Abstract
Objective:
To evaluate the overall survival of patients with operable stage IA non-small-cell lung cancer (NSCLC) who undergo “early” SBRT (within 0–30 days after diagnosis) versus “delayed” surgery (90–120 days after diagnosis).
Summary of Background Data:
During the COVID-19 pandemic, national guidelines have recommended patients with operable stage IA NSCLC to consider delaying surgery by at least 3 months or, alternatively, to undergo SBRT without delay. It is unknown which strategy is associated with better short- and long-term outcomes.
Methods:
Multivariable Cox proportional hazards modeling and propensity score-matched analysis was used to compare the overall survival of patients with stage IA NSCLC in the National Cancer Data Base from 2004 to 2015 who underwent “early” SBRT (0–30 days after diagnosis) versus that of patients who underwent “delayed” wedge resection (90–120 days after diagnosis).
Results:
During the study period, 570 (55%) patients underwent early SBRT and 475 (45%) underwent delayed wedge resection. In multivariable analysis, delayed resection was associated with improved survival [adjusted hazard ratio 0.61; (95% confidence interval (CI): 0.50–0.76)]. Propensity-score matching was used to create 2 groups of 279 patients each who received early SBRT or delayed resection that were well-matched with regard to baseline characteristics. The 5-year survival associated with delayed resection was 53% (95% CI: 45%–61%) which was better than the 5-year survival associated with early SBRT (31% [95% CI: 24%–37%]).
Conclusion:
In this national analysis, for patients with stage IA NSCLC, extended delay of surgery was associated with improved survival when compared to early treatment with SBRT.
Keywords: COVID-19, lung cancer, NSCLC, SBRT, stereotactic body radiation therapy, surgery, wedge resection
The first case of COVID-19 in the United States was detected in Washington State on January 20, 2020.1 By May of 2020, there were ∼1.5 million confirmed cases and over 90,000 deaths in all 50 states.2 In response to the pandemic, many hospitals postponed elective surgeries3,4,5,6 and delayed both surgical and systemic treatments5,7,8,9,10 of cancer to preserve limited hospital resources and protect patients from potential exposure to COVID-19. For patients with operable stage IA non-small-cell lung cancer (NSCLC), several American and European medical societies have recommended delaying surgery for an extended period of time.4,11,12,13 In lieu of surgery, stereotactic body radiotherapy (SBRT) has also been recommended for patients with operable stage IA NSCLC during the COVID pandemic.4,9,12,13,14,15
Because the current crisis is unprecedented, there is very little, if any, data to support the above-noted recommendations. It is unclear whether timely treatment of stage IA NSCLC with SBRT is better than extended delay of surgery. The objective of this study was to use data from the National Cancer Data Base (NCDB) to evaluate the overall survival of patients given SBRT within 0–30 days after diagnosis versus the overall survival of patients who underwent wedge resection 90–120 days after diagnosis. We aimed to provide clinicians with data that could be used to inform treatment decision-making for patients with stage IA NSCLC either during the present COVID-19 pandemic or in preparation of any future pandemic waves.
METHODS
Data Source: NCDB
The NCDB is a clinical oncology database and a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data collected from the NCDB are estimated to include >80% of newly diagnosed lung cancer cases in the U.S.16 Staging was reclassified using best available data according to AJCC eighth edition criteria.17
Study Design
All patients with clinical stage IA NSCLC with adenocarcinoma, squamous cell carcinoma, or large cell histology (identified via International Classification of Diseases for Oncology, third edition histology and topography codes) from 2004 to 2015 who were treated with extended “delayed” wedge resection (ie, wedge resection performed 90–120 days after diagnosis) or “early” SBRT (ie, SBRT performed 0–30 days after diagnosis) were included. The time intervals chosen for “early” and “delayed” were based on guidelines and observations noted from previous literature, and were defined a priori as 0–30 days15,18,19 and 90–120 days.4,11,12 As described previously,20 in an effort to minimize bias, with the surgical cohort, we chose to limit the inclusion criteria to only patients who underwent wedge resection, as opposed to anatomic lung resection (eg, with segmentectomy or lobectomy).
In addition, we excluded patients who were coded in the NCDB as having undergone SBRT because surgery “was not recommended/performed because it was contraindicated due to patient risk factors (comorbid conditions, advanced age, etc).” We also restricted the analysis to patients with no history of prior malignancy. Patients with other neuroendocrine tumors and previously-termed “bronchioloalveolar” tumors were excluded because these histologic subtypes are usually associated with better prognoses than other types of NSCLC.21,22 The primary outcome was overall survival.
Statistical Analysis
Patients were grouped based on whether they received “early” SBRT or “delayed” wedge resection. Pearson Chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables were used to determine differences in patient and post-treatment characteristics. Survival was measured from the start date of treatment to death or date of last follow-up.
We examined differences in cumulative survival in patients who received “early” SBRT versus “delayed” wedge in the NCDB, using the Kaplan-Meier method and the log-rank test. In addition, a multivariable Cox proportional hazards model was used to evaluate differences in overall survival between the SBRT and wedge groups, adjusting for age, sex, race, Charlson Deyo comorbidity score (CDCC score), clinical T status, tumor size, tumor location, histology, insurance status, facility type, facility treatment volume, distance from the hospital, income, education, and year of diagnosis. The proportional hazards assumption was evaluated for the Cox models using smooth scaled Schoenfeld residual plots and there were no violations of assumptions found.
Next, we used propensity scores to match patients in the “early” SBRT and “delayed” wedge treatment groups.23 Briefly, patients were stratified into 2 groups (ie, those who underwent “early” SBRT and “delayed” wedge) and a logistic regression model was used to calculate propensity scores based on patient- and disease-related variables that were determined to most likely act as confounders. These variables were determined a priori and included the same above-mentioned variables used in our Cox proportional hazards model. We applied a greedy nearest neighbor matching algorithm without replacement with a caliper of 0.01 to calculate propensity scores. Balance of the match was assessed using standardized differences. We examined the cumulative survival in the matched “early” SBRT and “delayed” wedge treatment groups using the Kaplan-Meier method and log-rank test.
Sensitivity Analyses
The above-mentioned matched analysis using propensity scores, applying the same covariates and matching algorithm detailed above, was performed for patients with no co-morbidities, defined as a CDCC score of 0.
All statistical analyses were performed using R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) and Stata version 13.0 (StataCorp LP, College Station, TX). This study was approved by the Institutional Review Boards at Duke and Stanford University.
RESULTS
During the study period, 570 (55%) patients underwent early SBRT and 475 (45%) underwent delayed wedge resection (Fig. 1). Patient and tumor characteristics are detailed in Supplemental Table 1. When compared to the early SBRT group, patients undergoing delayed wedge were younger, were less likely to be White, had a higher CDCC score, were less likely to have T1c tumors, and were more likely to have a histology of adenocarcinoma.
FIGURE 1.
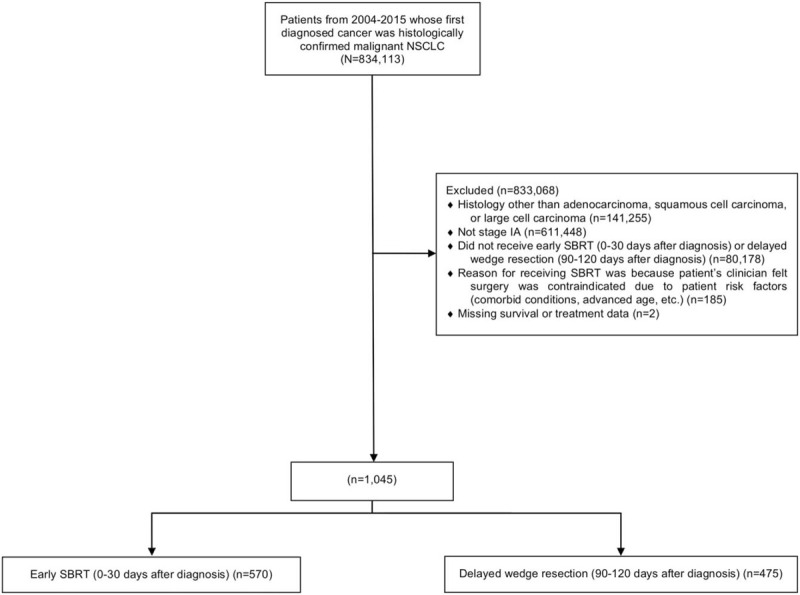
Flow diagram showing schema of study subject selection of patients with stage IA adenocarcinoma, squamous cell carcinoma, or large cell carcinoma who received early SBRT (0-30 d after diagnosis) versus delayed wedge resection (90–120 d after diagnosis). SBRT indicates stereotactic body radiotherapy.
The median follow-up time was 27.6 months (interquartile range 14.4–47.0 months). There were 251 deaths in the early SBRT group and 224 deaths in the delayed wedge group. There were no significant differences in 30-day mortality between the delayed wedge [1.1% (n < 10)] and SBRT [0.4% (n < 10)] groups (P = 0.26). There were no significant differences in 90-day mortality between the delayed wedge [2.9% (n = 14)] and SBRT [1.9% (n = 11)] groups (P = 0.28).
In unadjusted analysis, delayed wedge resection was associated with better survival than early SBRT (5-year survival 49% [95% CI: 43%–54%] versus 31%% [95% CI: 24%–36%], log-rank, P < 0.001, Fig. 2). In multivariable analysis, delayed wedge resection was associated with improved survival when compared to early SBRT [adjusted hazard ratio 0.61; 95% CI: (0.50–0.76): P < 0.001] (Supplemental Table 2).
FIGURE 2.
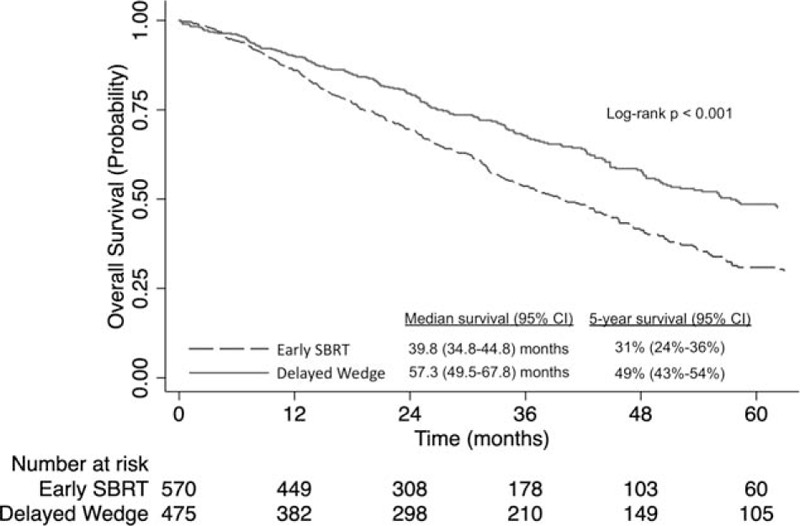
Kaplan-Meier analysis of overall survival for patients with stage IA NSCLC who received early SBRT (0–30 d after diagnosis) versus delayed wedge resection (90–120 d after diagnosis). NSCLC indicates non-small-cell lung cancer; SBRT, stereotactic body radiotherapy.
Propensity Score-matched Analysis
Propensity-score matching was used to create 2 groups of 279 patients each who had early SBRT or delayed wedge resection that were well-matched with regard to baseline characteristics (Supplemental Table 3). All standardized mean differences were less than or equal to 8.7%. The 30-day [1.1% (n < 10)] and 90-day mortality [2.9% (n < 10)] of the delayed wedge group was not significantly different from the 30-day [0.4% (n < 10)] and 90-day mortality [2.9% (n < 10)] associated with the SBRT group (P = 0.62 and P = 1.00, respectively). Delayed wedge resection was associated with better survival than early SBRT (5-year survival 53% [95% CI: 45%–61%] vs 31% [95% CI: 24%–37%], log-rank, P < 0.001, Fig. 3).
FIGURE 3.
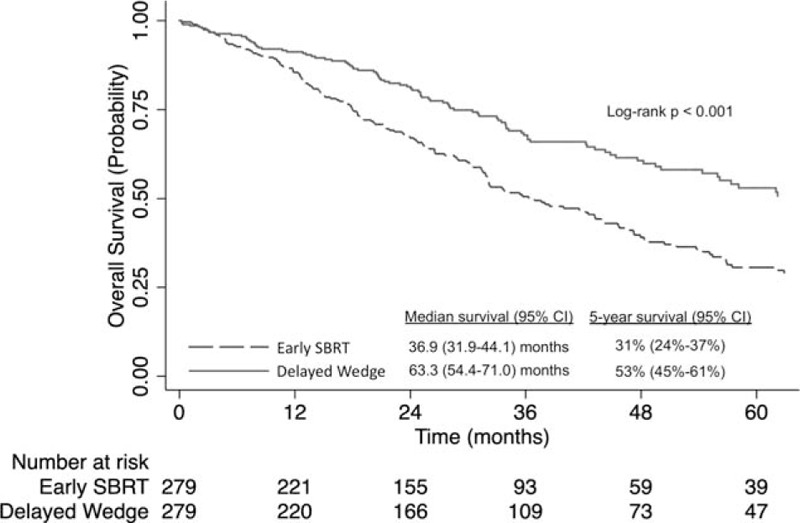
Kaplan-Meier analysis of overall survival for propensity score-matched patients with stage IA NSCLC who received early SBRT (0–30 d after diagnosis) versus delayed wedge resection (90–120 d after diagnosis). NSCLC indicates non-small-cell lung cancer; SBRT, stereotactic body radiotherapy.
Sensitivity Analysis
Propensity-score matching was used to create 2 groups of 95 patients each who had early SBRT or delayed wedge resection and no co-morbidities. These groups were well-matched with regard to baseline characteristics (data not shown). The 30-day [0% (n = 0)] and 90-day mortality [2.1% (n < 10)] of the delayed wedge group was not significantly different from the 30-day [1.1% (n < 10)] and 90-day mortality [1.1% (n < 10)] associated with the SBRT group (P = 1.00 and P = 1.00, respectively). Delayed wedge resection was associated with better survival than early SBRT (5-year survival 61% [95% CI: 46%–73%]) versus 31% [95% CI: 22%–42%], log-rank, P < 0.001, Fig. 4).
FIGURE 4.
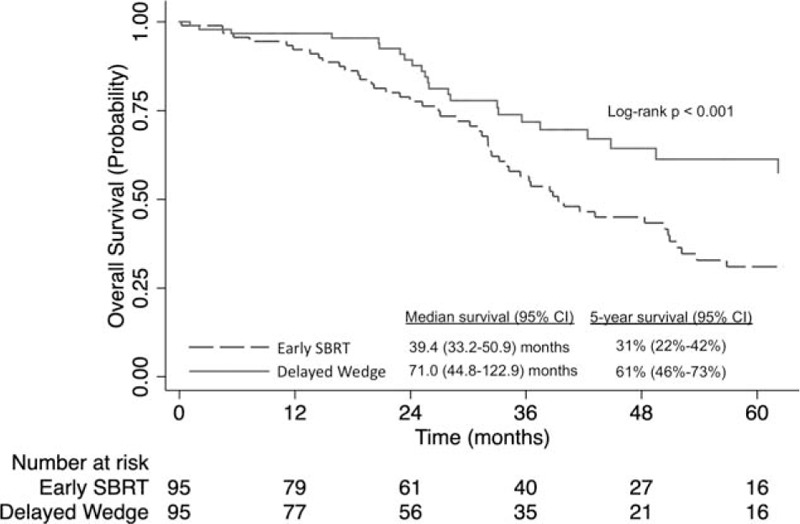
Kaplan-Meier analysis of overall survival for propensity score-matched patients with stage IA NSCLC who had a CDCC score of 0 (no major comorbidities) and received early SBRT (0–30 d after diagnosis) versus delayed wedge resection (90–120 d after diagnosis). CDCC indicates Charlson comorbidity score; NSCLC indicates non-small-cell lung cancer; SBRT, stereotactic body radiotherapy.
DISCUSSION
In this study, we evaluated the perioperative mortality and long-term survival of patients who underwent “early” SBRT (ie, SBRT within 30 days of diagnosis) versus “delayed” wedge resection (ie, wedge resection 90–120 days after diagnosis) for stage IA NSCLC in the NCDB. In unadjusted, multivariable, and propensity score-matched analysis, we found that delayed wedge resection was associated with similar 30- and 90-day mortality and improved long-term survival when compared to early SBRT. This finding was consistent in our sensitivity analysis where we performed the same propensity score-matched analysis, as above, but limited the comparison to only patients with no major comorbidities.
Numerous studies have compared outcomes of SBRT to surgery for early stage NSCLC with conflicting results24,25,26,27,28,29,30,31,32,33,34,35,36 and currently there are 4 randomized trials in progress further evaluating SBRT vs surgery.37,38,39,40 However, none of the data from these prior or ongoing studies are particularly relevant for decision-making during the COVID-19 pandemic. Unlike pre-COVID times, when patients may be presented with a choice between timely receipt of surgery versus timely receipt of SBRT, during the pandemic, the questions facing some patients, particularly in areas severely strained by COVID-19, may be whether to undergo delayed surgery or timely SBRT. Recommendations from medical societies have noted that either delayed surgery or early SBRT is appropriate, although, to date, there have been no data supporting these guidelines. The present study findings suggest that, in the setting of the COVID pandemic, if a patient has stage IA NSCLC and cannot readily and safely undergo surgery immediately, then waiting for surgery 3–4 months after diagnosis is a strategy that can be carefully considered in a multidisciplinary setting.
Limitations
Because of the study's observational design, there is potential for residual confounding and selection bias to exist. To avoid a possible scenario where the surgery cohort is healthier or comprises patients medically operable by criteria not captured by the NCDB and to avoid a situation where the SBRT group is less healthy or comprises patients who were medically inoperable due to factors not captured by the NCDB, we excluded patients who had received SBRT because their physician thought surgery was contraindicated due to patient risk factors (eg, comorbid conditions, elderly age, etc).
Of note, in this study, for the surgery group, we specifically evaluated patients who underwent wedge resection, to try to limit confounding. Standard-of-care for stage IA NSCLC is lobectomy with mediastinal lymph node dissection or systematic lymph node sampling, and wedge resection is generally reserved for patients with poor pulmonary function or major comorbidities.11 We chose not to compare SBRT to lobectomy though, because of concerns previously raised. A critique of previous studies evaluating lobectomy vs SBRT is that results may be biased in favor of surgery because patients who undergo lobectomy may be healthier than patients who undergo SBRT.41 We aimed to minimize bias by selecting, for the surgery group, patients who underwent wedge resection, as these patients generally have more underlying comorbidities than patients undergoing anatomic lung resection. Of note, in the present study, the wedge resection group had more comorbidities and were presumably less fit than the SBRT group. By including only wedge resections, the surgery cohort likely included higher-risk patients and may be more similar to the SBRT cohort than a surgery cohort that included patients undergoing anatomic lung resections.
Although there are important covariates such as pulmonary function data that are not available in the NCDB, in our multivariable analysis and propensity-score matching, we were able to include key covariates such as comorbidity scores. Of note, as a sensitivity analysis, we performed a matched analysis using propensity scores limited to patients with no major comorbidities and found results that were consistent with the primary analysis.
There are several more limitations to the study. First, our results are not necessarily generalizable to stage IB and other stages of NSCLC. In addition, they are not necessarily generalizable to other types of anatomic lung resection (eg, segmentectomy and lobectomy). However, given that anatomic lung resection is standard-of-care for early-stage NSCLC and associated with better outcomes than wedge resection, the study findings can likely be extrapolated to anatomic lung resection as well. Second, cancer-specific and recurrence-free survival are not available in the NCDB. Third, the NCDB does not have data on whether the tumors were peripheral or central, and patients who underwent SBRT may have had central tumors not amenable to wedge resection.
Lastly, for future lines of investigation, it will be important to evaluate whether patients who have SBRT followed by delayed surgery have better or worse short- and long-term outcomes than patients with delayed surgery alone, given that “salvage” or “completion” wedge resection after SBRT could be a consideration for patients who, during the COVID-19 pandemic, underwent SBRT but otherwise would have been candidates for surgery.
CONCLUSIONS
In this national analysis, for patients with stage IA NSCLC, extended delay of surgery was associated with improved survival when compared to early treatment with SBRT. This finding can be used to help inform the treatment decision-making process for early-stage NSCLC during the COVID-19 pandemic and in future pandemic waves.
Supplementary Material
Acknowledgments
The National Cancer Data Base (NCDB) data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Footnotes
TAD is a consultant for Scanlan (<$10,000). The remaining authors have no conflicts of interest to declare.
REFERENCES
- 1.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSEE) at Johns Hopkins University (JHU). Johns Hopkins University. Available at: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed May 21, 2020. [Google Scholar]
- 3.Commins J. Surgeon General Urges Providers to ‘Consider Stopping Elective Surgeries.’ Hospitals Push Back. Health Leaders. Available at: https://www.healthleadersmedia.com/clinical-care/surgeon-general-urges-providers-consider-stopping-elective-surgeries-hospitals-push. Accessed May 21, 2020. [Google Scholar]
- 4.Thoracic Surgery Outcomes Research Network Ig. COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network, the Society of Thoracic Surgeons and The American Association for Thoracic Surgery. J Thoracic Cardiovasc Surg 2020; 160:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stahel PF. How to risk-stratify elective surgery during the COVID-19 pandemic? Patient Saf Surg 2020; 14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacobucci G. Covid-19: all non-urgent elective surgery is suspended for at least three months in England. BMJ 2020; 368:m1106. [DOI] [PubMed] [Google Scholar]
- 7.Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw 2020; 1:1–4. [DOI] [PubMed] [Google Scholar]
- 8.Karen Weise MB, Nicholas BB. The Coronavirus Is Forcing Hospitals to Cancel Surgeries. New York City, NY: New York Times; 2020. [Google Scholar]
- 9.Guckenberger M, Belka C, Bezjak A, et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. Radiother Oncol 2020; 146:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghavan D, Chai SJ, Plate K, et al. Levine cancer institute approach to pandemic care of cancer patients. J Clin Oncol Oncol Pract 2020; 16:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehens. Short-Term Recommendations for Non-Small Cell Lung Cancer Management During the COVID-19 Pandemic (Version 1. 2020). National Comprehensive Cancer Network. Available at: https://www.nccn.org/covid-19/pdf/COVID_NSCLC.pdf. Accessed May 21, 2020. [Google Scholar]
- 12.COVID-19: Elective Case Triage Guidelines for Surgical Care. Thoracic Cancer Surgery. American College of Surgeons. Available at: https://www.facs.org/-/media/files/covid19/guidance_for_triage_of_nonemergent_surgical_procedures_thoracic_cancer.ashx. Accessed May 21, 2020. [Google Scholar]
- 13.Mazzone PJ, Gould MK, Arenberg DA, et al. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST expert panel report. Chest 2020; 158:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banna G, Curioni-Fontecedro A, Friedlaender A, et al. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open 2020; 5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ESMO Management and Treatment Adapted Recommendations in the COVID-19 ERA: Lung Cancer. European Society for Medical Oncology. Available at: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/lung-cancer-in-the-covid-19-era. Accessed May 20, 2020. [Google Scholar]
- 16.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008; 15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017; 151:193–203. [DOI] [PubMed] [Google Scholar]
- 18.The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. Thorax 1998; 53: Suppl 1: S1–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devbhandari MP, Bittar MN, Quennell P, et al. Are we achieving the current waiting time targets in lung cancer treatment? Result of a prospective study from a large United Kingdom teaching hospital. J Thorac Oncol 2007; 2:590–592. [DOI] [PubMed] [Google Scholar]
- 20.Yerokun BA, Yang CJ, Gulack BC, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2017; 154:675–86.e4. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6:244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010; 134:1628–1638. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian S, Higgins KA, Curran WJ, et al. Stereotactic body radiation therapy vs. surgery in early-stage non-small cell lung cancer: lessons learned, current recommendations, future directions. J Thorac Dis 2018; 10:1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yerokun BA, Yang C-FJ, Gulack BC, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non–small cell lung cancer. J Thorac Cardiovasc Surg 2017; 154:675–86.e4. [DOI] [PubMed] [Google Scholar]
- 26.Ackerson BG, Tong BC, Hong JC, et al. Stereotactic body radiation therapy versus sublobar resection for stage I NSCLC. Lung Cancer 2018; 125:185–191. [DOI] [PubMed] [Google Scholar]
- 27.Albano D, Bilfinger T, Nemesure B. 1-, 3-, and 5-year survival among early-stage lung cancer patients treated with lobectomy vs SBRT. Lung Cancer (Auckl) 2018; 9:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi A, Fang W, Sun Y, et al. Comparison of long-term survival of patients with early-stage non-small cell lung cancer after surgery vs stereotactic body radiotherapy. JAMA Netw Open 2019; 2:e1915724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 83:348–353. [DOI] [PubMed] [Google Scholar]
- 30.Lin Q, Sun X, Zhou N, et al. Outcomes of stereotactic body radiotherapy versus lobectomy for stage I non-small cell lung cancer: a propensity score matching analysis. BMC Pulm Med 2019; 19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokhles S, Verstegen N, Maat AP, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer 2015; 87:283–289. [DOI] [PubMed] [Google Scholar]
- 32.Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011; 81:1352–1358. [DOI] [PubMed] [Google Scholar]
- 33.Ostheimer C, Evers C, Palm F, et al. Mortality after radiotherapy or surgery in the treatment of early stage non-small-cell lung cancer: a population-based study on recent developments. J Cancer Res Clin Oncol 2019; 145:2813–2822. [DOI] [PubMed] [Google Scholar]
- 34.Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014; 149:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013; 24:1543–1548. [DOI] [PubMed] [Google Scholar]
- 36.Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014; 90:603–611. [DOI] [PubMed] [Google Scholar]
- 37.National Library of Medicine (U.S.). (2016-present). Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR). Identifier: NCT02984761. Available at: https://clinicaltrials.gov/ct2/show/NCT02984761. Accessed May 20, 2020. [Google Scholar]
- 38.National Library of Medicine (U.S.). (2015-present). SBRT (Stereotactic Body Radiation Therapy) vs. Surgery in High Risk Patients With Early Stage Lung Cancer. Identifier: NCT02562027. Available at: https://clinicaltrials.gov/ct2/show/NCT02562027. Accessed May 20, 2020. [Google Scholar]
- 39.National Library of Medicine (U.S.). (2020). JoLT-Ca Sublobar Resection (SR) Versus Stereotactic Ablative Radiotherapy (SAbR) for Lung Cancer (STABLE-MATES). Identifier: NCT02468024. Available at https://clinicaltrials.gov/ct2/show/NCT02468024. Accessed May 20, 2020. [Google Scholar]
- 40.National Library of Medicine (U.S.). (2019). Radical Resection Vs. Ablative Stereotactic Radiotherapy in Patients With Operable Stage I NSCLC (POSTILV). Identifier: NCT01753414. Available at: https://clinicaltrials.gov/ct2/show/NCT01753414. Accessed May 20, 2020. [Google Scholar]
- 41.Poullis M. Treatment outcomes in stage I lung cancer: a comparison of surgery and stereotactic body radiation therapy. J Thorac Oncol 2016; 11:e64–e65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


