Curable sexually transmitted infections in pregnant women may cause poor maternal and newborn outcomes worldwide. Syndromic management is practiced in many settings yet fails to identify most infections. Etiologic screening has promise, but further effectiveness and cost-effectiveness studies are needed.
Supplemental digital content is available in the text.
Background
Sexually transmitted infections (STI), such as chlamydial, gonorrheal, and trichomonal infections, are prevalent in pregnant women in many countries and are widely reported to be associated with increased risk of poor maternal and neonatal outcomes. Syndromic STI management is frequently used in pregnant women in low- and middle-income countries, yet its low specificity and sensitivity lead to both overtreatment and undertreatment. Etiologic screening for chlamydial, gonorrheal, and/or trichomonal infection in all pregnant women combined with targeted treatment might be an effective intervention. However, the evidence base is insufficient to support the development of global recommendations. We aimed to describe key considerations and knowledge gaps regarding chlamydial, gonorrheal, and trichomonal screening during pregnancy to inform future research needed for developing guidelines for low- and middle-income countries.
Methods
We conducted a narrative review based on PubMed and clinical trials registry searches through January 20, 2020, guidelines review, and expert opinion. We summarized our findings using the frameworks adopted by the World Health Organization for guideline development.
Results
Adverse maternal-child health outcomes of potential interest are wide-ranging and variably defined. No completed randomized controlled trials on etiologic screening and targeted treatment were identified. Evidence from observational studies was limited, and trials of presumptive STI treatment have shown mixed results. Subgroups that might benefit from specific recommendations were identified. Evidence on harms was limited. Cost-effectiveness was influenced by STI prevalence and availability of testing infrastructure and high-accuracy/low-cost tests. Preliminary data suggested high patient acceptability.
Discussion
Preliminary data on harms, acceptability, and feasibility and the availability of emerging test technologies suggest that etiologic STI screening deserves further evaluation as a potential tool to improve maternal and neonatal health outcomes worldwide.
The curable sexually transmitted infections (STIs) Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Trichomonas vaginalis (TV) are common in pregnant women in many countries. Regional estimates of STI prevalence among pregnant women vary1: NG, 1.2% (Latin America) to 4.6% (Southern Africa); CT, 0.8% (Asia) to 11.2% (Latin America); and TV, 3.9% (Latin America) to 24.6% (Southern Africa). Although it is difficult to fully elucidate their relative impact, multiple studies have found associations between these 3 STIs and increased risk of poor maternal and neonatal outcomes (e.g., miscarriage, stillbirth, preterm birth, low birth weight, and mother-to-child HIV transmission).2–6
Few countries recommend routine screening for chlamydial, gonorrheal, or trichomonal infection in pregnant women.7 The World Health Organization (WHO) recommends screening for HIV infection and syphilis8 but has no specific guidelines for other STIs beyond syndromic management, which limits treatment to symptomatic women.9 The frequently asymptomatic nature of STIs in women is well established,10 and syndromic management fails to identify most infected women. Syndromic management has modest sensitivity (40%–75%) and specificity (54%–76%) for detecting chlamydial and/or gonococcal infection.10 A study of HIV-infected pregnant women in South Africa found that only 24% of women who tested positive for a chlamydial, gonococcal, or trichomonal infection had vaginal symptoms (sensitivity), whereas 47% of those with symptoms were negative for all 3 infections (specificity).11 The poor specificity and sensitivity of syndromic management lead to both overtreatment and undertreatment. Poor antimicrobial stewardship may increase the risk of antibiotic resistance.12
The prevalence of and likely adverse outcomes associated with curable STIs in pregnant women suggest that etiologic STI screening of all pregnant women followed by targeted treatment might be beneficial. However, the evidence base around that intervention is insufficient to support the development of global recommendations. The WHO uses a systematic process for developing guidelines13 based, in part, on the Population, Intervention, Comparator, Outcomes (PICO) and Grading of Recommendations, Assessment, Development and Evaluations (GRADE) frameworks for formulating the question and assessing the benefits, harms, and other relevant factors. This narrative review aimed to describe key considerations and knowledge gaps regarding etiologic STI screening during pregnancy using the PICO and GRADE frameworks. We also aimed to identify key studies in progress that may contribute to addressing these knowledge gaps. Our goal was to inform future research contributing to the evidence needed for developing guidelines, particularly for low- and middle-income countries.
MATERIALS AND METHODS
This narrative review drew on focused PubMed literature searches, review of WHO and other agency guidelines, and expert opinion. International public health and clinical experts from academia, government, industry, and community-based organizations met on July 14, 2019, in Vancouver, British Columbia, Canada, to frame the initial inquiry. Presentations and discussion during the meeting were the initial source of information for the review. PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and clinical trials registry (https://clinicaltrials.gov and http://www.isrctn.com) searches conducted through January 20, 2020, were developed iteratively based on initial searches using the terms “pregnancy” and “screening,” and “sexually transmitted infections,” “chlamydia,” “gonorrhea,” or “trichomonas.” PubMed searches initially focused on review articles. Reference lists were examined to identify relevant studies. Randomized controlled trials, observational studies, modeling, and qualitative studies related to STI screening/treatment and presumptive STI treatment were examined. We limited our review to studies where the full-text was available in English. Because this was not a systematic review, literatures searches were not conducted systematically, and identified studies and articles were not assessed using standardized criteria.
We presented findings using the PICO and GRADE Evidence to Decision frameworks for health system/public health decisions14 and for tests in clinical practice and public health.15 The population (pregnant women in low- and middle-income countries), intervention (etiologic screening for CT, NG, and/or TV of all pregnant women followed by treatment and case management of those with positive test results), and comparator (syndromic STI management) were predetermined by the authors to delineate the scope of the project. We examined the following GRADE domains: priority/importance, test accuracy, desirable effects (benefits), undesirable effects (harms), resource requirements/cost-effectiveness, equity, acceptability, and feasibility. We did not formally address the quality of available evidence or develop recommendations.
Findings
Formulating the key question using the PICO format and selecting outcomes are critical initial steps in the WHO guideline process.13 (Fig. 1) The population, intervention, and comparator were selected a priori by the authors. The adverse outcomes of potential interest were wide-ranging. In meta-analyses of associations between chlamydial infection and adverse pregnancy outcomes, women with chlamydial infection had increased risk of preterm labor/birth, perinatal mortality, stillbirth, intrauterine fetal demise, and newborn low birth weight/birth size compared with those without chlamydial infection.4,5 The strength of those associations was attenuated in adjusted analyses and higher-quality studies. Chlamydial infection was found to increase mother-to-child HIV transmission by almost 50% in one study.3 A meta-analysis of trichomonal infection in pregnancy found that infected women had a 41% increased risk of preterm birth and 51% increase in having small for gestational age newborns compared with those without trichomonal infection.2 We did not identify any meta-analyses on maternal gonococcal infection; however, maternal gonococcal infection has been associated with preterm birth, low birth weight, and neonatal eye infections.6
Figure 1.
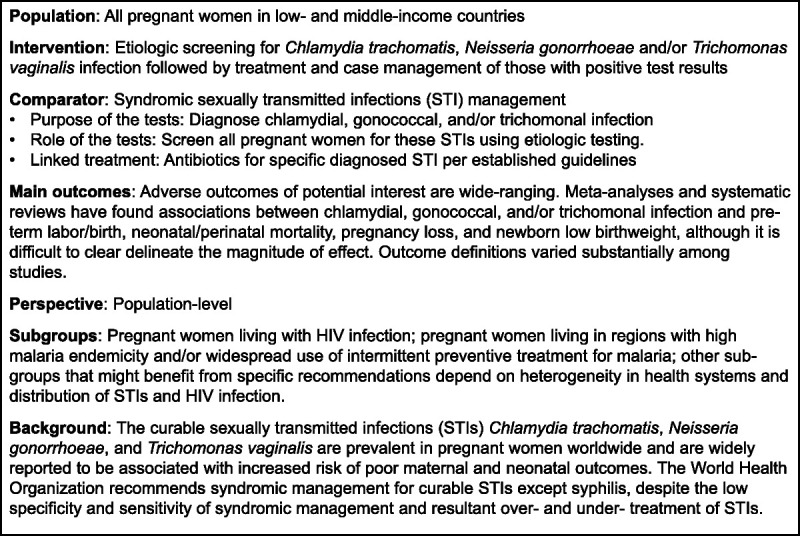
Population, intervention, comparison, and outcome model for etiologic screening for chlamydial, gonorrheal, and/or trichomonal infection in pregnant women in low- and middle-income countries.
Outcome definitions varied substantially among studies. Outcomes related to birth size have been examined using (1) mean birth weight,16 (2) low birth weight categorization based in weight (<2500 g)17,18 or chest/head circumference,19 or (3) intrauterine growth restriction categorization based on weight or height (<10th percentile).18 In some studies, gestational age was measured using ultrasound, a highly accurate method,20 whereas others used self-reported date of last menstrual period or fundal height, which is less accurate. Other outcome measures had similarly variable definitions across studies.
Subgroups
We identified several patient and population-level subgroups that might benefit from specific recommendations. Pregnant women living with HIV infection may have higher STI prevalence21 and a higher risk of poor birth outcomes22 than those without HIV infection, which may modify the effect of screening interventions. In malaria-endemic areas, sulfadoxine-pyrimethamine for intermittent preventive treatment in pregnant women may have some efficacy against CT and NG and associated adverse birth outcomes.18 As such, local implementation of intermittent preventive treatment of malaria23 could also influence the need for specific recommendations. Geographic heterogeneity in health systems and the distribution of STIs and HIV infection1 might indicate other identifiers of subgroups.
Figure 2 summarizes the GRADE Evidence to Decision domains.
Figure 2.
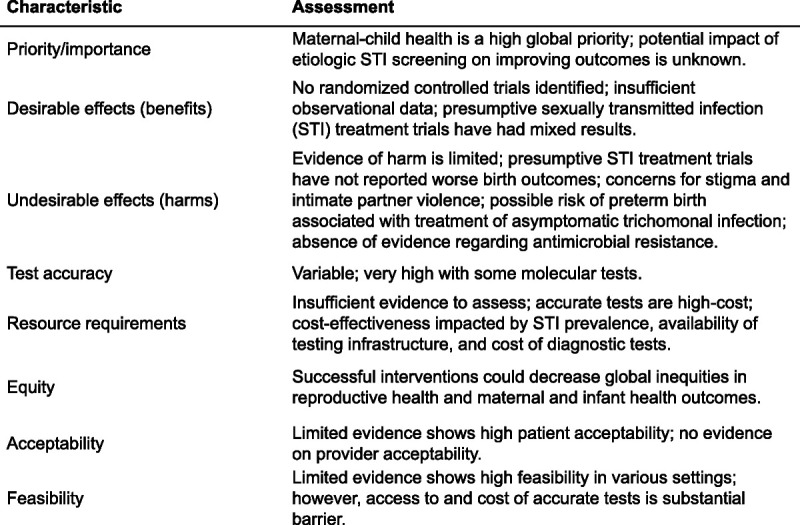
Summary of GRADE Evidence to Decision characteristics for etiologic screening and treatment of chlamydial, gonorrheal, and/or trichomonal infection in pregnant women in low- and middle-resource countries.
Priority/Importance
Improved maternal-child health is a primary target of the UN Sustainable Development Goals,24 and addressing STIs will contribute to meeting these targets. The high prevalence of STIs in pregnant women in low- and middle-income countries is established,1 and treatments are widely available and easy to administer.25,26 However, the magnitude of the impact of treating STIs in pregnant women on poor maternal-child outcomes has not yet been fully elucidated (see Benefits), a necessary step for establishing this area as a priority for intervention.
Benefits and Desirable Effects
The benefits of etiologic screening and treating curable STIs in pregnancy in low- and middle-income countries, apart from syphilis,9 have not been rigorously examined. We did not identify any completed clinical trials on etiologic gonococcal, chlamydial, or trichomonal screening in pregnant women in low- and middle-income countries. Some observational studies from high-income countries support chlamydial screening for improving pregnancy outcomes, but generalizability to low- and middle-income countries is unclear.21 Multiple authors of reviews and meta-analyses reported that insufficient information on confounders, including timing of infection versus testing/treatment, diagnosis of other infections, and other causes of poor maternal-child health outcomes, complicated the interpretation of the available evidence.4,21,27
Trials of presumptive STI treatment in pregnant women to improve maternal/neonatal outcomes provide information that may help elucidate the potential impact of screening and treatment.16,17,19 A cluster randomized controlled trial among ~4000 pregnant women in Uganda19 found that one-time treatment with azithromycin 1 g, cefixime 400 mg, and metronidazole 2 g, which were effective against NG, CT, TV, chancroid, and bacterial vaginosis, as well as several non-STI pathogens, resulted in a 17% decrease in early neonatal deaths and 47% improvement in birth weight compared with syndromic STI management. No effects on stillbirth, maternal deaths, or preterm delivery were identified. Three randomized trials of intermittent treatment of malaria in pregnancy were relevant. In Malawi,17 pregnant women received sulfadoxine-pyrimethamine 1500 mg/75 mg for malaria prevention and azithromycin 1 g, effective against NG, CT, and a variety of non-STI pathogens, during the second and third trimesters or placebo. The authors found a 34% decrease in preterm delivery and a 36% decrease in low birth weight among those who received azithromycin compared with placebo. No differences in perinatal or neonatal mortality were found. A second trial in Malawi16 that compared presumptive azithromycin 1 g + sulfadoxine-pyrimethamine 1000 mg/50 mg during the second and third trimesters with placebo + sulfadoxine-pyrimethamine found no significant impacts on preterm birth, gestational age at birth, mean birth weight, or perinatal death. In Papua New Guinea, presumptive azithromycin 1 g + sulfadoxine-pyrimethamine 1500 mg/75 mg compared with sulfadoxine-pyrimethamine and chloroquine 450 to 600 mg in ~2000 pregnant women resulted in a 26% lower prevalence of low birth weight and 38% lower risk of preterm delivery.28
Insufficient knowledge of the effects of STIs at different gestational ages on birth outcomes limits our ability to optimize the timing of etiologic screening and treatment. Administration of presumptive STI treatment in the aforementioned trials varied from one-time treatment at any point during pregnancy19 to monthly treatment during 14 to 26 weeks’ gestation until delivery.17 Even if successfully treated, women can be reinfected during pregnancy if partners are not treated. Unfortunately, the effectiveness of partner management in these settings has not been fully examined. In addition, the physiologic mechanisms by which chlamydial, gonococcal, and trichomonal infection impact birth outcomes are complex and unclear.21,29
Table 1 shows the status of ongoing studies on the effectiveness of etiologic screening and treatment in pregnancy. Randomized controlled trials are underway in China30 and Papua New Guinea,29 and in the planning stages in Botswana and South Africa. A prospective cohort study was recently completed in Brazil.31s A comparative-effectiveness study is in the planning stages in Ethiopia. Studies in Cameroon,32s Kenya, Tanzania, and Malawi,33s Mali,34s and Zambia35s are examining the impact of presumptive STI treatment, usually coupled with preventive malaria therapy.
TABLE 1.
Key Characteristics of Known Studies in Progress on Nonsyndromic Management of Chlamydia, Gonorrhea, and/or Trichomonas in Pregnant Women in Low- and Middle-Income Countries
| PI (Country) [Reference], Study Name, Status |
Study Design and Target Sample Size | Study Population and Inclusion Criteria | Study Groups and Interventions | Outcomes |
|---|---|---|---|---|
| Etiologic screening interventions | ||||
| Lee and Berhane (Ethiopia), ENAT, status: not yet recruiting |
Pragmatic comparative effectiveness study 2 × 2 factorial design Target sample size: 3600 |
Pregnant women with first ANC visit at study health centers at ≤24 wk of gestation based on last menstrual period and/or fundal height | Health center randomization Group 1: strengthening Ethiopian MOH/WHO-recommended nutrition interventions, including iron, folate, iodized salt, and local corn soya blend supplement to women with MUAC <23 cm Group 2 (control): nutrition standard of care Individual randomization All groups: routine screening for HIV, syphilis, malaria Group 1: urine culture and AST, molecular CT/NG testing (GeneXpert*), symptomatic women screened for BV (BVBlue*) and TV (OSOM*) at enrollment using self-collected vaginal swabs; treat per test results; deworming in second and third trimesters. Group 2: standard of care screening urine dipstick; syndromic STI management |
Primary Birth weight; birth length Secondary Gestational age at delivery; preterm birth; small-for-gestational age; low birth weight; length-for age (at birth and 6 mo); weight for age (at birth and 6 mo); gestational weight gain; maternal anemia; stillbirth; cost-effectiveness |
| Klausner, Morroni, Wynn (Botswana), status: preparation | Cluster randomized controlled crossover trial in 2 antenatal clinics 500 women |
Pregnant women aged ≥18 y attending first ANC visit who are asymptomatic for CT/NG | Group 1: molecular CT/NG (GeneXpert) screening using self-collected vaginal swabs at first ANC visit and again after 27 wk of gestation; treat per test results; partner treatment provided when possible Group 2 (control): syndromic STI management |
Primary Mother-to-child CT/NG transmission; newborn eye infection; newborn pneumonia Secondary Preterm birth; low birth weight; premature rupture of membranes; maternal STI diagnosed and treated; incremental cost-effectiveness ratios, acceptability among women and health care workers |
| Medina-Marino and Klausner (South Africa), status: not yet recruiting |
3-arm (1:1:1) individually randomized-controlled hybrid-effectiveness trial with economic evaluation 2500 women (834 per arm) |
Pregnant women aged ≥18 y attending first ANC visit at public antenatal clinic at <20 wk of gestation by ultrasound | All groups: routine screening for HIV and syphilis Group 1: molecular screening for CT, NG, and TV (GeneXpert) at first ANC visit using nurse-collection vaginal swabs; treat per test results; tests-of-cure at 3 wk of posttreatment Group 2: molecular screening and treatment of CT, NG, and TV (GeneXpert) at first ANC visit using nurse-collection vaginal swabs; repeat screening at 30–34 wk of gestation; treat per test results Group 3 (control): syndromic STI management |
Primary Change in maternal STI status between first ANC visit and birth; composite outcome: low birth weight, premature rupture of membranes, preterm birth, stillbirth/spontaneous abortion Secondary Implementation process evaluation; STI risk factors; mother-to-child STI transmission; neonatal STI colonization; cost-effectiveness substudy |
| Tang (China),30† status: recruiting |
Individually randomized controlled trial in hospital-based antenatal clinic 200 women |
Pregnant women aged 18–45 y at first ANC visit to hospital-based clinic | Group 1: molecular CT/NG screening (Cobas of urine or vaginal swab on enrollment and during 37–40 wk of gestation; azithromycin 1 g as per test results; test of cure at 1 mo, and 3 mo after treatment as needed; patients offered expedited partner therapy Group 2 (control): syndromic STI management; molecular CT/NG testing (Cobas) during 37–40 wk of gestation |
Primary Composite outcome: stillbirth, spontaneous abortion, preterm labor, premature rupture of membranes, small for gestational age, low birth weight, infant death, birth defects, neonatal conjunctivitis Secondary Stillbirth; spontaneous abortion; preterm labor; premature rupture of membranes; infant death; birth defects; neonatal conjunctivitis and pneumonia; screening rate; treatment rate; cure rate; partner treatment; costs of testing and treatment |
| Vallely and Pomat (Papua New Guinea),29 WANTAIM, status: recruiting |
Cluster randomized controlled crossover trial in 10 health centers 4600 women |
Women aged ≥16 y attending ANC at ≤26 wk of gestation by ultrasound | All groups: HIV and syphilis screening Group 1: molecular CT/NG and TV (GeneXpert) and BV (BVBlue) screening using self-collected vaginal swabs at 4 wk of postenrollment and 34–36 wk of gestation; treat per test results; partner treatment provided when possible Group 2 (control): syndromic STI management |
Primary Composite outcome: preterm birth, low birth weight Secondary Premature rupture of membranes; maternal STI diagnosed and treated; incremental cost-effectiveness ratios; health system implementation requirements; acceptability among women and health care workers; newborn eye infection; newborn pneumonia; mother-to-child STI transmission; test accuracy for neonatal infection |
| Presumptive treatment interventions | ||||
| Chico and Chandramohan (Zambia),35s ASPIRE, status: recruiting |
3-arm individually randomized controlled trial 5436 pregnant women (1812 per group) |
HIV-negative pregnant women who have not yet started IPTp‡ 16–28 wk of gestation by ultrasound | All groups: HIV and syphilis screening; syndromic STI management Group 1: monthly IPTp-SP‡; metronidazole 2 g at first and second ANC visit Group 2: monthly IPTp-DP‡; metronidazole 2 g at first and second ANC visit Group 3 (control): monthly IPTp-SP; placebo at first and second ANC |
Primary Composite outcome: spontaneous abortion, stillbirth, small for gestational age, low birth weight, preterm delivery, neonatal mortality Secondary Individual components of composite outcome; neonatal length and stunting; clinical malaria; malaria parasitemia; placental malaria; maternal anemia; congenital anemia; congenital malaria; TV and BV treatment efficacy; GI side effects; maternal NG, CT, TV, and syphilis infection; maternal vaginal microbiota; inflammation markers; AST of cultured isolates from vaginal swabs in symptomatic women; intervention costs; maternal and health care preferences for treatments |
| Dionne-Odom (Cameroon),32s PREMISE, status: recruiting |
Individually randomized controlled trial 310 pregnant women |
HIV-positive pregnant women 16–55 <28 wk of gestation by dates/fundal height or ultrasound |
Group 1: IPTp with daily trimethoprim-sulfamethoxazole DS; monthly azithromycin 1 g × 3 d Group 2 (control): IPTp with daily trimethoprim-sulfamethoxazole DS; monthly placebo × 3 d |
Primary Plasmodium falciparum peripheral parasitemia; composite outcome: CT, NG, and syphilis infection Secondary Birth weight; symptomatic malaria; parasite density; placental malaria; maternal anemia; Group B streptococcus colonization; Mycoplasma genitalium infection; composite adverse birth outcome: low birth weight, miscarriage, preterm delivery, small-for-gestational age, congenital anomaly, early neonatal mortality; maternal adherence |
| Kotloff (Mali),34s status: not yet recruiting |
3-cohort individually randomized controlled trial Cohort 1 (rural) 2 × 2 factorial design with mothers and infants randomized separately (groups 1–4) Cohort 2 (rural infant-only): infants randomized (groups 5 and 6) Cohort 3 (urban): mothers/infants randomized in tandem (groups 7 and 8) 99,700 participants |
Pregnant women attending ANC visit during 13–37 wk of gestation by fundal height and/or maternal report of quickening Unborn infants enrolled with mothers. Cohort 2 infants enrolled during routine vaccination visits |
Groups 1 and 7 (cohorts 1 and 3, respectively): maternal oral azithromycin 2 g at 2nd- and 3rd-trimester ANC visits and during delivery; infant oral azithromycin at 6- and 14-wk visits Group 2 (cohort 1): maternal oral azithromycin 2 g at 2nd- and 3rd-trimester ANC visits and during delivery; infant placebo at 6- and 14-wk visits Group 3 (cohort 1): maternal placebo at 2nd- and 3rd-trimester ANC visits and during delivery; infant oral azithromycin at 6- and 14-wk visits Groups 4 and 8 (cohorts 1 and 3, respectively): maternal placebo at 2nd- and 3rd-trimester ANC visits and during delivery; infant placebo at 6- and 14-wk visits Groups 5 and 6 (cohort 2): no maternal intervention; infant oral azithromycin at 6- and 14-wk visits versus placebo |
Primary Infant mortality from 6 wk to 6–12 mo of age; composite outcome: stillbirth, infant mortality through 6–12 mo of age; Secondary Gestational age at birth; birth weight; incremental cost-effectiveness ratio |
| ter Kuile and Madanitsa (Kenya, Tanzania, Malawi),33s status: done recruiting |
3-arm (1:1:1) individually randomized controlled trial 4680 pregnant women (1560 per group) |
HIV-negative pregnant women 16–28 wk of gestation assessed by ultrasound who have not yet started IPTp | Group 1: monthly IPTp-DP; placebo at first ANC visit Group 2: monthly IPTp-DP; azithromycin 2 g at first ANC visit Group 3 (control): monthly IPTp-SP at ANC |
Primary Composite outcome: spontaneous abortion, stillbirth, small for gestational age, low birth weight, preterm delivery, neonatal mortality Secondary Individual components of composite measure; neonatal length and stunting; clinical malaria; malaria parasitemia; placental malaria; maternal anemia; congenital anemia; congenital malaria; TV and BV treatment efficacy; GI side effects; maternal NG, CT, TV, and syphilis infection; maternal vaginal microbiota; inflammation markers; intervention costs |
| Other interventions | ||||
| Yeganeh, Brazil,31s status: completed, analysis ongoing |
Prospective cohort 400 women and their partners |
Pregnant women aged >18 y with sexual partner for longer than 3 mo seen at community antenatal care clinics§ | Cohort: women and partners screened for HIV, syphilis, hepatitis B and C (by lateral flow assay) and molecular CT/NG and TV (GeneXpert) screening using self-collected vaginal swabs; STIs treated per test results | Primary STI diagnosis; partner attendance at ANC visits for STI testing; gestational age; birth weight; congenital anomalies Secondary Demographics and behavioral factors; acceptance of testing; partner presence at ANC visits; partner STI diagnosis; partner acceptance of treatment and referral |
*GeneXpert, Cepheid, Sunnyvale, CA; BVBlue, Gryphus Diagnostics, Knoxville, TN, US; OSOM, Sekisui Diagnostics, Burlington, MA; Cobas Roche Diagnostics, Rotkreuz, Switzerland.
†Referenced protocol is for completed pilot study; authors are using same protocol for current randomized controlled trial.
‡IPTp, intermittent preventive therapy for malaria in pregnancy; IPTp-SP, intermittent preventive therapy in pregnancy using sulfadoxine-pyrimethamine; IPTp-DP, intermittent preventive therapy in pregnancy using dihydroartemisinin-piperaquine. Sulfadoxine-pyrimethamine is recommended by the WHO to protect against adverse birth outcomes attributable to malaria in endemic countries.23 Sulfadoxine is a sulfanomide and may confer some protective effect against adverse birth outcomes among pregnant women with NG, CT, and TV and bacterial vaginosis.18
§Women with a history of intimate partner violence were excluded.
ANC indicates antenatal care visit; AST, antimicrobial susceptibility testing; BV, bacterial vaginosis; CT, Chlamydia trachomatis; DALY, disability-adjusted life year; NG, Neisseria gonorrhoeae; STI, sexually transmitted infection; TV, Trichomonas vaginalis.
Source: Studies were identified through searches of ClinicalTrials.gov and ISRCTN.org, and coauthor’s personal knowledge.
Harm and Undesirable Effects
Evidence of harm from etiologic STI screening and treatment studies in low- and middle-income countries was limited. Publications of large clinical trials on presumptive STI treatment have not reported worse birth outcomes compared with control interventions.16,17,19,28 In a trial of presumptive treatment in Papua New Guinea, numbers of adverse events were similar between in the control and intervention arms.28 In one trial in the United States, treatment of asymptomatic trichomonal infection in pregnant women was associated with increased preterm birth36s but the selected intervention (two 2-g doses of metronidazole 48 hours apart at 16–23 and 24–29 weeks’ gestation) was nonstandard.
Harm attributable to antibiotic use during pregnancy is possible; however, STI treatment guidelines were designed to minimize potential harm.25,26 Although increasing antibiotic use can lead to increased antimicrobial resistance, treatment based on etiological test results rather than syndromic management should reduce overtreatment and decrease selective pressure for antimicrobial resistance. However, the effects on STI antimicrobial resistance have not been studied empirically. The presumptive STI treatment trial in Zambia35s is investigating antimicrobial resistance in the vaginal microbiome.
Sexually transmitted infections are often stigmatized12 and have been associated with intimate partner violence37s and fear of intimate partner violence.38s,39s However, many studies have reported very high rates of acceptance of partner notification,38s–41s suggesting that concerns about intimate partner violence and stigma around STIs were not a significant barrier for most women. The trial underway in Papua New Guinea29 is examining intimate partner violence as an adverse event.
Test Accuracy
Culture-based STI testing requires trained laboratory staff, specialized specimen transport, and equipment, and has long turn-around times and low sensitivity.42s,43s Consequently, STI diagnostics have moved toward molecular testing in many settings.42s Although molecular tests also require specialized equipment, some can be conducted in low-resource clinical settings at or near the point-of-care rather than in a laboratory.43s,44s Reported accuracy of those tests varied substantially, but studies of some platforms (e.g., GeneXpert, Cepheid, Sunnyvale, CA) have shown accuracies of >95%.42s,44s Studies in progress on etiologic STI screening are all using molecular methods for chlamydial and gonococcal screening (Table 1). Tests for trichomonal infection are either molecular or immunochromatographic assays.
Resource Requirements
Cheap antibiotics for STI treatment are widely available, but accurate diagnostic tests are relatively expensive. In 2019, the Foundation for Innovative New Diagnostics negotiated a GeneXpert CT/NG test cartridge price of US$16.20/test for low- and middle-income countries,45s which excludes specimen collection supplies and the test platform (US$17,000 one-time cost). Such pricing is well above the US$1/test threshold some experts have suggested is needed to implement etiologic STI testing in low- and middle-income countries.43s Substantially lower test accuracies have been reported for more affordable test options.42s,44s
Cost-effectiveness studies in Australia and the United States compared chlamydial screening in younger pregnant women with no screening.46s,47s When considering costs associated with adverse outcomes averted, screening was cost saving at chlamydial prevalences of 16.9% and 11%, respectively. Neither study considered overhead/capital costs or long-term population impacts of infections averted. In addition, the evidence used in both studies to inform parameters related to short-term health outcomes was limited.
Different testing strategies could reduce costs and maximize impact. A modeling analysis of cost and effectiveness compared different antenatal chlamydial and gonococcal screening strategies in Botswana with syndromic management.48s Having GeneXpert equipment available at every antenatal care facility was the most expensive option but resulted in the most infections treated and cured. Syndromic management was the least expensive strategy, but it resulted in fewer infections cured and considerable overtreatment. A hub-and-spoke approach, where testing occurred at high-volume facilities and low-volume facilities collected specimens and sent them to high-volume facilities for testing, offered the optimal cost per infection averted. Further examination of the costs associated with etiologic screening and treatment in pregnancy is planned for the etiologic STI screening trials in Botswana, Papua New Guinea, and South Africa, as well as the comparative-effectiveness study in Ethiopia and presumptive STI treatment trials in Mali and Zambia (Table 1)
Equity
A 2015–2016 survey of ministries of health found only 14 countries, of which 11 were high income, with national antenatal screening policies for gonorrhea or chlamydia.7 Given that pregnant women in low- and middle-income countries suffer from a disproportionate burden of STIs and poor maternal/neonatal outcomes,24 access to etiologic STI screening could help improve health equity around reproductive health and maternal/neonatal outcomes, although the potential magnitude of the impact global etiologic STI screening on health equity is unclear.
Acceptability
Etiologic STI screening and treatment has been shown to be highly acceptable to pregnant women in low- and middle-income countries. In a combined analysis of 1817 pregnant women from 6 different studies, 93.3% of women approached agreed to be tested.49s Most participants preferred self-collected vaginal swabs over physician-collected vaginal swabs; same-day test results and treatment might have increased participation. In a Papua New Guinea pilot study using self-collected vaginal swabs, nearly all women approached wanted to participate.50s Findings from a South African study also suggest very high (>95%) levels of acceptability.51s Studies in Botswana, Brazil, and Papua New Guinea are examining acceptability among women, partners, and health care providers (Table 1).
Including sex partners in etiologic screening and treatment is necessary to prevent reinfection. Partner notification and treatment has been acceptable to pregnant women in multiple settings in low- and middle-income countries.38s–41s In one study from Brazil, 97% of women reported feeling comfortable asking their partners to attend antenatal care and 54% to 56% of partners did attend.40s A follow-up study to examine further partner involvement and etiologic screening was recently completed31s (Table 1).
Feasibility
Feasibility must be considered at both the facility and health system levels. Some etiologic STI tests can be conducted at or near the point of care, allowing for decentralized diagnostic services and enabling same-day testing and treatment in low-resource settings.52s The 6-study combined analysis discussed previously reported high levels of feasibility across study sites (overall 96.7%) defined as the percentage of diagnosed women who received treatment.49s The pilot in Papua New Guinea found that etiologic STI testing and treatment could be successfully implemented with same-day treatment.50s In South Africa, 92% of 172 pregnant women with positive STI test results received same-day treatment.51s Although all of those studies used molecular test platforms that require electricity, the findings suggest that etiologic STI screening and treatment can be operationalized in a variety of settings.
Despite successes in research studies, access to test technologies is a substantial barrier to implementing sustainable etiologic screening globally. The WHO has recommended the GeneXpert platform to diagnose tuberculosis in low- and middle-income countries since 2013.53s As a result, many low- and middle-income countries have some laboratory infrastructure to support molecular testing using GeneXpert,53s which could be applied to STI diagnosis. However, the costs associated with those tests remain high, making the need for cost-effectiveness studies critical for determining feasibility.
Studies in Papua New Guinea29 and South Africa will examine operational feasibility of etiologic screening in select clinics (Table 1). In Ethiopia, investigators are examining feasibility at the regional health system level.54s
DISCUSSION
This review examined the evidence gaps around etiologic STI screening in pregnancy in low- and middle-income countries for each of the GRADE criteria to guide ongoing research that could support the development of international guidelines. We did not find direct evidence on the impact of etiologic screening and treatment of gonococcal, chlamydial, and/or trichomonal infections on pregnancy outcomes in low- and middle-income countries. We found that differences in outcome definitions may contribute to future challenges with evaluating the evidence for eventual guidelines. Preliminary data on harms, acceptability, and feasibility suggest that etiologic STI screening and treatment hold promise and merit further investigation, although a key challenge facing potential widespread implementation of this intervention is the high cost of and infrastructure needed for accurate etiologic STI tests. Potential harms should continue to be investigated but should not be considered a substantial barrier to further research. Current studies are further examining many of these challenges and knowledge gaps.
This review had several limitations. First, the focused search strategies and study selection processes of a narrative review might have missed some relevant studies. Second, we did not formally rate the quality of the existing evidence because our focus was on identifying current research gaps. Third, this review was limited to a subset of common curable STIs, gonorrheal, chlamydial, and trichomonal infection. Guidelines for the detection and treatment of other treatable STIs that impact maternal and neonatal outcomes, most notably Mycoplasma genitalium and bacterial vaginosis, are also lacking,1255s but were beyond the scope of this effort because of limited evidence and testing options, lack of complete understanding of pathophysiology, and/or limited treatment options,
Robust intervention trials examining the efficacy and potential harms of etiologic STI screening in antenatal settings are a priority. Additional research needs include the following:
Development of consistent outcome measures, particularly for gestational age, birth weight, and pregnancy loss, that allow for comparisons across studies and in meta-analyses of individual-level data
Systematic recording of malaria prevalence and use of intermittent preventive therapy in affected areas given that such therapy may be protective against adverse birth outcomes among women with some STIs
Integration of partner notification and treatment into intervention trials to examine the role of reinfection and the effectiveness of strategies such as at-home testing, expedited partner therapy, and incentives for partner testing
Investigation of the influence of timing of etiologic STI screening and treatment during pregnancy as the ideal timing of STI screening during pregnancy is currently unknown, and there is ongoing risk of reinfection
Collection of data on intimate partner violence at study enrollment and incidents of violence during studies since intimate partner violence is both a potential confounder and adverse effect
Collection of detailed cost data for performing cost-effectiveness analyses that consider STI prevalence, risk-based profile approaches to etiologic screening in low-resource settings, and availability of testing infrastructure to potentially guide the development of prevalence- or risk-based recommendations in resource-limited settings where the unit cost per test may otherwise be prohibitive
Collection and dissemination of population-based STI prevalence data to inform national estimates of STI burden in pregnancy
Collection of robust data on other factors associated with adverse pregnancy outcomes including other infections such as HIV, syphilis, M. genitalium, and bacterial vaginosis, maternal nutrition, maternal history of adverse birth outcomes, and anemia
Implementation research to examine operational and health system optimization for intervention delivery
Global health inequities and the association between STIs and poor pregnancy and newborn outcomes support the need to continue to develop and evaluate more effective interventions to improve maternal and neonatal health worldwide. Although the magnitude of the effect of STIs on poor maternal-child health outcomes in low- and middle-income countries is still unknown, there is potential for substantial population-level impacts given the high prevalence of STIs in pregnant women in low- and middle-income countries and the relative ease of treatment. The current syndromic approach to STI management in pregnant women in low- and middle-income countries leads to both underdiagnosis and overdiagnosis and treatment. Emerging technologies have created new opportunities for implementing more effective STI screening and treatment approaches, which are now being evaluated in large randomized controlled trials. Research focused on addressing key knowledge gaps identified here will be central to generating a robust evidence base to inform the development of effective and sustainable interventions aimed at reducing the burden and consequences of curable STIs in pregnancy in low- and middle-income countries.
Supplementary Material
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A539.
Footnotes
Acknowledgments: The authors thank Remco Peters for his critical discussions on sexually transmitted infection screening interventions and Emily Hartman for her assistance with this article. The authors wish to thank the reviewers for their comments, which have improved the manuscript.
Conflict of Interest and Sources of Funding: J.D.K. reports receiving personal fees and nonfinancial support from Cepheid, Inc; Hologic, Inc; and SpeeDx, Pty Ltd, for activities outside the submitted work. A.C.L. reports receiving grants from Bill and Melinda Gates Foundation and the National Institute of Child Health and Human Development during the conduct of the study. No other authors have conflicts to disclose.
This work was supported by Team Klausner Saving Lives, University of California, Los Angeles, and the Center for AIDS Research at the National Institutes of Health (grant number 5P30 AI028697).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Juliana S. Grant, Email: juliana@publichealthnerds.com.
R. Matthew Chico, Email: Matthew.Chico@lshtm.ac.uk.
Anne CC. Lee, Email: anne.cc.lee@gmail.com.
Nicola Low, Email: nicola.low@ispm.unibe.ch.
Andrew Medina-Marino, Email: AndrewM@foundation.co.za.
Rose L. Molina, Email: rmolina@bidmc.harvard.edu.
Chelsea Morroni, Email: chelseaamorroni@gmail.com.
Doreen Ramogola-Masire, Email: doreen.masire@gmail.com.
Chrysovalantis Stafylis, Email: cstafylis@mednet.ucla.edu.
Weiming Tang, Email: weiming_tang@med.unc.edu.
Andrew J. Vallely, Email: avallely@kirby.unsw.edu.au.
Adriane Wynn, Email: adriane.wynn@gmail.com.
Nava Yeganeh, Email: NYeganeh@mednet.ucla.edu.
Jeffrey D. Klausner, Email: JDKlausner@mednet.ucla.edu.
REFERENCES
- 1.Joseph Davey DL Shull HI Billings JD, et al. . Prevalence of curable sexually transmitted infections in pregnant women in low- and middle-income countries from 2010 to 2015: A systematic review. Sex Transm Dis 2016; 43:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver BJ Guy RJ Kaldor JM, et al. . Trichomonas vaginalis as a cause of perinatal morbidity: A systematic review and meta-analysis. Sex Transm Dis 2014; 41:369–376. [DOI] [PubMed] [Google Scholar]
- 3.Adachi K Klausner JD Bristow CC, et al. . Chlamydia and gonorrhea in HIV-infected pregnant women and infant HIV transmission. Sex Transm Dis 2015; 42:554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang W Mao J Li KT, et al. . Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: A global systematic review and meta-analysis. Sex Transm Infect 2019; 96:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson-Chen C, Balaram K, Hackney DN. Chlamydia trachomatis and adverse pregnancy outcomes: Meta-analysis of patients with and without infection. Matern Child Health J 2018; 22:812–821. [DOI] [PubMed] [Google Scholar]
- 6.Mullick S Watson-Jones D Beksinska M, et al. . Sexually transmitted infections in pregnancy: Prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect 2005; 81:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medline A, Joseph Davey D, Klausner JD. Lost opportunity to save newborn lives: Variable national antenatal screening policies for Neisseria gonorrhoeae and Chlamydia trachomatis. Int J STD AIDS 2017; 28:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 9.World Health Organization Standards for maternal and neonatal care. In: Department of Making Pregnancy Safer. Geneva, Switzerland: World Health Organization:2007. [Google Scholar]
- 10.van Gemert C Hellard M Bradshaw CS, et al. . Syndromic management of sexually transmissible infections in resource-poor settings: A systematic review with meta-analysis of the abnormal vaginal discharge flowchart for Neisseria gonorrhoea and Chlamydia trachomatis. Sex Health 2018; 15:1–12. [DOI] [PubMed] [Google Scholar]
- 11.Mudau M Peters RP De Vos L, et al. . High prevalence of asymptomatic sexually transmitted infections among human immunodeficiency virus–infected pregnant women in a low-income South African community. Int J STD AIDS 2018; 29:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unemo M Bradshaw CS Hocking JS, et al. . Sexually transmitted infections: Challenges ahead. Lancet Infect Dis 2017; 17:e235–e279. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization WHO Handbook for Guideline Development, 2nd ed. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 14.Moberg J Oxman AD Rosenbaum S, et al. . The GRADE Evidence to Decision (EtD) framework for health system and public health decisions. Health Res Policy Syst 2018; 16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schunemann HJ Mustafa R Brozek J, et al. . GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol 2016; 76:89–98. [DOI] [PubMed] [Google Scholar]
- 16.van den Broek NR White SA Goodall M, et al. . The APPLe study: A randomized, community-based, placebo-controlled trial of azithromycin for the prevention of preterm birth, with meta-analysis. PLoS Med 2009; 6:e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luntamo M Kulmala T Mbewe B, et al. . Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: A randomized controlled trial. Am J Trop Med Hyg 2010; 83:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chico RM Chaponda EB Ariti C, et al. . Sulfadoxine-pyrimethamine exhibits dose-response protection against adverse birth outcomes related to malaria and sexually transmitted and reproductive tract infections. Clin Infect Dis 2017; 64:1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray RH Wabwire-Mangen F Kigozi G, et al. . Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am J Obstet Gynecol 2001; 185:1209–1217. [DOI] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists Committee Opinion No. 700: Methods for estimating the due date. Obstet Gynecol 2017; 129:e150–e154. [DOI] [PubMed] [Google Scholar]
- 21.Adachi K, Nielsen-Saines K, Klausner JD. Chlamydia trachomatis infection in pregnancy: The global challenge of preventing adverse pregnancy and infant outcomes in sub-Saharan Africa and Asia. Biomed Res Int 2016; 2016:9315757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wedi CO Kirtley S Hopewell S, et al. . Perinatal outcomes associated with maternal HIV infection: A systematic review and meta-analysis. Lancet HIV 2016; 3:e33–e48. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. In: World Malaria Report 2019. Geneva: World Health Organization, 2019. [Google Scholar]
- 24.United Nations Goal 3: Ensure healthy lives and promote well-being for all at all ages 2019. 2019. Available at: https://www.un.org/sustainabledevelopment/health/. Accessed August 18, 2019.
- 25.World Health Organization WHO Guidelines for the Treatment of Chlamydia trachomatis. Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 26.World Health Organization WHO Guidelines for the Treatment of Neisseria gonorrhoeae. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 27.Vallely LM Egli-Gany D Pomat W, et al. . Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: A systematic review and meta-analysis protocol. BMJ Open 2018; 8:e024175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger HW Ome-Kaius M Wangnapi RA, et al. . Sulphadoxine-pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: A randomised controlled trial. BMC Med 2015; 13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallely A Pomat W Homer C, et al. . Point-of-care testing and treatment of sexually transmitted infections to improve birth outcomes in high-burden, low-income settings: Study protocol for a cluster randomized crossover trial (the WANTAIM Trial, Papua New Guinea) [version 2; peer review: 2 approved] 2019. Available at: https://wellcomeopenresearch.org/articles/4-53/v2. Accessed December 15, 2019. [DOI] [PMC free article] [PubMed]
- 30.Nie J, Tang W. Genital CT treatment to pregnant women to prevent adverse pregnancy outcomes: A pilot RCT (RCT). 2019. Available at: https://clinicaltrials.gov/ct2/show/NCT03862495. Accessed August 20, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


