Abstract
Background
Based on inhibition of viral replication and limited reports on clinical efficacy, hydroxychloroquine (HCQ) is being considered as prophylaxis and treatment of COVID-19. Although HCQ is generally considered safe during pregnancy based on studies in patients with systemic lupus erythematous and other rheumatic conditions, there may still be reluctance to institute this antimalarial during pregnancy for the sole purpose of antiviral therapy.
Methods
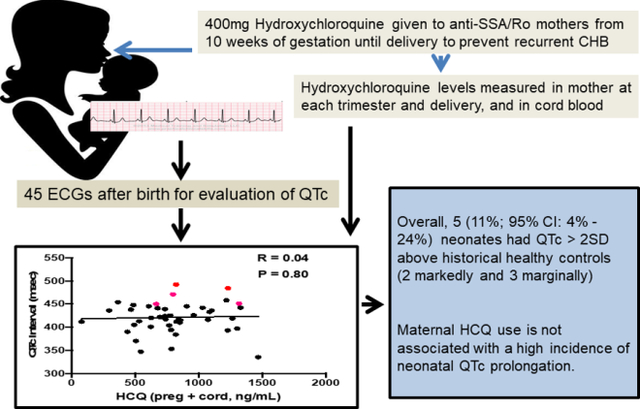
To provide data regarding any potential fetal/neonatal cardiotoxicity, we leveraged a unique opportunity in which neonatal electrocardiograms (ECGs) and HCQ blood levels were available in a recently completed study evaluating the efficacy of HCQ 400mg daily to prevent the recurrence of congenital heart block associated with anti-SSA/Ro antibodies.
Results
Forty-five ECGs were available for QTc measurement, and levels of HCQ were assessed during each trimester of pregnancy and in the cord blood, providing unambiguous assurance of drug exposure. Overall, there was no correlation between cord blood levels of HCQ and the neonatal QTc (R = 0.02, P = 0.86) or the mean of HCQ values obtained throughout each individual pregnancy and the QTc (R = 0.04, P = 0.80). In total 5 (11%; 95% CI: 4% - 24%) neonates had prolongation of the QTc > 2SD above historical healthy controls (2 markedly and 3 marginally) but ECGs were otherwise normal.
Conclusions
In aggregate, these data provide reassurances that the maternal use of HCQ is associated with a low incidence of infant QTc prolongation. However, if included in clinical COVID-19 studies, early postnatal ECGs should be considered.
Registration
https://clinicaltrials.gov; Unique Identifier: NCT01379573.
Keywords: QT interval electrocardiography, pediatrics, pregnancy, hydroxychloroquine, COVID-19
Journal Subject Terms: Clinical Studies, Pregnancy, Cardiotoxicity
Graphical Abstract
Introduction
As the world faces the pandemic of the novel coronavirus SARS-CoV-2 and its resulting illness (COVID-19), the reach for therapeutics assumes the highest of priorities. Two anti-malarials, hydroxychloroquine (HCQ) and chloroquine (CQ), have surfaced as promising candidates. By raising endosomal pH, these weak bases have been shown in vitro to decrease SARS-CoV-2 viral replication.1 It has been suggested that endosome maturation is blocked at intermediate stages of endocytosis, which would then result in decreased transport of virions to the ultimate releasing site.2 Since the race against time has precluded mature placebo-controlled trials, favorable results from China support efficacy of CQ against COVID-19-associated pneumonia3 and a very limited French study using HCQ reported a significant reduction in viral carriage.4 Although such positive results were not reproduced in a limited study comparing HCQ to placebo,5 the United States has embraced massive testing of HCQ for both prophylaxis of high-risk patients and treatment (for example, see https://clinicaltrials.gov/ct2/show/NCT04308668). As the use of HCQ continues to expand, two relevant points surface: that of toxicity, and that of inclusion or exclusion of pregnant women. Concerns regarding cardiac toxicity have surfaced based on the FDA-approved package insert for HCQ, which states that the drug may prolong the QTc. Accordingly, data are needed to address cardiac safety in the neonate exposed to HCQ.
This is especially true since there is a known transplacental passage of HCQ6 and the terminal elimination half-life of HCQ is long7. After absorption, the half-life of HCQ is approximately 40 days owing to the large volume of distribution in the blood. Moreover, HCQ can distribute to aqueous cellular and intercellular compartments, resulting in long mean residence times.8
Physicians caring for patients with systemic lupus erythematosus (SLE) are intimately familiar with HCQ. It is virtually the most frequently prescribed long-term medication used to treat this disease, a practice driven by extensive literature supporting the prevention of flares and reduction of mortality.8, 9 Predictably, these clinically favorable effects have led to the strong recommendation by the American College of Rheumatology to maintain HCQ during pregnancy in these patients.10 Furthermore, based on promising retrospective and case-control studies, maternal use of HCQ may extend to the prevention of anti-SSA/Ro-associated congenital heart block (CHB).11, 12 The overall safety of HCQ during pregnancy is supported by the absence of congenital malformations in over 400 pregnancies.13, 14
To provide further data on the fetal cardiac effects of exposure to HCQ, we leveraged a recently completed open-label study in which HCQ was prospectively evaluated to reduce the recurrence rate of CHB.15 Herein reported are the results of available electrocardiograms (ECGs) obtained within four months of postnatal life of fetuses exposed to HCQ from 10 weeks of gestation until delivery. Supporting maternal compliance and unambiguous fetal exposure, levels of HCQ during each trimester and delivery as well as in cord bloods are presented.
Methods
Description of Parent Study
The authors declare that all supporting data are available within the article. In brief, the Preventive Approach To Congenital Heart Block With Hydroxychloroquine (PATCH) study is an open-label single-arm Phase II trial to assess whether HCQ is effective for the prevention of CHB recurrence (https://clinicaltrials.gov/ct2/show/NCT01379573).15 Treatment with HCQ 400mg was required by completion of 10 weeks of gestation and the dose was maintained throughout pregnancy. If the mother was already on HCQ the dose remained at 400mg, and if the mother was taking 200mg the dose had to be escalated to 400mg by 10 weeks. The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and philanthropic foundations. The protocol was approved by the NYU School of Medicine (NYUSOM) Institutional Review Board (IRB), and all study participants gave written informed consent. The trial was overseen by a data and safety monitoring board (DSMB) of independent experts which convened every six months by teleconference. Patients were eligible if they had anti-SSA/Ro with or without anti-SSB/La antibodies irrespective of rheumatologic maternal diagnosis and a previous pregnancy with any of the following complications: fetal 2° or 3° CHB, serious cardiac injury defined as autopsy evidence of a mononuclear infiltrate in the endocardium, myocardium and pericardium, and/or the presence of severe endocardial fibroelastosis (EFE) associated with cardiac dysfunction seen on fetal echocardiography.15
Maternal blood was obtained at baseline, second trimester and birth to determine antibody levels by ELISA using native Ro60, recombinant Ro52 and recombinant La48 in the lab of JPB as previously described.16 To assess maternal compliance, HCQ levels were measured when possible at baseline, second trimester, third trimester, delivery and in cord bloods in Paris, France as previously described.17, 18 Values reported at < 100 ng/ml, which imply that the measurement of HCQ is below the level of detection, were assigned a specific value of 50 ng/ml in the calculation of mean values.
The primary endpoint of the main study was recurrence of 2° or 3° block in utero or at birth. The cardiac secondary endpoints consisted of prolonged fetal Doppler mechanical PR interval (>150msec) documented on the fetal echocardiogram (AV interval) or PR interval on ECG done at or after birth. Evaluation of QT/QTc intervals (see below) were not included in the study outcomes. Infants had an ECG and/or echocardiogram performed at birth and/or up to one year after birth.
Included in the current sub-study were all neonates in which an ECG result and HCQ levels were available. Several additional neonates who were screen failures based on maternal dosing past 10 weeks were also included as the appropriate information was still collected. Available ECGs (birth to four months of age) with sufficient quality to evaluate the QTc were read by the pediatric cardiologist (DMF). The ECGs of affected newborns who met the primary outcome of advanced block were not included in this safety study so that the results only reflect those infants with no clinical cardiac disease. Using the Bazett formula to correct for heart rate, corrected QT (QTc) intervals were calculated and compared to age-matched normal values.19 For the median (2nd percentile – 98th percentile), values for QTc were 413 (378–448) msec in males, and 420 (379–462) msec in females. QTc intervals were recorded in the absence of knowledge of the HCQ levels. Values exceeding 448 msec for males and 462 msec for females were considered abnormal. We are aware of the common usage of Schwartz to calculate the QTc but chose the recent simplified method as our historical control.20
Statistical Analysis
The Pearson correlation coefficient was used to estimate the association between blood HCQ levels and the neonatal QTc intervals. In addition, HCQ levels were divided into quartiles and neonatal QTc levels were compared between the highest and lowest HCQ quartiles with the two sample T-test and Mann-Whitney test. Since results were nearly identical, only those based on the former are reported. For all analyses, two HCQ levels were used: a) HCQ levels in cord blood or close to birth and b) the average of the maternal HCQ levels obtained at each trimester and delivery plus cord levels. A two-sided P-value of < 0.05 was considered statistically significant. All data analyses were performed utilizing GraphPad Prism (GraphPad Software Inc, La Jolla, CA).
Results
As detailed in Table 1, there were 45 ECGs available for interpretation within the first 4 months of life in unaffected infants. Levels of HCQ obtained throughout pregnancy, albeit not available in 5 (11%) of the cord bloods, confirmed fetal exposure. Given that the terminal elimination half-life of HCQ is very long,7 the unavailability of a cord blood HCQ level did not preclude inclusion in this study. Overall, there was no correlation between cord blood levels of HCQ and the QTc (R = 0.02, P = 0.86) or the average value of HCQ levels obtained during each individual pregnancy and cord blood and the QTc (R = 0.04, P = 0.80), as shown in Figure 1A and Figure 1B. Likewise there was no correlation between the average of the maternal HCQ levels obtained at each trimester and delivery plus cord levels and the QTc on the ECGs of the 31 infants evaluated on day of life 1–4 (R = 0.08, P = 0.63) or those of the 14 children older than 4 days (R = 0.01, P = 0.95).
Table 1:
Newborn Electrocardiographic QT Intervals and Hydroxychloroquine Exposure
| ID | Gestational Age (wks) |
Sex | Birth Weight (kg) |
Maternal 1T HCQ | Maternal 2T HCQ | Maternal 3T HCQ | Maternal Delivery HCQ | Cord HCQ | QTc Interval |
|---|---|---|---|---|---|---|---|---|---|
| 3A | 34 | M | 2.8 | NA | 1306 | NA | < 100 | 156 | 370 |
| 3B | 34 | F | 2.2 | 114 | 405 | ||||
| 10 | 41 | F | 3.01 | 161 | 1205 | < 100 | 311 | 693 | 448 |
| 11 | 37 | F | 2.55 | 830 | 870 | 662 | 711 | 1198 | 415 |
| 14 | 41 | F | 3.36 | < 100 | 1153 | 161 | < 100 | <100 | 436 |
| 17 | 35 | F | 2.07 | 656 | 718 | 1176 | 1330 | 320 | 417 |
| 20 | 39 | M | 3.74 | 1270 | 801 | 795 | 742 | 632 | 410 |
| 21 | 39 | F | 3.32 | < 100 | 604 | 688 | 453 | 389 | 390 |
| 22 | 41 | F | 3.92 | < 100 | < 100 | 179 | < 100 | <100 | 412 |
| 24 | 38 | F | NA | NA | NA | NA | 640 | 609 | 420 |
| 26 | 39 | F | 3.27 | 569 | 1132 | 1601 | 1637 | 1143 | 458 |
| 29 | 39 | F | 3.59 | 669 | 525 | 925 | 1097 | 868 | 384 |
| 33 | 35 | M | 2.90 | 869 | 603 | 798 | 723 | 507 | 422 |
| 36 | 39 | M | 3.18 | 671 | 660 | 1992 | 1204 | 975 | 436 |
| 40 | 39 | M | 3.03 | 951 | 3264 | 890 | 717 | 682 | 397 |
| 41 | 37 | F | 2.18 | 1195 | 900 | 905 | 571 | 1778 | 416 |
| 42 | 39 | M | 3.06 | 611 | 891 | 969 | NA | NA | 412 |
| 44 | 39 | F | 3.90 | 1256 | 1309 | 1058 | 1661 | 1349 | 442 |
| 45 | 35 | F | 2.14 | 1308 | 1149 | NA | NA | NA | 484 |
| 49 | 36 | F | 2.32 | < 100 | 341 | 566 | 735 | 631 | 438 |
| 52 | 39 | M | 3.51 | 509 | 1063 | 905 | 810 | 375 | 424 |
| 54 | 39 | M | 3.17 | 671 | 601 | 615 | 854 | 1130 | 425 |
| 55 | 38 | F | 2.77 | 1251 | 1445 | 983 | NA | NA | 393 |
| 57 | 39 | F | 2.83 | 134 | 1235 | 900 | 1513 | 738 | 445 |
| 60 | 39 | F | 3.49 | < 100 | 700 | NA | 839 | 836 | 445 |
| 61 | 38 | M | 3.42 | 678 | 884 | 1017 | 938 | 585 | 492 |
| 62 | 40 | M | 2.99 | NA | NA | NA | 1202 | 966 | 433 |
| 69 | 37 | F | 2.00 | 721 | 794 | 805 | 855 | 486 | 439 |
| 71 | 37 | M | 3.03 | 527 | 1409 | 1999 | 537 | 671 | 442 |
| 73 | 35 | M | NA | 597 | 459 | 481 | 363 | 746 | 413 |
| 74 | 35 | M | 2.63 | 761 | 916 | 783 | 660 | 348 | 422 |
| 75 | 37 | F | 3.73 | 1152 | 1406 | 1251 | 1842 | 1678 | 335 |
| 77 | 35 | M | 2.04 | 274 | 552 | NA | 1007 | 655 | 401 |
| 80 | 37 | M | 3.04 | 707 | 831 | 335 | 688 | 154 | 347 |
| 82 | 37 | M | 3.77 | 1866 | 1251 | 829 | NA | NA | 451 |
| 83 | 39 | M | 3.69 | 930 | 213 | 849 | 715 | 1124 | 404 |
| 84 | 39 | M | 2.54 | 362 | 740 | 866 | 819 | 533 | 450 |
| 85 | 38 | M | 3.42 | 405 | 1234 | 854 | 790 | 627 | 394 |
| 87 | 39 | M | 2.69 | 609 | 527 | 626 | 957 | 658 | 441 |
| 88 | 38 | M | NA | 1005 | 1153 | 801 | 1173 | 658 | 426 |
| 89 | 39 | F | NA | 808 | 552 | 642 | 1168 | 811 | 471 |
| 90 | 38 | F | NA | 1363 | 862 | 1694 | 1028 | 1366 | 419 |
| 91 | 36 | F | 3.07 | 363 | NA | NA | NA | NA | 454 |
| 93 | 39 | F | 3.01 | 250 | 914 | 1287 | 834 | 637 | 353 |
| 95 | 37 | F | 2.86 | 663 | 711 | 788 | 832 | 696 | 417 |
The five infants with prolonged QTc intervals are indicated in bold. All hydroxychloroquine (HCQ) levels are displayed in ng/mL, and they correspond to levels during the first, second and third trimesters and at delivery, as well as levels in the infant’s cord blood at birth. For samples < 100 ng/ml, which meant below level of detection, 50ng g/ml was used to calculate average exposure. QTc intervals are displayed in msec.
Figure 1. Correlation between blood HCQ levels and neonatal QTc.
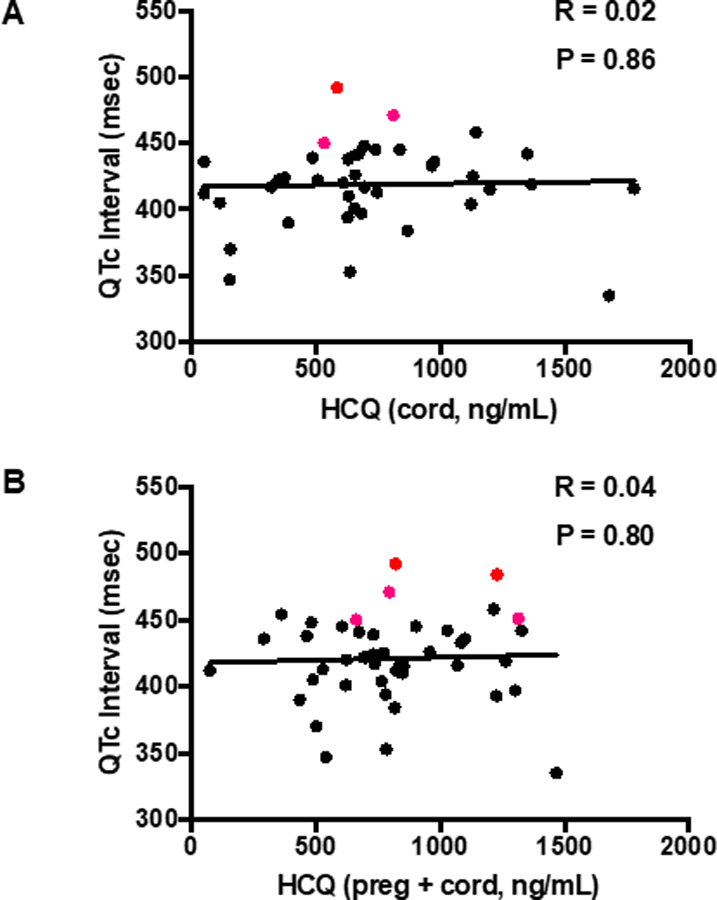
(Panel A) Cord HCQ is plotted against QTc. (Panel B) Overall mean HCQ levels (obtained by averaging all HCQ levels throughout an individual pregnancy and cord blood level) are plotted against QTc. Subjects with an abnormally prolonged QTc are designated with red bold circles. All QTc intervals were calculated using the Bazett formula (QTcB).
Maternal values of HCQ were sustained throughout pregnancy and delivery (Figure 2). Mean QTc values were nearly identical between those in the highest and lowest quartiles of cord blood HCQ levels (P = 0.57) and between the highest and lowest quartiles of average HCQ levels during pregnancy (P = 0.54) (Figure 3A and 3B).
Figure 2.
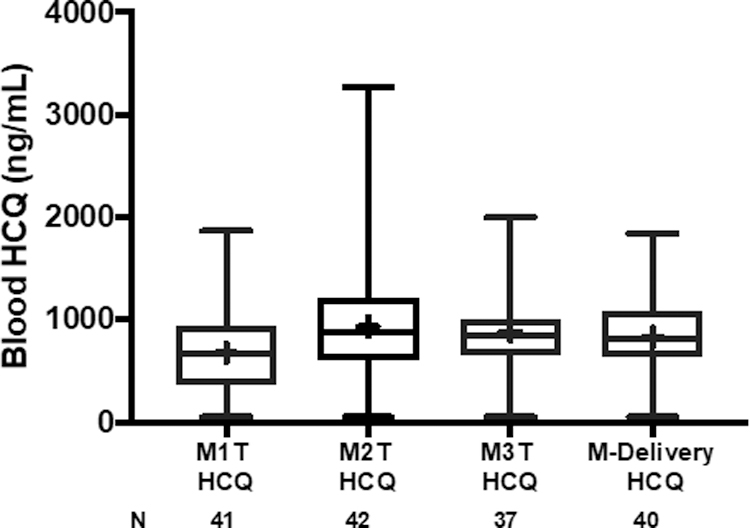
Box plots of maternal blood levels of HCQ during each trimester of pregnancy and delivery. M1T is baseline first trimester. M2T is second trimester. M3T is third trimester. M-Delivery is at the time of delivery. Median levels of HCQ (interquartile range) for M1T: 669 ng/mL (363–941); M2T: 877 ng/mL (604–1212); M3T: 849 ng/mL (652–1000); M-Delivery: 815 ng/mL (645–1080). Mean values denoted by + in box plot.
Figure 3.
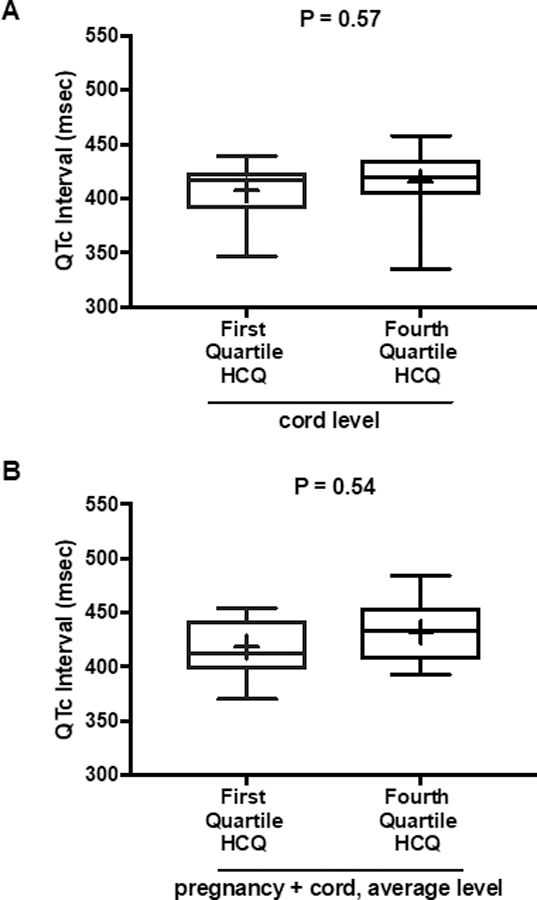
Box plots of QTc interval data for subjects in first and fourth quartiles of cord blood HCQ levels and average HCQ levels during pregnancy. (Panel A) Comparison of QTc between the first and fourth quartiles of HCQ cord blood levels. Median QTc (interquartile range) in first HCQ quartile: 417 msec (390–424); in fourth HCQ quartile: 419 msec (404–436). (Panel B) Comparison of QTc between the first and fourth quartiles of HCQ levels averaged over pregnancy and cord blood. Median QTc (interquartile range) in first HCQ quartile: 413 msec (398, 443); in fourth HCQ quartile: 433 msec (407, 455). Mean values denoted by + in box plots. All QTc intervals were calculated using the Bazett formula (QTcB).
Among these 45 infants, only 5 had prolongation of the QTc (11%; 95% CI: 4% - 24%), 2 marked and 3 marginal. One (#45) was an asymptomatic female born prematurely at 35 weeks 3 days gestation whose QTc on the first day of life was 484 msec. No follow-up ECG is presently available. Although cord blood HCQ levels were not obtained in this case, the mother reported taking the drug throughout pregnancy and provided bloods during the first and second trimesters which substantiated compliance with values > 1,000ng/ml. The second case (#61) was a full-term asymptomatic male newborn whose QTc on the first day of life was 503 msec. A repeat ECG on day 2 of life revealed sustained prolongation at 492 msec. No further ECG data are available; however verbal contact with the mother confirmed no cardiac concerns. Cord blood HCQ was 585ng/ml with an average level of HCQ at 879 ng/ml throughout pregnancy. Of relevance, with regard to these two infants (#45 and #61), their ECG tracings were more challenging to interpret due to baseline artifact, with prominent u waves, such that the QTc may have been incorrectly read as prolonged. In fact, review by the local pediatric cardiologist was “normal.” The third case (#89) was a full-term asymptomatic female whose QTc was 471 msec on the first day of life. Cord blood HCQ was 811ng/ml with an average level of HCQ at 793ng/ml throughout pregnancy. While the mother reports no health issues with the child, no further ECGs are available. The two other cases (# 82, # 84), both males, had minimal QTc prolongation of 451 msec on day 1 of life and 450 msec on day 2 of life, respectively. Cord blood levels were unknown in #82 but maternal levels were close to 1,000ng/ml during the first, second and third trimesters. The cord blood level was 533ng/ml in case # 84 with an average HCQ level of 697ng/ml throughout pregnancy. No arrhythmias occurred in any neonate that was not known to have heart block.
Discussion
The safety of HCQ use in pregnancy is a critical issue especially in consideration of its now very extended and immediate application to prevent and treat high-risk and positively infected individuals with SARS-CoV-2. Gathering information to balance against potential antiviral efficacy is needed. Albeit limited in numbers, the data presented in this sub-study revealed that, of fetuses with no evidence of CHB who were exposed to HCQ, 89% of their neonatal ECGs exhibited normal QTcs.
Moreover, assurances of compliance with the medication strengthen the value of the data since many previous reports in patients with SLE indicate non-adherence to HCQ in up to 31%.21 Relevant to antiviral efficacy, the blood levels observed throughout pregnancy and in the cord blood were largely equivalent to or even exceeded the mean serum level of HCQ (serum 460 ng/ml which approximates whole blood 920 ng/ml per personal communication, NC: mean ratio of serum/whole blood levels of HCQ is 0.53 ± 0.15; manuscript in review) reported in the one open label COVID-19 study which suggested efficacy.4
Previous studies have considered the cardiac safety in offspring exposed to maternal HCQ. Costedoat-Chalumeau and colleagues reported on the ECGs of 92 newborns at the third day of life, 47 of whom were exposed to maternal HCQ (doses 200mg to 400mg) and 45 control newborns whose mothers did not receive HCQ.22 There were no statistical differences in the mean ± SD QTc intervals between the 2 groups (406 ± 34 msec vs 407 ± 32 msec, HCQ-exposed and HCQ-unexposed respectively, P = 0.40), with all newborns having normal ECGs. However, this study did not systematically verify maternal compliance by measuring HCQ maternal or cord blood levels. Further evidence for safety is addressed in an autopsy performed on one fetus in the PATCH study in which complete heart block was detected at 19 weeks despite HCQ. Reassuringly, there were no histologic findings consistent with HCQ toxicity such as myelin figures.23
One potential limitation in the study reported herein is that a control group of anti-SSA/Ro pregnancies unexposed to HCQ was not available. Therefore, an effect of maternal autoantibodies on the neonatal QTc cannot be entirely excluded. The contribution of anti-SSA/Ro is considered in part by Costedoat-Chalumeau in a study which included an evaluation of neonatal ECGs in 58 anti-SSA/Ro-exposed children compared to 85 anti-SSA/Ro-negative mothers with a known connective tissue disease. There were no differences in mean QTc ± SD between these groups (mean QTc values of 397 ± 27 msec anti-SSA/Ro positive and 395 ± 25 msec anti-SSA/Ro negative, P = 0.57).24 Although HCQ levels were not obtained, in 60% of pregnancies in both groups, the mother took HCQ. Data were not specifically provided on anti-SSA/Ro mothers who did not take HCQ. A second study also affirms that our results are not confounded by maternal anti-SSA/Ro antibodies and are likely to be applicable to the general population of otherwise healthy pregnant women who may be treated with HCQ.25 Specifically, in a prospective study by Gerosa and colleagues, ECGs were done in infants 20 – 90 days of age. The mean QTc interval ± SD was 414 ± 17 in 46 anti-SSA/Ro exposed infants, 420 ± 23 in 25 non-anti-SSA/Ro exposed infants born to mothers with an autoimmune connective tissue disease, and 416 ± 16 in 200 infants of otherwise healthy mothers. A QTc interval > 470ms was observed in none of the anti-SSA/Ro-exposed infants. HCQ was used in about a fifth of these mothers.
Two other considerations are also relevant for clinicians to put these data in perspective. Study subjects were at increased risk of potential prolongation of QTc shortly after birth because they were exposed to high levels of HCQ throughout pregnancy in contrast to the short course of drug being used in COVID-19 trials. Historical controls were used, and specifically referred to the entire newborn period (birth to one month of life) and not specifically to days 1, 2, or 3 of life in which the QT interval is more variable and often somewhat prolonged. These considerations, combined with some difficulties related to baseline artifact and u waves, may have resulted in an over-reading of QTc intervals.
Although the present study was limited to high titer anti-SSA/Ro positive mothers who were not ill with a severe potentially multi-system virus and not simultaneously taking other QT prolonging drugs, the data should be applicable to otherwise healthy pregnant women being considered for preventive anti-COVID-19 strategies, especially since HCQ will not likely be given in sustained doses. In aggregate, these data provide reassurances that the maternal use of HCQ is associated with a low incidence of infant QTc prolongation. However, since 11% of the neonates (with levels unequivocally assuring exposure to HCQ) did manifest QTc prolongation shortly after birth, although asymptomatic, consideration should be given to an early postnatal ECG with appropriate follow-up ECG monitoring as determined by the initial findings.
Supplementary Material
What Is Known:
Hydroxychloroquine (HCQ) is being tested widely for therapeutic/prophylactic efficacy against COVID-19, but it is not known if pregnant women can be safely entered into trials given potential prolongation of the infant QTc.
What the Study Adds:
Five of 45 (11%) of infants exposed to HCQ 400mg in utero had prolongation of the QTc > 2SD but were otherwise asymptomatic, and there was no correlation with maternal or cord HCQ levels.
Maternal HCQ use is associated with a low incidence of infant QTc prolongation.
Sources of Funding:
This research was supported by the Lupus Foundation of Minnesota, the Lupus Foundation of America (LIFELINE Grant), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R03HD069986, J.P.B.; R01HD079951, J.P.B.).
Nonstandard Abbreviations and Acronyms
- CHB
congenital heart block
- COVID-19
coronavirus disease
- ECG
electrocardiogram
- EFE
endocardial fibroelastosis
- HCQ
hydroxychloroquine
- QTc
Corrected QT interval
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SLE
systemic lupus erythematosus
Footnotes
Disclosures: None.
References:
- 1.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao J, Tian Z and Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. [DOI] [PubMed] [Google Scholar]
- 4.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, Ling Y, Huang D, Song S, Zhang D, et al. [A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19]. Randomized Controlled Trial. 2020;49:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costedoat-Chalumeau N, Amoura Z, Aymard G, Le TH, Wechsler B, Vauthier D, Dermer ME, Darbois Y, Piette JC. Evidence of transplacental passage of hydroxychloroquine in humans. Arthritis Rheum 2002;46:1123–4. [DOI] [PubMed] [Google Scholar]
- 7.Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol 1989;27:771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020;16:155–166. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. [DOI] [PubMed] [Google Scholar]
- 10.Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, Marder W, Guyatt G, Branch DW, Buyon J, et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol 2020;72:529–556. [DOI] [PubMed] [Google Scholar]
- 11.Izmirly PM, Kim MY, Llanos C, Le PU, Guerra MM, Askanase AD, Salmon JE, Buyon JP. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis 2010;69:1827–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, Friedman D, Llanos C, Piette JC, Buyon JP. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diav-Citrin O, Blyakhman S, Shechtman S, Ornoy A. Pregnancy outcome following in utero exposure to hydroxychloroquine: a prospective comparative observational study. Reprod Toxicol 2013;39:58–62. [DOI] [PubMed] [Google Scholar]
- 14.Costedoat-Chalumeau N, Amoura Z, Huong DL, Lechat P, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun Rev 2005;4:111–5. [DOI] [PubMed] [Google Scholar]
- 15.Izmirly P, Kim M, Friedman DM, Costedoat-Chalumeau N, Clancy R, Copel JA, et al. Hydroxychloroquine to Prevent Recurrent Congenital Heart Block in Fetuses of Anti-SSA/Ro-Positive Mothers. J Am Coll Cardiol. 2020. July 21;76(3):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed JH, Clancy RM, Lee KH, Saxena A, Izmirly PM, Byron JP. Umbilical cord blood levels of maternal antibodies reactive with p200 and full-length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus. Arthritis Care Res 2012;64(9):1373–1381. 10.1002/acr.21704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costedoat-Chalumeau N, Galicier L, Aumaitre O, Frances C, Le Guern V, Liote F, Smail A, Limal N, Perard L, Desmurs-Clavel H, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 2013;72:1786–92. [DOI] [PubMed] [Google Scholar]
- 18.Noe G, Amoura Z, Combarel D, Lori L, Tissot N, Seycha A, Funck-Brentano C, Zahr N. Development and Validation of a Fast Ultra-High Performance Liquid Chromatography-Fluorescent Method for the Quantification of Hydroxychloroquine and Its Metabolites in Patients With Lupus. Ther Drug Monit 2019;41:476–482. [DOI] [PubMed] [Google Scholar]
- 19.Rijnbeek PR, Witsenburg M, Schrama E, Hess J, Kors JA. New normal limits for the paediatric electrocardiogram. Eur Heart J 2001;22:702–11. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costedoat-Chalumeau N, Houssiau F, Izmirly P, Guern VL, Navarra S, Jolly M, Ruiz-Irastorza G, Baron G, Hachulla E, Agmon-Levin N, et al. A Prospective International Study on Adherence to Treatment in 305 Patients With Flaring SLE: Assessment by Drug Levels and Self-Administered Questionnaires. Clin Pharmacol Ther 2019;106:374–382. [DOI] [PubMed] [Google Scholar]
- 22.Costedoat-Chalumeau N, Amoura Z, Duhaut P, Huong DL, Sebbough D, Wechsler B, Vauthier D, Denjoy I, Lupoglazoff JM, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty-three cases compared with a control group. Arthritis Rheumatol 2003;48:3207–11. [DOI] [PubMed] [Google Scholar]
- 23.Friedman D, Lovig L, Halushka M, Clancy RM, Izmirly PM, Buyon JP. No histologic evidence of foetal cardiotoxicity following exposure to maternal hydroxychloroquine. Clin Exp Rheumatol 2017;35:857–859. [PMC free article] [PubMed] [Google Scholar]
- 24.Costedoat-Chalumeau N, Amoura Z, Lupoglazoff JM, Huong DL, Denjoy I, Vauthier D, Sebbouh D, Fain O, Georgin-Lavialle S, Ghillani P, et al. Outcome of pregnancies in patients with anti-SSA/Ro antibodies: a study of 165 pregnancies, with special focus on electrocardiographic variations in the children and comparison with a control group. Arthritis Rheumatol 2004;50:3187–94. [DOI] [PubMed] [Google Scholar]
- 25.Gerosa M, Cimaz R, Stramba-Badiale M, Goulene K, Meregalli E, Trespidi L, Acaia B, Cattaneo R, Tincani A, Motta M, et al. Electrocardiographic abnormalities in infants born from mothers with autoimmune diseases--a multicentre prospective study. Rheumatology (Oxford). 2007;46:1285–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


