervention de soins buccodentaires (ou des soins buccodentaires habituels) réduira-t-il le risque d’infections respiratoires? » Seules les revues systématiques (RS) avec ou sans méta-analyse (MA) d’essais contrôlés randomisés, publiées en anglais entre 2007 et 2019, ont été comprises. Les bases de données consultées comprenaient PubMed, MEDLINE, EbscoHost, CINAHL, Scopus, le Registre de revues systématiques Cochrane, et le Registre des essais cliniques. Les évaluations de la qualité ont été menées par les 2 auteurs à l’aide de la liste de vérification PRISMA. Les critères de Bradford Hill ont été utilisés pour déterminer les preuves de causalité.
Résultats
Sur les 47 études respiratoires relevées, après élimination des doublons et des études ne répondant pas aux critères d’inclusion, 10 RS ont été sélectionnées, dont 9 comprenaient des MA. Bien que des preuves existaient que l’administration de gluconate de chlorhexidine (CHG) avait réduit le risque de PVA, il n’en existait aucune pour les PCH. Les limites comprenaient des incohérences parmi les études auprès des groupes de population, la concentration de CHG, la fréquence d’administration, le nombre d’applications, et l’insuffisance de preuves pour l’utilisation de povidone-iodine ou de brossage dentaire chez les patients ventilés. Bien que certaines études aient fait état d’autres résultats centrés sur le patient (p. ex., mortalité en USI, durée du séjour en USI ou durée de la ventilation mécanique), les résultats n’étaient positifs que pour les patients de chirurgie cardiaque ventilés qui ne répondaient pas aux critères d’inclusion.
Conclusion
L’analyse des critères de Bradford Hill a échoué à soutenir un lien de cause à effet entre les microbes parodontaux ou les soins de santé buccodentaire et les maladies respiratoires telles que la pneumonie.
CANADIAN DENTAL HYGIENISTS ASSOCIATION POSITION STATEMENT .
The Canadian Dental Hygienists Association acknowledges that, although associations between periodontal and respiratory diseases such as pneumonia (VAP and NV-HAP) have been well established, there is insufficient evidence that periodontal microbes cause these diseases.
INTRODUCTION
This position paper is the third in a series reviewing the state of the evidence of a causal relationship between periodontal disease and a systemic condition, in this case respiratory diseases. Because each of these papers forms the evidence for Canadian Dental Hygienists Association (CDHA) position statements, the information about causality in each paper’s introduction section is essentially the same. It is important to clarify this concept for those who may not be familiar with or have the background to distinguish the difference between associations and causality, or for those who may not have read the earlier papers published in previous issues of this journal.
Relationships between periodontal disease/inflammation and a number of systemic diseases have been proposed since the late 1800s when physicians speculated that bacteria from the mouth caused everything from brain abscesses to arthritis.1, 2 With the onset of “ periodontal medicine ” in the early 1990s, studies investigating the relationships between numerous oral and systemic conditions have increased, with inflammation now recognized as a common factor. Despite the amount of research published over the last 30 years, questions remain about the exact nature of these relationships. While relationships may be in the form of associations or correlations, they should not be assumed as causal.
Unfortunately, the differences between associations and causality are not well understood, and the terms are often used interchangeably. A relationship merely describes how 2 variables might somehow be related or connected to each other. For instance, lung cancer rates are higher for people without a postsecondary education (who tend to smoke more), but that does not mean that someone can reduce his or her cancer risk just by getting a college or university education.3 An “ association ” refers to “ a relationship between an exposure (or a characteristic) and a disease that is statistically dependent; that is, the presence of one alters the probability of observing the presence of the other. An as,sociation is a necessary condition of a causal relationship, but not all associations are causal. If there is no association, the variables are said to be independent.”4
In order for a relationship to be coined as “causal,” actual “cause and effect” must be determined through a very rigorous set of epidemiological criteria. One must be able to state with certainty that a specific exposure has been shown to cause a specific outcome.4 Randomized clinical trials (RCTs) provide the strongest evidence for demonstrating cause and effect, rather than the outcome happening by chance. These experimental studies are the most methodologically challenging and ones in which the researcher controls or manipulates the variables (i.e., the intervention, its timing and dose) under investigation, such as in testing the effectiveness of a treatment, as compared to another treatment or a placebo.5
Often, when clinicians read a research article that is reporting a correlation or an association between an oral disease and a particular outcome of interest, they automatically, and incorrectly, jump to the conclusion that the relationship is causal. Prime examples of such misinterpretations are frequently found with proposed oral–systemic linkages, such as the assumption that periodontitis is one cause of heart disease or of adverse pregnancy outcomes, or that stress causes periodontitis. It is important for clinicians to understand that correlations and associations do not imply or equal causality. In fact, incorrect assumptions of causality are a major public health concern. From a public health perspective, any evidence should not be considered causal unless it has gone through very rigorous scrutiny using standard public health guidelines such as the Bradford Hill criteria for causality6 (Table 1).
CDHA published position papers on oral–systemic linkages in 20047,8 , followed by updates in 20069 and 200710 with similar outcomes, reporting associations between periodontal disease and several systemic diseases. In particular, these papers identified strong evidence for an association between pneumonia and health-compromised seniors living in nursing homes and chronic care facilities.8
A recent systematic mapping of registers of clinical research trials conducted on periodontal medicine revealed 57 conditions that are currently hypothesized to be linked with periodontal diseases.11 While it is beyond the scope of this current series of position papers to explore all of these proposed linkages, the status of 10 of these hypotheses will be evaluated in 5 position papers written by the same authors. The first 2 papers in this series analysed the state of the evidence of a causal relationship between periodontal disease and cardiovascular diseases12 and between periodontal disease and adverse pregnancy outcomes13 . This third paper focuses on the evidence related to whether a causal relationship exists between periodontal microbes and respiratory diseases, with an emphasis on pneumonia, both ventilator-associated pneumonia (VAP) and hospital-acquired pneumonia (HAP)—more recently termed non-ventilator hospital-acquired pneumonia (NV-HAP). Chronic obstructive pulmonary diseases (COPD) were excluded from this paper as the evidence of an association reported in the literature was weak and search results did not reveal any systematic reviews (SRs) or meta-analyses (MAs) on the topic.
Should you choose to read any of the individual SRs or the research articles discussed within them, you will come across the terms relative risk (RR), absolute risk ratio (ARR), numbers needed to treat (NNT), heterogeneity, and the symbol I 2 . The following are some basic definitions of each term.
Table 1.
The Bradford Hill criteria for causality6
|
Criteria |
Meaning |
|
Strength of association |
A strong association is more likely to have a causal component than is a modest association. Strength of the association is determined by the types of existing studies. The highest level studies from the evidence pyramid would represent the strongest associations (i.e., RCTs and systematic reviews with meta-analyses) Results from these studies must demonstrate an odds ratio or relative risk of at least 2.0 or above in order to be meaningful. Anything between 1 and 2 is weak while >2 is moderate and >4 is considered strong. |
|
Consistency |
A relationship is repeatedly observed in all available studies. |
|
Specificity |
A factor influences specifically a particular outcome or population. The more specific an association between a factor and an effect, the greater the probability that it is causal. |
|
Temporality |
The cause must precede the outcome it is assumed to affect (e.g., smoking before the appearance of lung cancer). Outcome measured over time (longitudinal study). |
|
Biological gradient (dose-response) |
The outcome increases monotonically with increasing dose of exposure or according to a function predicted by a substantive theory (e.g., the more cigarettes one smokes, the greater the chance of the cancer occurring). |
|
Plausibility |
The observed association can be plausibly explained by substantive matter (i.e., biologically possible). |
|
Coherence |
A causal conclusion should not fundamentally contradict present substantive knowledge. (Studies must not contradict each other.) |
|
Experiment |
Causation is more likely if evidence is based on randomized experiments or a systematic review of randomized experiments. However, these RCTs may not be ethically possible and thus prospective rather than experimental studies, such as cohort studies, may be the highest level of evidence available. |
|
Analogy |
For analogous exposures & outcomes an effect has already been shown (e.g., effects first demonstrated on animals or an effect previously occurring on humans such as the effects of thalidomide on a fetus during pregnancy). |
Source: Lavigne SE. From Evidence to Causality: How Do We Determine Causality? [Online course]. 2018. Available from: www.dentalcare.com/en-us/professional-education/ce-courses/ce530
Relative risk (RR): the ratio of the probability of an event occurring (e.g., developing a disease, preventing a negative outcome) in an exposed group to the probability of the event occurring in a comparison, non-exposed group (B/A). A RR >1 indicates a positive benefit and a RR <1 indicates a negative risk.
Absolute risk ratio (ARR): the arithmetic difference between two rates, i.e., an event occurring in an exposed group minus the event occurring in a comparison, non-exposed group (A – B).
Numbers needed to treat (NNT): the number of clients (or teeth, surfaces, periodontal pockets, pneumonia) that need to be treated with the experimental treatment or intervention in order to have one additional client (or tooth, surface, periodontal pocket, pneumonia) benefit, or to prevent one adverse outcome. NNT is calculated as 1/ARR.
Heterogeneity: any variability or differences among studies brought together in a systematic review, such as in the intervention regimens or protocols (e.g., different concentrations of CHX); different delivery mechanisms (e.g., rinse, gel or foam); different frequency of application (e.g., once/day, twice/day or 3x/daily), and its study outcomes. SRs need ways to assess the variability in order to make decisions about pooling data or making comparisons.14
I 2 : the percentage of variation across studies that is due to heterogeneity rather than chance. This statistic is used to quantify inconsistency among studies in a meta-analysis. It often is found on forest plots displaying the results of a meta-analysis. A rough guide for interpretation is as follows:14
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
The purpose of this series of updated position papers is to review the research undertaken since the publication of the last CDHA position papers in 2006 and early 2007 on these proposed relationships. Unlike the methodology used for the previous position papers and updates, this series of investigations is targeted more specifically at identifying whether the evidence has evolved from one of association to one of actual causality. In order to establish a causal relationship, interventional studies are required, thus only the highest levels of evidence have been sought for these updates. This position paper is the third in the series and investigates whether a causal relationship exists between periodontal microbes and respiratory diseases.
METHODOLOGY
The overarching PICO question explored in this series of position papers was customized for this paper on respiratory diseases, specifically pneumonia: “ For patients in hospitals, nursing homes or long-term care facilities who are at high risk for respiratory infections ( Population ), will an oral care intervention such as toothbrushing, administration of antimicrobial agents, and/or professional care ( Intervention ), as compared to no oral care intervention (or usual oral care) ( Comparison group ), reduce the risk for respiratory infections?( Outcome )”
Eligibility criteria
Both authors independently searched the literature, limiting the search to SRs with or without MAs of intervention studies using the inclusion and exclusion criteria described in Table 2 . SRs and MAs of observational studies were excluded.
Table 2.
Inclusion and exclusion criteria
|
Inclusion criteria |
Exclusion criteria |
|
Published between 2007 and 2019 |
Published before 2007 |
|
English language |
Languages other than English |
|
SRs with or without MAs of RCTs |
Abstracts, posters, conference proceedings, editorials or commentaries, duplicate studies, narrative reviews, RCTs, observational studies/both cohort and case-control and systematic reviews of observational studies and/or case-control studies. |
|
Studies involving humans |
Animal studies (in vivo, ex-vivo) and in vitro studies |
Search strategy
Databases searched were PubMed, MEDLINE, EbscoHost, CINAHL, Scopus, Cochrane Registry of Systematic Reviews, and Clinical Trials Registry (clinicaltrials.gov). Additionally, bibliographies of retrieved articles were hand searched for further relevant SRs and MAs and added when appropriate.
Keywords used for each search were as follows: respiratory diseases; pneumonia; COPD, oral care; antiseptics, periodontal disease; periodontitis; periodontal treatment; oral health; VAP; VAP prevention; AND systematic reviews; meta-analysis
Search strategies (limited to publications after 2007 and in the English language) were carried out according to the conventions required by each database. Within the same database, multiple strategies were used. For example, searches within PubMed were as follows:
(periodontal disease OR periodontitis) AND (VAP) AND systematic reviews
(periodontal disease OR periodontitis) AND (ventilator-associated pneumonia) AND systematic reviews
(periodontal disease OR periodontitis) AND (VAP) and the filter “Article Type,” which provides check-off boxes including one for SRs and another for MAs
Study selection
Titles and abstracts of all articles retrieved using the specified inclusion criteria were screened independently by both authors. Their choices were then discussed to arrive at a consensus regarding their suitability for full-text reading. The selected articles were then independently reviewed, and consensus reached on their inclusion or exclusion.
Quality assessment
The methodological quality of the selected SRs and MAs was assessed blindly by both authors using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist tool,15 available at www.prismastatement.org. Where inconsistencies occurred, scores were compared and discussed to reach consensus.
Data extracted
Information extracted from each selected SR or MA was compiled and presented in table format: year published, number of RCTs included, country of origin, methods used for assessing risk of bias, heterogeneity, outcomes measured, and conclusions of the findings.
RESULTS
Forty-seven (47) SRs were retrieved from database searches and articles identified within these reviews. After eliminating duplicates, summaries of studies, and articles that did not meet the inclusion criteria, 10 studies16-25 remained eligible for review, 9 of which included MAs.16-22,24,25 A flow diagram ( Figure 1 ) illustrates the details of the selection process; Table 3 reports the reasons for elimination of full-text articles.
Figure 1.
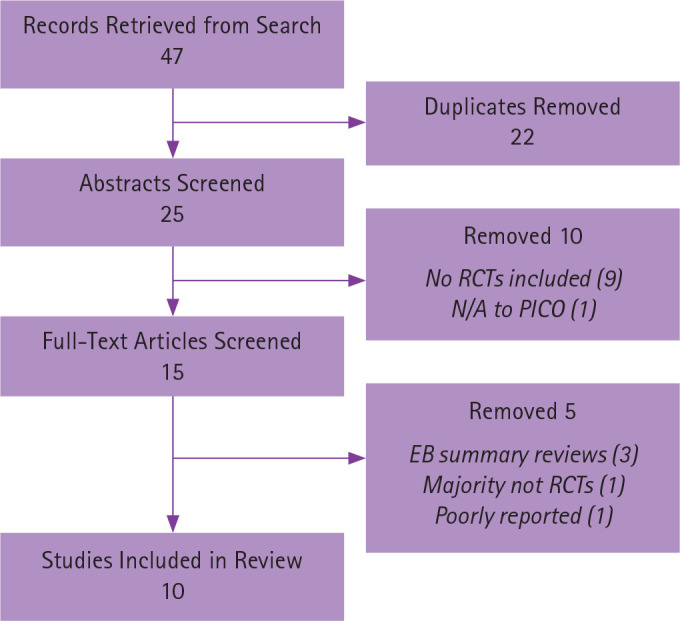
Respiratory diseases search flow diagram
Results of the quality appraisal of the 10 included SRs and MAs are shown in Table 4 . Based on the PRISMA checklist’s 27 items, scores ranged from 17 to 25. Agreement between the 2 independent evaluators was close to 100%, with a few scores being off by only 1 or 2 points. The quality of the systematic reviews was generally moderate to high, although 1 review did not report risk of bias19 and 1 review did not include a quality assessment tool.18
Of the 10 included SRs and MAs, 7 were specific to prevention of VAP while 3 studies addressed both NV-HAP and NV-Nursing Home Acquired Pneumonia (NV-NHAP). The majority of these SRs showed mixed results for a variety of reasons. Weaknesses identified by the systematic review authors included different study designs, methodology, settings, mixed populations and interventions, along with the quality of reporting, and lack of power calculations ( Table 5 ). Of the VAP SRs, most of the included studies investigated the effects of chlorhexidine (CHX) and/or povidone iodine on reducing the incidence of VAP. Three reviews included the effects of both manual and power toothbrushing on reduction of VAP. In the 3 NV-HAP/NHAP SRs and MAs, a variety of interventions were included, such as professional oral care, toothbrushing by professionals, toothbrushing by staff, application of antimicrobial agents such as CHX, and povidone iodine brushing of the pharynx. Control groups in most studies were either usual care or placebo with the majority being usual care, which could comprise several interventions. Detailed results are presented in Tables 6 and 7.
DISCUSSION
It has been well established for over 2 decades that a relationship exists between periodontally associated microbes and respiratory infections such as pneumonia.7-10,33,35 The purpose of this umbrella review was to take this knowledge one step further to determine if new evidence exists to establish the nature of this relationship as causal. The relationship is based on the premise that oropharyngeal microorganisms are aspirated into the lower respiratory tract, colonize in the lungs, and develop pneumonia.26 A search of the scientific literature revealed that the most common diseases for which there are high-level research studies (RCTs) are hospital-acquired pneumonia (HAP/NV-HAP), nursing home-acquired pneumonia (NHAP/NV-NHAP), and ventilator-associated pneumonia (VAP). There is a lack of high-quality studies related to COPD and, thus, this relationship was not included in this umbrella review.
The occurrence of nosocomial pneumonia in health care facilities and nursing homes is a major public health concern. It is one of the major causes of mortality in nursing homes27; the prevalence of VAP is 8% to 28%28 in mechanically ventilated hospital patients. Systematic reviews of the individual RCTs focused on different oral hygiene procedures delivered by different people such as oral health professionals, hospital staff, nursing home and long-term care staff, and individual patients or residents. Among the different procedures and products employed were antiseptics at different strengths and frequencies, with CHX being the most common intervention. In fact, regular oral care with CHX gluconate in hospitals has become the gold standard of care for the prevention of VAP in many countries in both North America and Europe.19 Some studies investigated the effects of toothbrushing on the prevention of nosocomial pneumonia, while others examined various interventions and strategies, ranging from professional oral care to toothbrushing by professionals and caregivers along with the application of a variety of antimicrobial agents.
Several of the VAP studies had mixed populations: some ventilated patients had had cardiovascular surgery while others were critically ill patients in intensive care units (ICU). The inclusion of cardiac surgery patients is problematic as they do not fit the definition for VAP which is “pneumonia developing in people who have receive mechanical ventilation for at least 48 hours.”17 Cardiac surgery patients typically are intubated in the operating room and are extubated within one day. Thus, any pneumonia that they would be susceptible to would be nosocomial in nature, not ventilator associated.
Results from these mixed studies are confounded by the lack of focus on patient-centred outcomes, such as the effects of these various strategies on mortality, length of ventilation, and length of stay in ICUs. Despite this lack of focus on patient-centred outcomes, some SRs did report that the intervention administered had no effect on mortality, duration of mechanical ventilation or duration of ICU stay. Conversely, Klompas et al.19 reported an increase in mortality among non-cardiac surgery patients in a meta-analysis of 12 RCTs, 9 of which included non-cardiac surgery patients randomized to receive CHX. Interestingly, this was not the case when compared with the 3 cardiac surgery studies in the review, where no effect on mortality was found among those using CHX. The authors proposed 2 potential explanations for these findings, the first being the possibility that patients may have inadvertently aspirated small amounts of CHX causing acute lung injury. The other explanation suggested use of CHX may have masked the actual diagnosis of pneumonia, resulting in false negative VAP tests precluding early antibiotic intervention.19 This second explanation is plausible as a Canadian study by Muscedere et al.29 demonstrated that culture-negative VAP diagnoses had higher mortality rates than culture-positive VAP. Although these mortality results from the Klompas et al.19 SR/MA were not statistically significant, the authors argued in favour of re-evaluating both the safety and efficacy of CHX for mechanically ventilated non-cardiac surgery patients.
Table 3.
Screened respiratory articles included and deleted
|
|
Author & Year |
Included |
Deleted |
Reason for deletion/Notes |
|
1. |
Agado & Bowen 201233 SR pneumonia or COPD |
|
XX |
RCTs included along with other studies; outcome association vs. causal |
|
2. |
Astvaldsdóttir et al. 201834 Knowledge |
|
XX |
Focus on knowledge vs. causal |
|
3. |
Azarpazhooh & Leake 200635 SR of association between respiratory diseases and oral health |
|
XX |
Association investigated vs. not a causal relationship; too old |
|
4. |
Cagnani et al. 201636 SR aspiration pneumonia |
|
XX |
No RCTs included in SR (case studies, cohort; lots of weaknesses) |
|
5. |
Gomes-Filho et al. 202037 SR/MA |
|
XX |
No RCTs included in SR |
|
6. |
Gu et al. 201216 SR/MA toothbrushing |
XX |
|
|
|
7. |
Hua et al. 201617 Cochrane, SR/MA VAP; OH care |
XX
|
|
|
|
8. |
Veitz-Keenan & Ferraiolo 201738 Summary review of Hua |
|
XX |
Summary review of Hua (which is included) |
|
9. |
Kaneoka et al. 201518 SR/MA NV-HAP w/o mechanical ventilation |
XX |
|
|
|
10.
|
Klompas et al. 201419 SR/MA VAP Reappraisal of routine use of CHX |
XX |
|
|
|
11. |
Labeau et al. 201120 SR/MA VAP-CHX, P-Iodine |
XX
|
|
|
|
12. |
Li et al. 201521 SR/MA Antiseptics prevention of VAP |
XX |
|
|
|
13. |
Liu et al. 201822 Cochrane, SR/MA Prevention nursing home-acquired pneumonia |
XX
|
|
|
|
14. |
Mitchell et al. 201939 SR NV-HAP |
|
XX |
Only 6 of 15 included studies are RCTs; no separate analysis of the 6 |
|
15. |
Scannapieco 201440 COPD summary review of Peter |
|
XX |
Summary review and no RCTs in the Peter study (case-control study) |
|
16. |
Sjögren et al. 200823 SR/MA OH effect pneumonia & respiratory diseases |
XX
|
|
|
|
17. |
Sjögren et al. 201641 SR/MA Older with pneumonia in hospitals or nursing homes |
|
XX |
Poorly reported; poor definition of groups and terms |
|
18. |
Shi et al. 201324 SR/MA, VAP, CHX |
XX
|
|
|
|
19. |
Richards 201342 EBD review of Shi |
|
XX |
Summary review of Shi (which is included)
|
|
20. |
Spreadborough et al. 201643 VAP
|
|
XX |
No RCTs included in SR |
|
21. |
van der Maarel-Wierink et al. 201144
|
|
XX |
No RCTs included in SR |
|
22. |
Villar et al. 201625 SR/MA, VAP (CHX)
|
XX |
|
|
|
23. |
Zeng et al. 201245 MA, COPD |
|
XX |
No RCTs included in SR
|
|
24. |
Zeng et al. 201646 Lung CA risk |
|
XX |
No RCTs included in SR Risk of lung CA association |
|
25. |
Zhou et al. 201147 COPD, quality of life |
|
XX |
N/A to PICO and no RCTs in SR |
Table 4.
Quality appraisal and summary of the systematic reviews/meta-analyses (n = 10)
|
Author (Country) |
PRISMA score (max 27) |
Heterogeneity |
Risk of bias |
Quality assessment instrument |
Number of RCTs & subjects; outcome measure |
Included meta-analysis |
|
Gu et al. 201216 (China) |
24 |
I2 = 61.1% |
Unclear risk of bias |
Jadad scale
CHBa |
4 RCTs, 828 subjects; toothbrushing, prevention of VAP |
Yes
|
|
Hua et al. 201617 (UK) Cochrane |
25 |
I2 = varies with different comparisons |
Low-quality evidence |
CHBa |
38 RCTs, 6016 subjects; oral health care & incidence of VAP |
Yes |
|
Kaneoka et al. 201518 (US) |
22.5 |
I2 = 0% |
High risk of bias |
CHBa |
5 RCTs, 1009 subjects; oral care in preventing pneumonia in non-ventilated patients |
Yes |
|
Klompas et al. 201419 (US) |
23 |
I2 = 0% for cardiac surgeries I2 = 42% for non-cardiac surgery studies |
11 of 16 studies were high quality |
Assessed random sequence generation & allocation concealment only |
16 RCTs, 3630 subjects; CHX & incidence of VAP; compared cardiovascular surgery patient outcomes with non-cardiovascular surgery patient outcomes |
Yes |
|
Labeau et al. 201120 (Belgium) |
23 |
I2 = 29% for CHX; 67% for Povi-Iod |
Not reported |
Checklist from Dutch Cochrane |
14 RCTs, 2481 subjects; oral antiseptics & prevention of VAP |
Yes |
|
Li et al. 201521 (China)
|
23 |
I2 = 49.7% for CHX; 54.8% for Povi-Iod |
Begg's test revealed no publication bias; Jadad score 4 (high quality) |
Jadad scale
|
17 RCTs, 4249 subjects; oral antiseptics & prevention of VAP |
Yes |
|
Liu et al. 201822(China) Cochrane |
24 |
Cochrane's Q and I2 = 67%
|
Low to very low quality evidence
All studies identified as high risk of bias |
CHBa |
4 RCTs, 3905/3546 subjects; oral care & incidence of nursing home acquired pneumonia |
Yes |
|
Sjögren et al. 200823 (Sweden) |
17 |
No MA due to heterogeneity of study designs |
Jadad scores between 3 and 5 for 3 of the RCTs |
Jadad scale |
15, of which only 5 studied interventions; 10 were non-RCT |
No |
|
Shi et al. 201324 (China) Cochrane |
24.5 |
Chi2 and I2 |
High = 17, Low = 5, Unclear = 13 |
CHBa |
35 RCTs, 5374 subjects; oral care & prevention of VAP in ICUs |
Yes |
|
Villar et al. 201625 (Brazil) |
25 |
I2 = 45% |
High = 8, Low = 1, Unclear = 4 |
CHBa |
13 RCTs, 1640 subjects; incidence of VAP |
Yes |
aCHB (Cochrane Handbook for Systematic Reviews of Interventions)
Although several SRs and MAs produced significant results for reducing the incidence of VAP with CHX at 0.12%17,21,24 , 2 studies demonstrated only positive effects with 2% concentrations20,25 and/or with 4 times daily applications25 . Villar et al.25 failed to produce significant results for the oral application of CHX in VAP incidence. However, a subgroup analysis showed CHX at 2% concentrations as well as CHX administered 4 times daily to have a significant effect o,n reducing the incidence of VAP. Whereas the substantivity of CHX has been attributed to its presence in the oral cavity for >12 hours, its antimicrobial activity has been shown to last for only 7 hours after a mouthrinse.30,31 This could account for the better results for the 4 times daily application and/or the use of a higher concentration.
Li et al.21 had positive outcomes for CHX in reducing the incidence of VAP. However, the authors pointed out that more than half of the pooled study population in the MA were cardiosurgical patients, which they suggest could have influenced the results. In congruence with the Klompas et al.19 MA, subgroup analysis showed positive effects of the CHX to be most marked on the cardiac surgery patients ( p = 0.001).21
Table 5.
Summary of issues identified by authors of systematic reviews of RCTs
|
1. Inconsistency in defining or even including mention of periodontal disease status of study participants 2. Inconsistent definitions of VAP, HAP, and NHAP 3. Gold standard for diagnosing VAP not always used 4. Inconsistency in the type(s) of treatment(s) provided, i.e., timing, concentration of antimicrobial agents, frequency, clinician, use of antibiotics, mixing various interventions 5. Different settings and population groups 6. No mention of nursing or caregiver staff training re: providing oral care 7. Variation in outcomes measured and measurement technique used 8. No uniform methods for adjustment of confounders such as comorbidities and hospital treatment bundles for prevention of VAP 9. Comparison group in most studies was "usual care"--anything from toothbrushing to mouthrinsing--which could confound results. Only a few included studies used a placebo 10. Quality of studies (methodological shortcomings) and reporting 11. Publication bias: so few studies involved in some SRs that publication bias was not assessed 12. Lack of power calculations in studies 13. More consistent use of CONSORT in the RCTs would improve the quality of the studies |
Interestingly, results were not significant for combining CHX with toothbrushing despite positive results for application of CHX alone.24 These results were consistent with findings from the other 2 SRs/MAs16,17 involving toothbrushing for the prevention of VAP. These mixed results for the use of CHX are disappointing, particularly from a health policy perspective. Reducing the incidence of VAP using a measure such as CHX in addition to usual care, rather than treating it with systemic antibiotics, would be more cost effective and help to reduce the use of systemic antibiotics, which have become a major public health concern given the rise in antibiotic-resistant bacteria.32
The lack of clear results for the prevention of VAP with the application of CHX could be explained by the fact that decontamination with chlorhexidine is only one of several interventions performed in ICUs by the nursing staff and may not be the sole preventive measure. There is a specific “ ventilator bundle” that is used in all ICUs to promote better ventilator care and patient outcomes.25 This bundle includes elevation of the head of the bed; daily sedation vacations and assessment of readiness to extubate; peptic ulcer disease prophylaxis; deep vein thrombosis prophylaxis; oral decontamination with CHX; coordinating spontaneous breathing trials with spontaneous awakening trials; early mobilization; conservative fluid management; and low tidal wave utilization.19,25 These confounders may significantly interfere with study results. It is also possible that the prevention of VAP may not be exclusively related to CHX.
What is of major interest and also very surprising is that numerous studies did not report the periodontal status of the study participants. In the Villar et al.25 study, as an example, only 2 RCTs out of 13 included this information. This gap creates a huge problem when it comes to determining causality, particularly if it is unknown whether the patient even had periodontal inflammation, which would be the source of the microorganisms that have been hypothesized to initiate the pneumonia.
Using the Bradford Hill criteria for causation to determine whether a causal relationship exists between periodontal microbes and VAP/NV-HAP, it is clear that several criteria have not yet been satisfied. In examining the “ strength of association,” moderate evidence was presented by 4 of the 10 SRs and MAs for the use of CHX in lowering the risk for pneumonia, there was no evidence to support the use of povidone iodine, and there was weak to no evidence for the use of toothbrushing unless combined with professional oral care. The best evidence was for the use of CHX at a 2% concentration used 4 times daily. The criterion of “ consistency” was not met since numerous inconsistencies in findings were reported. This situation also leads one to question whether studies with negative outcomes were turned away resulting in publication bias. Similarly, the criterion of “ specificity,” which requires similar outcomes in every instance, has not been demonstrated. The criterion of “ temporality,” where periodontal disease would be required to precede the respiratory disease, has not been established in these 10 SRs and MAs, definitely weakening the cause and effect hypothesis. In fact, few studies in these reviews even mentioned the periodontal or oral inflammatory status of the patient prior to administration of the intervention, which is problematic. Without this information, it is impossible to determine whether the oral cavity was the source of the microbes that initiated the pneumonia.
When considering the criterion of “ dose–response,” none of the studies included in these SRs compared results with various magnitudes of periodontitis, demonstrating that those with more severe periodontal inflammation would be at greater risk for pneumonia. The criterion of “ biological plausibility,” however, has been met, since nu merous studies hypothesized that microorganisms from the oral cavity can serve as reservoirs for colonization and could be the source of infection travelling from the oral cavity to the lungs through aspiration. The criterion of “ coherence” also has been previously met as numerous laboratory, animal, and human studies have established that a relationship does indeed exist between periodontal microbes and respiratory infections such as pneumonia. The criterion of “ experiment ” was not met in this review. Although numerous RCTs were conducted and evaluated in these 10 SRs and MAs, the results were mixed, and no study determined that periodontal microbes were the source of the infection. “ Analogy,” the weakes t criterion, was not explored in this review. Thus, of the 9 criteria, only 2 (biological plausibility and coherence) can be said to have been fulfilled. Table 8 provides a summary of these results.
Table 6.
Primary outcomes of retained studies for ventilator-associated pneumonia (7 studies)
|
Interventions (CHX, Povidone Iodine, Toothbrushing) | ||
|
Chlorhexidine gluconate (CHX) | ||
|
Outcome 1 No relationship |
Outcome 2 Possible relationship (mixed results) |
Outcome 3 Positive relationship |
|
Klompas et al.19 No reductions in VAP with CHX
Villar et al.25 No overall reductions in VAP with CHX at 0.12% |
Labeau et al.20 Subgroup analysis favoured only the 2% CHX application; results for cardio surgery patients were stronger
For 0.12% CHX, risk reduction was not significant |
Villar et al.25 Only at 2% or administered 4x daily
Hua et al.17 CHX gel or mouthrinse as part of OHC reduced risk of VAP by 18%
Shi et al.24 Moderate evidence that CHX mouthrinse or gel as part of OHC reduced risk of VAP by 40%
Li et al.21 Overall oral care including CHX reduced risk of VAP but half the study group comprised cardiosurgical patients who do not qualify for VAP diagnosis, which may have influenced the results. |
|
Povidone iodine | ||
|
Outcome 1 No relationship |
Outcome 2 Possible relationship (mixed results) |
Outcome 3 Positive relationship |
|
Labeau et al.20 Effects not significant
Li et al.21 Effects not significant |
Shi et al.24 Weak evidence that Povidone Iodine is better than saline
Hua et al.17 Very weak evidence that Povidone Iodine is better than saline |
|
|
Toothbrushing | ||
|
Outcome 1 No relationship |
Outcome 2 Possible relationship (mixed results) |
Outcome 3 Positive relationship |
|
Hua et al.17 No effect of either manual or power toothbrushing on reductions in VAP
Shi et al. 24 No effect of either manual or power toothbrushing on reductions in VAP
Gu et al.16 Did not significantly reduce incidence of VAP, mortality or length of ICU stay or days on ventilator | ||
Based on this analysis, it is concluded that there is insufficient evidence at this time to support a causal relationship between periodontal microbes and nosocomial pneumonia .
In spite of these results, one must not assume that there is no relationship or association between periodontal microbes and respiratory infections, nor should these results negate the numerous studies showing strong associations. Results from this umbrella review demonstrate that the existing relationships cannot be determined to be “ causal ” given the evidence available. Based on this analysis, since there is both biological plausibility as well as coherence, it will be very important to continue to conduct better interventional studies that address some of the shortcomings identified in Table 7.
None of the current studies included periodontal instrumentation, such as scaling and root planing, targeting the elimination and/or control of periodontal disease itself. These studies only targeted the removal of microbes with mouthrinses and/or toothbrushing which cannot eliminate periodontal disease alone without mechanical treatment. Ideally, for a true effect, studies should be designed to eliminate periodontal inflammation at or prior to admission to the hospital or nursing home, and to ensure that oral hygiene for these individuals is maintained on a daily basis by staff and supported by intermittent professional maintenance appointments. However, these types of studies are difficult to design as it would be considered unethical to have a control group without any oral intervention compared with a test group that maximizes oral care to determine if they would be less likely to develop pneumonia than those with existing inflammation. No research ethics board would ever approve such as study, where the control group would receive no biofilm removal or daily plaque control, thus potentially making them more vulnerable to developing pneumonia. As a result, RCTs studying this subject tend to have a control group that receives what is coined as “ standard or usual care,” which could end up being quite different for each participant thus confounding the results.
Table 7.
Primary outcomes of retained studies for nosocomial pneumonia (3 studies)
|
Interventions (Professional oral care versus usual care) | ||
|
Outcome 1 No relationship |
Outcome 2 Possible relationship (mixed results) |
Outcome 3 Positive relationship |
|
|
Liu et al.22 Used too many combinations of interventions. Unable to draw any conclusions from the 4 included studies as to which of the various interventions actually worked. Follow-up studies should be at least 24 months. Usual care should be replaced with placebo. |
|
|
|
Kaneoka et al.18 Same as above. Too many combinations of interventions that included professional care and brushing in nursing homes sometimes with Povidone Iodine. In hospitals, used brushing and chlorhexidine. Pooled results for 4 studies showed positive effects but unable to differentiate which worked the best. |
Sjögren et al.23 Best results from weekly professional care, brushing after each meal, and scrubbing pharynx with Povidone Iodine. |
RCTs are necessary for satisfying the criterion of “ experiment ” to determine causality, but are extremely difficult to administer in hospitals and nursing homes due not only to the issue of ethics approval, as mentioned, but also to the level of illness of the individual patients or residents and to difficulties in obtaining consent, especially from individuals who may not have the cognitive ability to give such consent. These issues do not, however, negate the importance of ensuring good oral hygiene for this very vulnerable population group. More emphasis in hospitals and nursing homes must be placed on providing adequate oral hygiene care for their patients and residents. Dental hygienists can play a very important role in these institutional settings, and policies need to be improved to include regular oral care for these vulnerable individuals.
Table 8.
Bradford Hill criteria results
|
Bradford Hill criterion |
Met |
Not Met |
|
Strength of association |
|
X |
|
Consistency |
|
X |
|
Specificity |
|
X |
|
Temporality |
|
X |
|
Dose-response |
|
X |
|
Biological plausibility |
X |
|
|
Coherence |
X |
|
|
Experiment |
|
X |
|
Analogy |
|
N/A |
CONCLUSION
Based on findings from the 10 SRs and MAs investigated in this review, one can state with confidence that the answer to the proposed PICO question, “ For patients in hospitals, nursing homes or long-term care facilities who are at high risk for respiratory infections, will an oral care intervention such as toothbrushing, administration of antimicrobial agents, and/or professional care, as compared to no oral care intervention (or usual oral care) reduce the risk for respiratory infections?” is “ unclear .” Current evidence is inconsistent and overall does not support oral care interventions such as toothbrushing or the administration of 0.12% CHX or povidone iodine to reduce the rate of HAP/NV-HAP or VAP. However, there is some evidence that administration of CHX at a 2% concentration or delivered 4 times daily does reduce the incidence of VAP. Additionally, the incidence of pneumonia was significantly reduced among cardiac surgery patients who received various concentrations of CHX application. Since these cardiac surgery studies were often included in the same SRs and MAs for VAP as were non-cardiac surgery patients, they were identified by the authors as confounders. They were also the source of positive outcomes for the other ICU group of patients. It is important to remember that pneumonia occurring in ventilated cardiac surgery patients does not fall under the definition of VAP and thus should not be combined with other mechanically ventilated patient studies.
Numerous issues in these published studies may have influenced the results. Future studies will need to focus on correcting the inconsistencies, particularly by 1) identifying the extent of periodontal disease in the study population; 2) using standard case definitions for periodontal disease, VAP, and NV-HAP; 3) providing a better explanation of the type and frequency of the intervention and control (i.e., usual care); 4) ensuring consistency of the target population; and 5) using the CONSORT guidelines to improve the quality of RCTs.
Two previous CDHA position papers on this topic have established associations between periodontal inflammation and respiratory diseases but neither of those papers investigated a causal link. This position paper explored whether periodontal microbes were causally related to respiratory diseases, in particular ventilator-associated pneumonia (VAP) and nosocomial pneumonia (NV-HAP). The results of this paper provide clear evidence that, although associations have been established, no causal link exists between periodontal microbes and respiratory diseases at this time.
Although a causal relationship has not been established by this umbrella review, there is still substantial evidence of an association between periodontally associated microbes and respiratory diseases, particularly VAP and nosocomial pneumonia (NV-HAP). Pneumonia causes significant morbidity and mortality, particularly to individuals who are hospitalized or who reside in nursing homes and chronic care facilities. It also places a heavy burden on Canada’s health care system, because of its high treatment costs. Consequently, dental hygienists could have a significant impact on reducing health care costs by helping to address the oral hygiene concerns of this very vulnerable population group.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest
Acknowledgments
This position paper was funded by the Canadian Dental Hygienists Association. Both authors received an honorarium for this work. We wish to thank the CDHA Steering Committee for their valuable input and guidance throughout the development of this paper.
REFERENCES
- 1. Kumar PS. From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J Physiol 2017;595(2):465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller WD.The micro-organisms of the human mouth: The local and general diseases which are caused by them.Philadelphia (PA):SS White Dental Mfg Co;1890. [Google Scholar]
- 3.SUNY Downstate Health Sciences University. EBM Tutorial Guide to Research Methods [Internet] [cited 2019 July 19]. Available from: https://guides.downstate.edu/c.php?g=856794&p=6152125
- 4.Brunette DM. Causation, association and oral health–systemic disease connections. In: The oral systemic health connection, edited by Michael Glick. Chicago:Quintessence Publishing Co. Inc;2014. pp. 13–26. [Google Scholar]
- 5.Forrest JL, Miller SA. EBDM in action: Developing competence in EB practice.Colbert (WA):ebdLibrary;2016. [Google Scholar]
- 6. Hill AB. The environment and disease: Association or causation? Proc Royal Soc Med 1965;58:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lux J, Lavigne S. Your mouth—Portal to your body CDHA position paper on the links between oral and general health Part I. Probe 2004;38(4):114–134 [Google Scholar]
- 8. Lux J, Lavigne S. Your mouth—Portal to your body CDHA position paper on the links between oral and general health Part II. Probe 2004;38(4):155–171 [Google Scholar]
- 9. Lux J. Review of the oral disease–systemic disease link Part I: Heart disease, diabetes. Can J Dent Hyg 2006;40(6):288–302 [Google Scholar]
- 10. Lux J. Review of the oral disease–systemic disease link Part II: Preterm low birth weight babies, respiratory disease. Can J Dent Hyg 2007;41(1):8–21 [Google Scholar]
- 11. Monsarrat P, Blaizot A, Kémoun P, Ravaud P, Nabet C, Sixou M, Vergnes J-N. Clinical research activity in periodontal medicine: a systematic mapping of trial registers. J Clin Periodontol 2016;43:390–400 doi: 10 1111/jcpe 12534 [DOI] [PubMed] [Google Scholar]
- 12. Lavigne SE, Forrest JL. An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and cardiovascular diseases: Position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg 2020;54(1):32–41 [PMC free article] [PubMed] [Google Scholar]
- 13. Lavigne SE, Forrest JL. An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and adverse pregnancy outcomes: Position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg 2020;54(2):92–100 [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan R, Cochrane Consumers and Communication Review Group. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: Planning the analysis at protocol stage. London (UK): Cochrane Consumers and Communication; December2016 [cited 2020 May 7]. Available from: http://cccrg.cochrane.org
- 15. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, The PRISMA -DTA Group(2018) Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA Statement. JAMA 2018 Jan 23;319(4):388–396 doi: 10 1001/jama 2017 19163 [DOI] [PubMed] [Google Scholar]
- 16. Gu WJ, Gong Y-Z, Pan L, Ni Y-X, Liu J-C. Impact of oral care with versus without toothbrushing on the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2012;16:R190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia [Review]. Cochrane Database Syst Rev 2016;(10):Art No :CD008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaneoka A, Pisegna JM, Miloro KV, Lo M, Saito H, Riquelme LF, et al. Prevention of healthcare-associated pneumonia with oral care in individuals without mechanical ventilation: a systematic review and meta-analysis of randomized controlled trials. Infect Control Hosp Epidemiol 2015;36(8):899–906 [DOI] [PubMed] [Google Scholar]
- 19. Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation Systematic review and meta-analysis. JAMA Intern Med 2014;174(5)751–61 [DOI] [PubMed] [Google Scholar]
- 20. Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI. Prevention of ventilator-asssociated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis 2011;11:845–54 [DOI] [PubMed] [Google Scholar]
- 21. Li L, Ai Z, Li L, Zheng X, Jie L. Can routine oral care with antiseptics prevent ventilator-associated pneumonia in patients receiving mechanical ventilation? An update meta-analysis from 17 randomized controlled trials. Int J Clin Exp Med 2015;8(2):1645–1657 [PMC free article] [PubMed] [Google Scholar]
- 22. Liu C, Lin J, Ng L, Needleman I, Walsh T, Li C. Oral care measures for preventing nursing home-acquired pneumonia (Review). Cochrane Database Syst Rev 2018;9:Art No :CDO12416 [Google Scholar]
- 23. Sjögren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: Effect estimates and methodological quality of randomized controlled trials. J Am Geriatr Soc 2008;56:2124–2130 [DOI] [PubMed] [Google Scholar]
- 24. Shi Z, Xie H, Wang P, Zhang Q, Wu Y, Chen E, Ng L, Worthington HV, Needleman I, Furness S. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia (Review). Cochrane Database Syst Rev 2013;(8):Art No :CD008367 [DOI] [PubMed] [Google Scholar]
- 25. Villar CC, Pannuti CM, Nery DM, Morillo CMR, Carmona MJ, Romito GA. Effectiveness of intraoral chlorhexidine protocols in the prevention of ventilator-associated pneumonia: meta-analysis and systematic review. Respir Care 2016;61(9):1245–1259 [DOI] [PubMed] [Google Scholar]
- 26.Bergmans D, Bonten M. Healthcare-associated pneumonia. In: in Mayhall CG. Hospital epidemiology and infection control. 4th ed.Philadelphia (PA):Lippincott Williams and Wilkins;2011:311–316. [Google Scholar]
- 27.Public Health Agency of Canada: Life and Breath: Respiratory Disease in Canada [Internet] 2007 [modified 2012 July 26]. Available from: https://www.canada.ca/en/public-health/services/reports-publications/2007/life-breath-respiratory-disease-canada-2007.html Accessed 2020-05-31.
- 28. Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867–903 [DOI] [PubMed] [Google Scholar]
- 29. Muscedere JG, McCall C, Shorr A, Jiang X, Marshall J, Heyland DK, Canadian Critical Care Trials Group Determinants of outcome in patients with a clinical suspicion of VAP J Crit Care 2008;23(1):41–49 [DOI] [PubMed] [Google Scholar]
- 30. Addy M, Jenkins S, Newcombe R. The effect of some chlorhexidine-containing mouthrinses on salivary bacterial counts. J Clin Periodontol 1991;18(2):90–93 [DOI] [PubMed] [Google Scholar]
- 31. Harper PR, Milsom S, Wade W, Addy M, Oran J, Newcombe RG. An approach to efficacy screening of mouthrinses: studies on a group of French products (II): inhibition of salivary bacteria and plaque in vivo. J Clin Periodontol 1995;22(9):723–727 [DOI] [PubMed] [Google Scholar]
- 32. Glyssens IC. Antibiotic policy. Int J Antimicrobial Agents 2011;38Suppl:11–20 [PUBMED:22018989] [DOI] [PubMed] [Google Scholar]
- 33. Agado B, Bowen D. Periodontal disease and respiratory disease: A systematic review of the evidence. Can J Dent Hyg 2012;46(2):103–114 [Google Scholar]
- 34. Ástvaldsdóttir A, Boström A, Davidson T, Gabre P, Gahnberg L, Englund GS, et al. Oral health and dental care of older persons—A systematic map of systematic reviews. Gerodontology undefined2018;35:290–304 [DOI] [PubMed] [Google Scholar]
- 35. Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol 2006;77:1465–1482 [DOI] [PubMed] [Google Scholar]
- 36. Cagnani A, Barros AM, Sousa LLA, Zanin L, Bergamaschi CC, Peruzzo DC, Flório FM. Periodontal disease as a risk factor for aspiration pneumonia: a systematic review. Bioscience J 2016;32(3):813–882 [Google Scholar]
- 37. Gomes-Filho IS, Seixas da Cruz S, Trindade SC, Passos-Soares JS, Carvalho-Filho PC, Godoy Figueiredo ACM, et al. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Diseases 2020;26(2):439–446 [DOI] [PubMed] [Google Scholar]
- 38. Veitz-Keenan A, Ferraiolo DM. Oral care with chlorhexidine seems effective for reducing the incidence of ventilator-associated pneumonia Summary review. Evid Based Dent 2017;18:113–114 doi:10 1038/sj ebd 6401272 [DOI] [PubMed] [Google Scholar]
- 39. Mitchell BG, Russo PL, Cheng AC, Stewardson AJ, Rosebrock H, Curtis SJ, Robinson S, Kiernan M. Strategies to reduce non-ventilator-associated hospital-acquired pneumonia: A systematic review. Infect Dis Health 2019;24:229–39 [DOI] [PubMed] [Google Scholar]
- 40. Scannapieco F. Individuals with chronic obstructive pulmonary disease (COPD) may be more likely to have more severe periodontal disease than individuals without COPD Summary review. J Evid Base Dent Pract 2014;14:79–81 [DOI] [PubMed] [Google Scholar]
- 41. Sjögren P, Wårdh I, Zimmerman M, Almståhl A, Wikström, M Oral care and mortality in older adults with pneumonia in hospitals or nursing homes: Systematic review and meta-analysis. J Am Geriatr Soc 2016;64:2109–2115 [DOI] [PubMed] [Google Scholar]
- 42. Richards D. Oral hygiene regimes for mechanically ventilated patients that use chlorhexidine reduce ventilator-associated pneumonia Summary review. Evid Based Dent 2013;14:91–92 doi:10 1038/sj ebd 6400957 [DOI] [PubMed] [Google Scholar]
- 43. Spreadborough P, Lort S, Pasquali S, Popplewell M, Owen A, Kreis I, et al. A systematic review and meta-analysis of perioperative oral decontamination in patients undergoing major elective surgery. Perioper Med 2016;5:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Maarel-Wierink CD, Vanobbergen JNO, Bronkhorst EM, Schols JMGA, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc 2011;12:344–54 [DOI] [PubMed] [Google Scholar]
- 45. Zeng XT, Tu ML, Liu DY, Zheng D, Zhang J, Leng W. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLoS One 2012;7(10):e46508 doi: 10 1371/journal pone 0046508 Epub 2012 Oct 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng X-T, Xia L-Y, Zhang Y-G, Li S, Leng W-D, Kwong JSW. Periodontal disease and incident lung cancer risk: a meta-analysis of cohort studies. J Periodontol 2016;87(10):1158–1164 [DOI] [PubMed] [Google Scholar]
- 47. Zhou X, Wang Z, Song Y, Zhang J, Wang C. Periodontal health and quality of life in patients with chronic obstructive pulmonary disease. Respir Med 2011;105: 67–73 [DOI] [PubMed] [Google Scholar]


