Abstract
Previous position papers have confirmed associations between periodontal disease and adverse pregnancy outcomes. Causal associations have not been confirmed and have been the source of much confusion for the profession and public.
Aim
To investigate whether sufficient evidence exists for a causal relationship between periodontal disease and adverse pregnancy outcomes.
Methods
The PICO question was “For adults in good general health who are diagnosed with periodontal disease, will receiving non-surgical periodontal therapy (NSPT), as compared to not receiving non-surgical periodontal therapy, lower their risk for adverse pregnancy outcomes?” Only systematic reviews (SRs) with or without meta-analyses (MAs) of randomized controlled trials published in the English language between 2007 and 2019 were included. Databases searched included PubMed, MEDLINE, EbscoHost, CINAHL, Scopus, Cochrane Registry of Systematic Reviews, and Clinical Trials Registry. Quality assessments were conducted by both authors using the PRISMA checklist. The Bradford Hill criteria were used to determine evidence for causality.
Results
Of 37 records retrieved for adverse pregnancy outcomes, 9 met the criteria for inclusion and were analysed. None showed that NSPT lowers the risk for adverse pregnancy outcomes.
Conclusion
Bradford Hill criteria analysis failed to support a causal relationship between periodontal disease and adverse pregnancy outcomes based on the most current evidence available.
Keywords: adverse pregnancy outcomes, low birth weight, meta-analysis, oral health AND systematic reviews, periodontal disease, periodontal treatment, preeclampsia, preterm birth
Abstract
Les énoncés de position précédents ont confirmé des associations entre la maladie parodontale et les effets indésirables de grossesse. Des associations causales n’ont pas été confirmées et ont été la source de beaucoup de confusion pour la profession et la population.
Objectiv
Étudier s’il y a suffisamment de preuves qu’un lien de causalité existe entre la maladie parodontale et les effets indésirables de grossesse.
Méthodologie
La question de PICO était : « Les adultes en bonne santé générale, qui ont reçu un diagnostic de maladie parodontale, auront-ils une réduction de leur risque d’effets indésirables de grossesse s’ils reçoivent une thérapie parodontale non chirurgicale (TPNC), par rapport à ne pas recevoir de thérapie parodontale non chirurgicale? » Seules les revues systématiques (RS) avec ou sans méta-analyse (MA) d’essais comparatifs randomisés publiés en anglais entre 2007 et 2019 ont été incluses. Les recherches de bases de données ont été effectuées, entre autres, dans PubMed, MEDLINE, EbscoHost, CINAHL, Scopus, le registre de revues systématiques Cochrane et le registre des essais cliniques. Des évaluations de la qualité ont été menées par les deux auteurs à l’aide de la liste de vérification PRISMA. Les critères de Bradford Hill ont été utilisés pour déterminer la preuve de causalité.
Résultats
Dans les 37 dossiers repérés sur les effets indésirables de grossesse, 9 répondaient aux critères d’inclusion et ont été analysées. Aucun dossier n’a montré que la TPNC réduit le risque d’effets indésirables de la grossesse.
Conclusion
Les critères d’analyse de Bradford Hill n’ont pas réussi à appuyer un lien de causalité entre la maladie parodontale et des effets indésirables de grossesse selon les preuves les plus récentes offertes.
CANADIAN DENTAL HYGIENISTS ASSOCIATION POSITION STATEMENT .
The Canadian Dental Hygienists Association acknowledges that, although associations between periodontal disease and adverse pregnancy outcomes have previously been established, there is insufficient evidence that periodontal disease causes adverse pregnancy outcomes.
INTRODUCTION
Relationships between periodontal disease and a number of systemic diseases have been proposed since the late 1800s when physicians speculated that bacteria from the mouth caused everything from brain abscesses to arthritis. 1, 2 With the onset of “periodontal medicine” in the early 1990s, studies investigating the relationships between numerous oral and systemic conditions have increased, with inflammation now recognized as a common factor. Despite the amount of research published over the last 30 years, questions remain about the exact nature of these relationships. While relationships may be in the form of associations or correlations, they should not be assumed as causal.
Unfortunately, the differences between associations and causality are not well understood and the terms are often used interchangeably. A relationship merely describes how 2 variables might somehow be related or connected to each other. For instance, lung cancer rates are higher for people without a postsecondary education (who tend to smoke more), but that does not mean that someone can reduce his or her cancer risk just by getting a college or university education. 3 An “association” refers to “a relationship between an exposure (or a characteristic) and a disease that is statistically dependent; that is, the presence of one alters the probability of observing the presence of the other. An association is a necessary condition of a causal relationship, but not all associations are causal. If there is no association, the variables are said to be independent.” 4
A correlation is a relationship in which there is a “Linear association between two continuous or ordinal variables. The measure of the correlation is the correlation coefficient, which ranges from 1 (perfect positive association, e.g., as one variable increases the second one also increases at the same rate) through 0 (no association) to a –1 (perfect negative association, e.g., as one variable increase, the second one decreases at the same rate).” 4
In order for a relationship to be coined as “causal,” actual “cause and effect” must be determined through a very rigorous set of criteria. One must be able to state with certainty that “A” causes “B” (a specific exposure has been shown to cause a specific outcome). 4 Randomized clinical trials (RCTs) provide the strongest evidence of cause and effect, rather than the outcome happening by chance. These experimental studies are the most methodologically challenging and ones in which the researcher controls or manipulates the variables (i.e., the intervention, its timing and dose) under investigation, such as in testing the effectiveness of a treatment, as compared to another treatment or a placebo. 5
Often, when clinicians read a research article that is reporting a correlation or an association between an oral disease and a particular outcome of interest, they automatically, and incorrectly, jump to the conclusion that the relationship is causal. Prime examples of such misinterpretations are frequently found with proposed oral–systemic linkages, such as the assumption that periodontitis is one cause of heart disease or of adverse pregnancy outcomes, or that stress causes periodontitis. It is important for clinicians to understand that correlations and associations do not imply or equal causality. In fact, incorrect assumptions of causality are a major public health concern. From a public health perspective, no evidence should be considered causal unless it has gone through very rigorous scrutiny using standard public health guidelines such as the Bradford Hill criteria for causality 6 (Table 1).
In 2004, Lux and Lavigne 7, 8 published a position paper for the Canadian Dental Hygienists Association (CDHA) in 2 parts, outlining the nature of the proposed linkages between periodontal disease and 4 systemic conditions: cardiovascular diseases, preterm low birth weight babies, respiratory diseases, and diabetes. Updates to those first position papers were published in the Canadian Journal of Dental Hygiene in November/December 2006 9 and January/February 2007, 10 in which the author reported associations between period,ontal disease and cardiovascular diseases, diabetes, adverse pregnancy outcomes, and respiratory diseases (in particular, pneumonia in health-compromised seniors).
A recent systematic mapping of trial registers of clinical research trials conducted on periodontal medicine revealed 57 conditions that are currently hypothesized to be linked with periodontal diseases. 11 While it is beyond the scope of this current position paper to explore all of these proposed linkages, the status of 10 of these hypotheses will be evaluated in a series of position papers written by the same authors and released in the coming months by CDHA. The first paper, evaluating the evidence of a causal relationship between periodontal disease and cardiovascular diseases was published in February 2020 in this journal. 12 Forthcoming position papers will assess the nature of the relationships between periodontal disease and respiratory diseases, diabetes, obesity, rheumatoid arthritis, Alzheimer disease, end-stage renal disease, inflammatory cancers, and influenza.
The purpose of these updated position papers is to review the research undertaken since the publication of the last CDHA position papers in 2006 and early 2007 on these proposed relationships. Unlike the methodology used for the previous position papers and updates, this investigation is more specific in looking at whether the state of the evidence has evolved from one of associations to one of actual causality. Determining a causal relationship requires studies that have examined an intervention, thus only the highest levels of evidence will be sought for this update. This position paper is the second in the series, and investigates whether a causal relationship exists between periodontal disease and adverse pregnancy outcomes.
Table 1.
The Bradford Hill criteria for causality 6
|
Criteria |
Meaning |
|
Strength of association |
A strong association is more likely to have a causal component than is a modest association. Strength of the association is determined by the types of existing studies. The highest-level studies from the evidence pyramid would represent the strongest associations (i.e., RCTs and systematic reviews with meta-analyses). Results from these studies must demonstrate an odds ratio or relative risk of at least 2.0 or above in order to be meaningful. Anything between 1 and 2 is weak while >2 is moderate and >4 is considered strong. |
|
Consistency |
A relationship is repeatedly observed in all available studies. |
|
Specificity |
A factor influences specifically a particular outcome or population. The more specific an association between a factor and an effect, the greater the probability that it is causal. |
|
Temporality |
The cause must precede the outcome it is assumed to affect (e.g., smoking before the appearance of lung cancer). Outcome measured over time (longitudinal study).- |
|
Biological gradient (dose-response) |
The outcome increases monotonically with increasing dose of exposure or according to a function predicted by a substantive theory (e.g., the more cigarettes one smokes, the greater the chance of the cancer occurring). |
|
Plausibility |
The observed association can be plausibly explained by substantive matter (i.e., biologically possible). |
|
Coherence |
A causal conclusion should not fundamentally contradict present substantive knowledge. (Studies must not contradict each other.) |
|
Experiment |
Causation is more likely if evidence is based on randomized experiments or a systematic review of randomized experiments. However, these RCTs may not be ethically possible and thus prospective rather than experimental studies, such as cohort studies, may be the highest level of evidence available. |
|
Analogy |
For analogous exposures & outcomes an effect has already been shown (e.g., effects first demonstrated on animals or an effect previously occurring on humans such as the effects of thalidomide on a fetus during pregnancy.) |
Source: Lavigne SE. From Evidence to Causality: How Do We Determine Causality? [Online course]. 2018. Available from: www.dentalcare.com/en-us/professional-education/ce-courses/ce530
METHODOLOGY
An overarching PICO question was developed for the first 5 oral–systemic connections to be explored in this series of position papers. For adverse pregnancy outcomes, the PICO question was customized as follows: “For adults in good general health who are diagnosed with periodontal disease ( Population ), will receiving non-surgical periodontal therapy (NSPT) ( Intervention ), as compared to not receiving NSPT ( Comparison group ), lower their risk for adverse pregnancy outcomes? ( Outcome )”
Eligibility criteria
Both authors independently searched the literature, limiting the search to systematic reviews (SRs) with or without meta-analyses (MAs) of intervention studies using the following inclusion and exclusion criteria. SRs and MAs of observational studies were excluded.
Search strategy
Databases searched included PubMed, MEDLINE, EbscoHost, CINAHL, Scopus, Cochrane Registry of Systematic Reviews, and Clinical Trials Registry (clinicaltrials.gov). Additionally, bibliographies of retrieved articles were searched for further relevant systematic reviews and meta-analyses and added when appropriate. The keywords used for each search were as follows: adverse pregnancy outcomes; preterm birth; low birth weight; preeclampsia; periodontal disease; periodontal treatment; oral health AND systematic reviews; meta-analysis.
Search strategies (limited to publications after 2007 and in the English language) were as follows:
adverse pregnancy outcomes and periodontal disease and systematic reviews
adverse pregnancy outcomes and periodontal treatment and systematic reviews
adverse pregnancy outcomes and oral health and systematic reviews
Study selection
Both authors independently screened the titles and abstracts of all articles retrieved by the search using the inclusion criteria and then discussed their choices to reach consensus regarding their suitability for full-text reading. Both authors independently reviewed the selected full-text articles and reached consensus on their inclusion or exclusion.
Quality assessment
The methodological quality of the selected systematic reviews and meta-analyses was assessed blindly by both authors using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist tool. 13 Scores were then compared and discussed where inconsistencies occurred to reach consensus.
Data extracted
The following information was extracted from each selected SR or MA and compiled in table format: year published, number of RCTs included, country of origin, methods used for assessing risk of bias, heterogeneity, outcomes measured, and conclusions of the findings.
Table 2.
Inclusion and exclusion criteria
|
Inclusion criteria |
Exclusion criteria |
|
Published between 2007 and 2019 |
Published before 2007 |
|
English language |
Languages other than English |
|
Systematic Reviews (SRs) with or without meta-analyses (MAs) of RCTs
|
Abstracts, posters, conference proceedings, editorials or commentaries, duplicate studies, narrative reviews, RCTs, observational studies/both cohort and case-control and systematic reviews of observational studies and/or case-control studies. |
|
Studies involving humans |
Animal studies (in vivo, ex vivo) and in vitro studies |
Table 3.
Adverse pregnancy outcomes screened articles included and deleted
|
|
Included |
|
Deleted |
Reason for deletion
|
|
1. |
Iheozor-Ejiofor et al. 2017 14 (15 RCTs) (Cochrane Review) |
|
Spivakovsky 2018 25 (critical summary of Iheozor-Ejiofor, Cochrane) |
Duplicate (critical summary) |
|
2. |
Polyzos et al. 2010 15 (1 RCT) (Greece) |
|
Baccaglini 2011 26 (critical summary of Polyzos) |
Duplicate (critical summary) |
|
3. |
Kim et al. 2012 16 (12 RCTs...6 post-2008) (US) |
|
Dasanyake 2013 27 (critical summary of Kim) |
Duplicate (critical summary) |
|
4. |
Chambrone et al. 2011 17 (13 RCTs) (Brazil) |
|
Leader 2011 28 (critical summary of Chambrone) |
Duplicate (critical summary) |
|
5. |
Rangel-Rincón et al. 2018 18 2-4 above included in this umbrella review (18 SRs, 19 intervention studies) (Columbia) |
|
Vivares-Builes et al. 2018 29 (Columbia) |
99 SRs but all observational studies. Purpose was to establish association. |
|
6. |
Shah et al. 2013 19 (SR of 13 RCTs) (India) |
|
Corbella et al. 2016 30 (Italy) |
All case-control or cohort studies |
|
7. |
da Silva et al. 2017 20 (4 RCTs) (Brazil)
|
|
Daalderop et al. 2018 31 (The Netherlands) |
23 SRs but all studies were cohort, case-control or CS |
|
8. |
Schwendicke et al. 2015 21 (13 RCTs) (Germany, US, Denmark) |
|
Ide et al. 2013 32 |
No intervention studies |
|
9. |
Lopez et al. 2015 22 (SR of 6 meta-analyses) |
|
|
|
|
10. |
|
|
Abati et al. 2013 33 |
Only case-control studies |
|
11. |
|
|
Teshome et al. 2016 34 |
Only case-control studies |
|
12. |
|
|
Macones et al. 2010 35 |
Editorial of Polyzos |
|
13. |
|
|
Otomo-Corgel et al. 2012 36 |
Literature review |
RESULTS
Thirty-seven records were retrieved in total from both the database searches and articles identified within these reviews. After eliminating duplicates and articles that were deemed ineligible based on the inclusion criteria, 9 studies 14- 22 remained eligible for review. A flow diagram (Figure 1) illustrates the details of the selection process; Table 3 reports the reasons for elimination of the full-text articles.
Results of the quality appraisal of the 9 included SRs and MAs are shown in Table 4. Based on the 27 PRISMA checklist items, scores ranged from 11 to 27. Agreement between the 2 independent evaluators was close to 100% with scores being off by only 1 to 3 points. The quality of the studies was generally moderate to high with the exception of one study 19 that was deemed by both reviewers to be of very poor quality because of multiple inaccuracies in references as well as grammatical errors throughout. This study was removed from further discussion in this review.
None of the outcomes of the 9 systematic reviews and meta-analyses showed a positive result for NSPT lowering the risk for adverse pregnancy outcomes. Six of the studies showed no relationship between adverse pregnancy outcomes and scaling and root planing as an intervention, while 3 studies reported a “possible relationship” and one study reported a possible relationship but only with low birth weight. These results are illustrated in Table 5.
Figure 1.
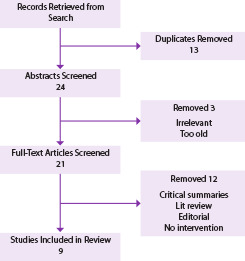
Adverse pregnancy outcomes search flow diagram
DISCUSSION
The purpose of this review was to explore the possible association between non-surgical periodontal therapy (NSPT) and the risk of preterm birth (PTB) and/or low birth weight (LBW) and/or pre-eclampsia. Nine systematic reviews, of which 7 contained a MA, were included in this review. Of the 2 studies without meta-analyses, one was an umbrella review 18 of 18 systematic reviews and the other, a systematic review of 6 meta-analyses. 22 There were numerous inconsistencies among the RCTs included in the 9 SRs with some reporting positive associations in their descriptive findings, but not supported in 4 of the 7 meta-analyses.
Conclusions reached in all 9 reviews were consistent in reporting no positive relationships between NSPT provided during the second trimester of pregnancy and the occurrence of preterm birth and/or low birth weight. The only systematic review that included studies on the effects of NSPT on pre-eclampsia was conducted by Kunnen and colleagues 23 and was included in the umbrella reviews by Rangel-Rinc ó n. 18 No effect of periodontal treatment on the risk of pre-eclampsia was found in their meta-analysis. Two MAs 16, 21 conducted subgroup analyses and found significant effects for preterm birth but only for studies conducted in low income countries where a high prevalence of low birth weight and preterm birth exists.
In the review by Chambrone et al. 17 , which included 13 trials involving 6813 women, despite positive results reported by more than half of the included studies, the MAs conducted in 11 of the trials showed that NSPT had little to no effect on pregnancy outcomes. Similarly, Polyzos et al. 15 concluded that treatment of periodontal disease could not be considered an efficient way to reduce the incidence of preterm birth based on the results of 3 separate meta-analyses performed on the 11 included studies involving 6558 pregnant women. One interesting finding in this review was that low-quality studies tended to overestimate the treatment effect in contrast to the high-quality trials which showed no effect. The authors cautioned the interpretation of, data from low-quality RCTs in making clinical decisions.
Lopez et al. 22 in their umbrella review of meta-analyses concluded that, in 4 of 5 MAs, periodontal treatment did not reduce preterm birth rates. However, one MA found that preterm birth rates were reduced following periodontal treatment but only for groups at high risk of preterm birth. This finding was also reported in the SRs conducted by Kim et al. 15 and Schwendicke et al. 21
The umbrella review by Rangel-Rincón et al. 18 of 18 systematic reviews, 11 of which included a meta-analysis, identified numerous methodological and conceptual gaps such as sample size, sociodemographic characteristics, type of masking, and control for confounders. Other glaring issues were the lack of a standard definition for periodontal disease as well as the inconsistency in the type and frequency of treatment provided in each study. For example, although this review investigated the effects of the most commonly used treatment (scaling and root planing) on adverse pregnancy outcomes, the gestational timeframes, frequencies for delivery of treatment, and individuals delivering the treatment varied, thus making it more difficult to draw valid inferences. Since the outcome of this umbrella review clearly did not support the effectiveness of scaling and root planing as an effective means of reducing the risk for adverse pregnancy outcomes, future research should explore other types of treatment.
Additionally, use of different indices to measure the magnitude of periodontal disease, as well as use of different outcome measurement criteria, could potentially have a major effect on the results. When uniform criteria are not used to define gestational age (for example, use of sonograms versus use of last menstrual period), there is potential for random or non-differential measurement error. An underestimation of the true effect could occur if equal amounts of measurement error are found in both treatment and control groups, which would then bias the risk ratios towards the null value. Variability was also noted in the definitions of adverse pregnancy outcomes, which makes comparisons difficult. In addition to the 3 adverse pregnancy outcomes explored in this review, other neonatal complications, such as still birth and spontaneous abortion, warrant further exploration.
A summary of inconsistencies between studies identified by several authors of the included systematic reviews is found in Table 6. These issues and inconsistencies will need to be addressed in future studies as they can affect case selection leading to selection bias, the overall quality of the study, and the ability to make comparisons. The umbrella review authors recommended that future research studies follow the recommendations for epidemiological surveillance of periodontal diseases in population studies.18
Using the 9 Bradford Hill criteria for causation outlined in Table 1, it is clear that several of the criteria have not been met in these studies. Therefore one can conclude at this time there is not sufficient evidence to support a causal relationship between periodontal disease and adverse pregnancy outcomes. For example, beginning with “strength of association,” although the highest levels of evidence (SRs & MAs) have been reviewed in this paper, none of the 9 SRs/MAs provided sufficient evidence of a strong enough association. The second criterion of “consistency” has definitely not been met as studies are inconsistent in their findings. Similarly, the criterion of “specificity” has not demonstrated that, in every instance, the outcome will be the same. The criterion of “temporality” has been met but only in some studies, where pregnant women in high-risk populations with periodontal disease have experienced adverse pregnancy outcomes. Studies investigated in this review also have not demonstrated a “dose–response” outcome comparing results with various magnitudes of periodontitis. The criterion of “biological plausibility,” however, has been met as it is possible that elevated levels of inflammatory cytokines present during periodontitis could have an effect on premature rupture of the membranes leading to preterm birth. The criterion of “coherence” has not been demonstrated based on the inconsistencies and contradictory findings. The criterion of “experiment” also has failed to demonstrate consistent results through RCTs and SRs /MAs of these studies. Finally, the last criterion of “analogy,” although the weakest, was not explored in this review. Thus, of the 9 criteria, only 2 can be said to have been fulfilled (Table 7).
Table 4.
Quality appraisal and summary of the systematic reviews/meta-analyses (n = 9)
|
Author (Country) |
PRISMA score |
Heterogeneity |
Risk of bias |
Quality assessment instrument |
Comments |
Included meta-analysis of the SR |
|
Rangel-Rinc&n et al. 2018 18 (Colombia) |
N/A (umbrella review of SRs) |
N/A (umbrella review of SRs) |
N/A (umbrella review of SRs) |
N/A (umbrella review of SRs) |
Very thorough umbrella review |
No (umbrella review) |
|
Iheozor-Ejiofor et al. 2017 14 (Cochrane Review) |
25/27 |
High |
High |
Cochrane's risk of bias tool |
15 RCTs (7161 participants) NSPT/PTB/LBW graded the evidence as low |
Yes |
|
da Silva 2017 20 (Brazil) |
24/27 |
Moderate |
Moderate/High |
Cochrane's risk of bias tool |
4 RCTs included (2006, 2013, 2015, 2015) NSPT/PTB/LBW |
Yes |
|
Schwendicke et al. 2015 21 (Germany, US, Denmark) |
18/27 |
High |
Unclear |
Cochrane's risk of bias tool |
13 RCTs included (6283 participants) NSPT/PTB/LBW |
Yes |
|
Lopez et al. 2015 22
|
18/27 |
Reported individually per clinical trial |
Reported individually per clinical trial |
Reported individually per clinical trial |
Systematic review of 6 meta-analyses |
No (SR of 6 MAs) |
|
Shah et al. 2013 19 (India) |
12/27 |
High |
Unclear |
Cochrane's risk of bias tool |
13 RCTs NSPT/PTB/LBW Poor Quality SR overall |
Yes |
|
Kim et al. 201216 (US) |
25/27 |
High |
Low |
Cochrane's risk of bias tool |
12 RCTs SRP/PTB |
Yes (11 studies included) |
|
Chambrone et al. 2011 17 (Brazil) |
22/27 |
High |
Low |
Cochrane's risk of bias tool |
13 RCTs SRP/SRP with antibiotics PTB/LBW |
11 of the 13 studies included in MA |
|
Polyzos et al. 2010 15 (Greece) |
24/27 |
High |
Low |
Cochrane's risk of bias tool |
11 RCTs (6558 participants) SRP/PTB/LBW
|
Yes (3 separate MAs on 11 studies) |
Table 5.
Primary outcomes of retained studies
|
Preterm Birth and Low Birth Weight | ||
|
Outcome 1 No relationship |
Outcome 2 Possible relationship |
Outcome 3 Positive relationship |
|
Polyzos et al. 2010 da Silva et al. 2017 Chambrone et al. 2011 Iheozor-Ejiofor et al. 2017 (preterm birth) Rangel-Rinc-n et al. 2018 Lopez et al. 2015 |
Kim et al. 2012 (only in high risk groups) Schwendicke et al. 2015 (only in populations with- high occurrence >20%) Iheozor-Ejiofor et al. 2017 (low birth weight) Shah et al. 2013 BUT (better studies needed to demonstrate cause) |
None |
Table 6.
Summary of issues identified by authors of systematic reviews of RCTs
|
1. Inconsistency in defining periodontal disease and periodontal status 2. Inconsistency in the type of periodontal treatment provided, timing, frequency, clinician 3. Quality of studies (methodological shortcomings):
4. Publication bias: studies showing no/negative effect may not have been published 5. Evidence does not support SRP for reducing the rate of PTB 6. Gingivitis and periodontitis in the same meta-analysis; questionable 7. Treatment effectiveness should be measured 8. Selection criteria: high-risk & low-risk individuals combined; taking medication and other dental treatment not reported 9. Is SRP the preferred treatment (vs. mouth wash or antibiotics)? 10. Other conditions, e.g., smoking, not reported or evaluated |
CONCLUSION
Based on findings from the 9 SRs/MAs investigated in this current review, one can state with confidence that the answer to the PICO question: “For pregnant women in good general health who are diagnosed with periodontal disease, will receiving non-surgical periodontal therapy, as compared to not receiving non-surgical periodontal therapy, lower their risk for adverse pregnancy outcomes?” is “No.” Numerous issues exist with published studies, which may have influenced these results. Future studies will need to focus on correcting these inconsistencies, particularly by identifying 1) a standard case definition of periodontal disease; 2) the type and frequency of the intervention; and 3) the target population. In addition, future studies should also investigate other types of interventions and measure their effectiveness.
It is interesting to note that, in August 2013, the American College of Obstetricians and Gynecologists Committee on Health Care of Underserved Women released an opinion piece entitled “Oral health care during pregnancy and through the lifespan.” 24 This piece stated there was a lack of evidence that prenatal oral health care improves pregnancy outcomes. The college did, however, indicate that receiving oral health care during pregnancy is safe, and acknowledged that improvements in oral health improve general health by reducing the risk of transmission of cariogenic bacteria to children.
While 2 previous CDHA position papers on this topic have established associations between periodontal disease and adverse pregnancy outcomes, neither of those papers investigated a causal link. This position paper explored whether periodontal disease was causally related to adverse pregnancy outcomes. Its findings provide clear evidence that, although associations have been established, no causal link exists at this time between periodontal disease and adverse pregnancy outcomes. This evidence will enable the dental hygiene practitioner to clarify the nature of this relationship with their clients based on the most current research.
Table 7.
Bradford Hill criteria analysis
|
Bradford Hill criteria |
Met |
Not Met |
|
Strength of association |
|
X |
|
Consistency |
|
X |
|
Specificity |
|
X |
|
Temporality |
X- (in a few studies) |
|
|
Dose-response |
|
X |
|
Biological plausibility |
X |
|
|
Coherence |
|
X |
|
Experiment |
|
X |
|
Analogy |
Not explored |
Not explored |
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
Acknowledgments
This position paper was funded by the Canadian Dental Hygienists Association. Both authors received an honorarium for this work. We wish to thank the CDHA Steering Committee for their valuable input and guidance throughout the development of this paper.
Footnotes
CDHA Research Agenda categories: risk assessment and management; capacity building of the profession
REFERENCES
- 1. Kumar PS. From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J Physiol 2017; 595( 2): 465– 476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller WD. The micro-organisms of the human mouth: The local and general diseases which are caused by them. Philadelphia: SS White Dental Mfg Co; 1890. [Google Scholar]
- 3.SUNY Downstate Health Sciences University. EBM Tutorial. Guide to Research Methods [Internet] [cited 2019 July 19]. Available from: https://guides.downstate.edu/c.php?g=856794&p=6152125
- 4. Brunette DM. Causation, association and oral health–systemic disease connections. In: The oral systemic health connection, edited by Michael Glick. Chicago: Quintessence Publishing Co. Inc; 2014. [Google Scholar]
- 5. Forrest JL, Miller SA. EBDM in action: Developing competence in EB practice. Colbert, WA: ebdLibrary; 2016. [Google Scholar]
- 6. Hill AB. The environment and disease: Association or causation? Proc Royal Soc Med 1965; 58: 295– 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lux J, Lavigne S. Your mouth—Portal to your body. CDHA position paper on the links between oral and general health Part I. Probe 2004; 38( 4): 114- 34 [Google Scholar]
- 8. Lux J, Lavigne S. Your mouth – Portal to your body CDHA position paper on the links between oral and general health Part II. Probe 2004; 38( 4): 155- 71 [Google Scholar]
- 9. Lux J. Review of the oral disease–systemic disease link Part 1: Heart disease, diabetes. Can J Dent Hyg 2006 40( 5): 288- 302 [Google Scholar]
- 10. Lux J. Review of the oral disease–systemic disease link Part II: Preterm low birth weight babies. Can J Dent Hyg 2007; 41( 1): 8- 21 [Google Scholar]
- 11. Monsarrat P, Blaizot A, Kémoun P, Ravaud P, Nabet C, Sixou M, Vergnes J-N. Clinical research activity in periodontal medicine: a systematic mapping of trial registers. J Clin Periodontol 2016; 43: 390– 400 doi: 10 1111/jcpe 12534 [DOI] [PubMed] [Google Scholar]
- 12. Lavigne SE, Forrest JL. An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and cardiovascular diseases: Position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg 2020; 54( 1): 32– 41 [PMC free article] [PubMed] [Google Scholar]
- 13. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, The PRISMA-DTA Group. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018Jan 23; 319( 4): 388– 396 doi: 10 1001/jama 2017 19163 [DOI] [PubMed] [Google Scholar]
- 14. Iheozor-Ejiofor Z, Middleton P, Esposito M, Glenny AM. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst Rev 2017; 6: CD005297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polyzos NP, Polyzos IP, Zavos A, Valachis A, Mauri D, Papanikolaou EG, Tzioras S, Weber D, Messinis IE. Obstetric outcomes after treatment of periodontal disease during pregnancy: systematic review and meta-analysis. BMJ 2010; 341: c7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: A systematic review and meta-analysis of randomized controlled trials. J Periodontol 2012; 83( 12): 1508– 1519 [DOI] [PubMed] [Google Scholar]
- 17. Chambrone L, Pannuti CM, Guglielmetti MR, Chambrone LA. Evidence grade associating periodontitis with preterm birth and/or low birth weight II A systematic review of randomized trials evaluating the effects of periodontal treatment. J Clin Periodontol 2011; 38: 902- 914 [DOI] [PubMed] [Google Scholar]
- 18. Rangel-Rincón LJ, Vivares-Builes AM, Botero JE, Agudelo-Suárez AA. An umbrella review exploring the effect of periodontal treatment in pregnant women on the frequency of adverse obstetric outcomes. J Evid Base Dent Pract 2018; 18( 3): 218– 239 [DOI] [PubMed] [Google Scholar]
- 19. Shah M, Muley A, Muley P. Effect of nonsurgical periodontal therapy during gestation period on adverse pregnancy outcome: a systematic review. J Matern Fetal Neonatal Med 2013; 26( 17): 1691– 1695 [DOI] [PubMed] [Google Scholar]
- 20. da Silva HE, Stefani CM, de Santos Melo N, de Lima AA, Kuchenbecker Rosing C, Porporatti AL, De Luca Canto G. Effect of intra-pregnancy nonsurgical periodontal therapy on inflammatory biomarkers and adverse pregnancy outcomes: a systematic review with meta-analysis Syst Rev 2017; 6: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwendicke F, Karimbux N, Allareddy V, Gluud C. Periodontal treatment for preventing adverse pregnancy outcomes: A meta- and trial sequential analysis. PLoS ONE 2015; 10( 6): e0129060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López NJ, Uribe S, Martinez B. Effect of periodontal treatment on preterm birth rate: a systematic review of meta-analyses. Periodontol 2000 2015; 67: 87– 130 [DOI] [PubMed] [Google Scholar]
- 23. Kunnen A, van Doormal JJ, Abbas F, Aarnoudse JG, van Pampus MG, Faas MM. Periodontal disease and pre-eclampsia: a systematic review J Clin Periodontol 2010; 37( 12): 1075– 1087 [DOI] [PubMed] [Google Scholar]
- 24. American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women. Committee opinion: Oral health care during pregnancy and through the lifespan. Obstet Gynecol 2013; 122( 2, pt1): 417– 422 [DOI] [PubMed] [Google Scholar]
- 25. Spivakovsky S. Periodontal treatment for the prevention of adverse pregnancy outcomes. Evid Based Dent 2018; 19: 12– 13 [DOI] [PubMed] [Google Scholar]
- 26. Baccaglini L. A meta-analysis of randomized controlled trials shows no evidence that periodontal treatment during pregnancy prevents adverse pregnancy outcomes. J Am Dent Assoc 2011; 142( 10): 1192– 1193 [DOI] [PubMed] [Google Scholar]
- 27. Dasanyake A. Scaling and root planing is effective in reducing preterm birth only in high-risk groups. J Evid Base Dent Pract 2013; 13: 42– 44 [DOI] [PubMed] [Google Scholar]
- 28. Leader DA. Critical summary of Chambrone L, Pannuti CM, Guglielmetti MR, Chambrone LA. Evidence grade associating periodontitis with preterm birth and/or low birth weight, II: a systematic review of randomized trials evaluating the effects of periodontal treatment. J Clin Periodontol. 2011; 38( 10): 902– 914 [DOI] [PubMed] [Google Scholar]
- 29. Vivares-Builes AM, Rangel-Rincón LJ, Botero JE, Agudelo-Suárez AA. Gaps in knowledge about the association between maternal periodontitis and adverse obstetric outcomes: an umbrella review. J Evid Base Dent Pract 2018; 18( 1): 1– 27 [DOI] [PubMed] [Google Scholar]
- 30. Corbella S, Taschieri S, Del Fabbro M, Francetti L, Weinstein R, Ferrazzi E. Adverse pregnancy outcomes and periodontitis: A systematic review and meta-analysis exploring potential association. Quintessence Int 2016; 47( 3): 193– 204 [DOI] [PubMed] [Google Scholar]
- 31. Daalderop LA, Wieland BV, Tomsin K, Reyes L, Kramer BW, Vanterpool SF, Been JV. Periodontal disease and pregnancy outcomes: overview of systematic reviews. JDR Clin Trans Res 2018; 3( 1): 10– 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ide M, Papapanou PN. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes—systematic review. J Periodontol 2013; 84( 4 Suppl): S181– [DOI] [PubMed] [Google Scholar]
- 33. Abati S, Villa A, Cetin I, Dessole S, Luglie PF, Strohmenger L, Ottolenghi L, Campus GG. Lack of association between maternal periodontal status and adverse pregnancy outcomes: a multicentric epidemiologic study. J Matern Fetal Neonatal Med 2013; 26( 4): 369– 372 [DOI] [PubMed] [Google Scholar]
- 34. Teshome A, Yitayeh A. Relationship between periodontal disease and preterm low birthweight: systematic review. Pan Afr Med J 2016; 24: 215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of pretern birth: results from the Periodontal Infections and Prematurity Study (PIPS). Am J Obstet Gynecol 2010; 202( 2): 147 e1–8 [DOI] [PubMed] [Google Scholar]
- 36. Otomo-Corgel J, Pucher JJ, Rethman MP, Reynolds MA. State of the science: Chronic periodontitis and systemic health. J Evid Base Dent Pract 2012; 12( 3 Suppl): 20– 28 [DOI] [PubMed] [Google Scholar]


