Supplemental Digital Content is available in the text.
Keywords: azithromycin, COVID-19, hydroxychloroquine, pandemic, torsade de pointes
Abstract
Background:
The severe acute respiratory syndrome coronavirus 2 (SARs-CoV-2) has resulted in a global pandemic. Hydroxychloroquine±azithromycin have been widely used to treat coronavirus disease 2019 (COVID-19) despite a paucity of evidence regarding efficacy. The incidence of torsade de pointes remains unknown. Widespread use of these medications forced overwhelmed health care systems to search for ways to effectively monitor these patients while simultaneously trying to minimize health care provider exposure and use of personal protective equipment.
Methods:
Patients with COVID-19 positive who received hydroxychloroquine±azithromycin across 13 hospitals between March 1 and April 15 were included in this study. A comprehensive search of the electronic medical records was performed using a proprietary python script to identify any mention of QT prolongation, ventricular tachy-arrhythmias and cardiac arrest.
Results:
The primary outcome of torsade de pointes was observed in 1 (0.015%) out of 6476 hospitalized patients with COVID-19 receiving hydroxychloroquine±azithromycin. Sixty-seven (1.03%) had hydroxychloroquine±azithromycin held or discontinued due to an average QT prolongation of 60.5±40.5 ms from a baseline QTc of 473.7±35.9 ms to a peak QTc of 532.6±31.6 ms. Of these patients, hydroxychloroquine±azithromycin were discontinued in 58 patients (86.6%), while one or more doses of therapy were held in the remaining nine (13.4%). A simplified approach to monitoring for QT prolongation and arrythmia was implemented on April 5. There were no deaths related to the medications with the simplified monitoring approach and health care provider exposure was reduced.
Conclusions:
The risk of torsade de pointes is low in hospitalized patients with COVID-19 receiving hydroxychloroquine±azithromycin therapy.
What Is Known?
In hospitalized COVID-19 patients, the use of hydroxychloroquine and azithromycin significantly increases the corrected QT interval (QTc).
A QTc greater than 500 ms increases the risk of torsade de pointes by two to three-fold.
Inpatient telemetry monitoring is warranted for patients at high risk of developing drug related torsade de pointes.
What the Study Adds?
A simplified approach to monitoring patients receiving hydroxychloroquine±azithromycin for the treatment of COVID-19 designed to reduce health care provider exposure and personal protective equipment use was safe
The incidence of life threatening tachy-arrhythmias associated with the inpatient use of hydroxychloroquine±azithromycin, with baseline screening, was exceedingly low.
The novel severe acute respiratory syndrome coronavirus 2 (SARs-CoV-2), first identified in China, has rapidly spread across the world resulting in over 2.3 million infections and over 150 000 deaths.1 There are multiple randomized control trials currently underway designed to find an effective therapy to combat the global pandemic. Based on limited in-vitro studies showing viral infection and replication inhibition, and a small study, that was subsequently withdrawn, hydroxychloroquine with and without azithromycin was widely promoted to treat patients with coronavirus disease 2019 (COVID-19).2,3 Of major concern has been the risk of QT prolongation and torsade de pointes (TdP) with the use of these agents individually, and even more so when used in combination. Widespread use of these medications created a dilemma as overwhelmed health care systems searched for ways to effectively monitor these patients while simultaneously trying to minimize health care provider (HCP) exposure and use of personal protective equipment (PPE). Initially, serial ECGs and telemetry were employed. We subsequently used mobile continuous telemetry (MCOT; BioTelemetry, Malvern, PA) patches to expand telemetry capabilities and reduce the need for serial ECGs.4 In a prospective observational study involving 201 patients, we did not observe any cases of TdP or arrhythmogenic death due to monotherapy with hydroxychloroquine or when hydroxychloroquine was combined with azithromycin.5 This suggested that a 5-day course of therapy may not warrant monitoring for cardiac arrhythmias in most inpatients with a normal baseline ECG. The findings led us to simplify our approach to monitoring patients treated with these medications; we recommended to only monitor patients with a baseline corrected QT interval (QTc) >500 ms. In this study, we report our evolving experience with monitoring patients receiving hydroxychloroquine±azithromycin and identify the incidence of drug-induced TdP and arrhythmic death due to these medications in a large health care network at the epicenter of the US COVID-19 crisis.
Methods
To minimize the possibility of unintentionally sharing information that can be used to reidentify private information, a subset of the data generated, and the analytical methods used for this study are available from the corresponding author to other researchers upon reasonable request
This study was approved by the Institutional Review Board of Northwell Health, which waived the requirement for individual informed consent. All patients >18 years of age with a polymerase chain reaction test confirmed diagnosis of COVID-19 illness admitted across 13 hospitals within the Northwell Health system that received hydroxychloroquine±azithromycin between March 1 and April 15, 2020 were identified. Criteria for initiating hydroxychloroquine±azithromycin within Northwell Health has been described previously.5 While there was treatment guidance from the health system, the treatment regimen was at the discretion of the individual clinician. Before initiating treatment, the QTc interval was optimized by stopping other QTc prolonging medications if possible and replacing electrolytes. Early on many patients received hydroxychloroquine+azithromycin. Over the course of the study period azithromycin was dropped from the regimen due to perceived lack of efficacy. In addition, clinicians were reminded that there was no data supporting the efficacy of hydroxychloroquine, and there should, therefore, be a low threshold for not starting and discontinuing therapy if there was a competing risk. Patients with COVID-19 positive who were chronically on hydroxychloroquine for autoimmune diseases such as lupus were excluded.
As mentioned above, monitoring over the period of the study evolved. Initially, patients receiving hydroxychloroquine±azithromycin were monitored via standard telemetry or with serial ECGs. MCOT patches were then employed to monitor the QTc interval and avoid serial ECGs when telemetry was not available.4 The use of MCOTs for this purpose has been previously described.4,5 Based on the early experience, a simplified approach to QTc monitoring was instituted system wide on April 5, 2020 (Figure). All patents received a baseline ECG. If the baseline QTc was ≤500 ms, therapy with hydroxychloroquine±azithromycin was initiated, and no further monitoring was performed. This included not performing serial ECGs. If the baseline QTc was >500 ms, clinicians were asked to determine if the risk of an unproven treatment was warranted. If therapy was begun, QTc monitoring was performed with telemetry if available, or with an MCOT patch if a telemetry bed was not available. Performing serial ECGs was discouraged in an effort to reduce HCP exposure and PPE usage.
Figure.
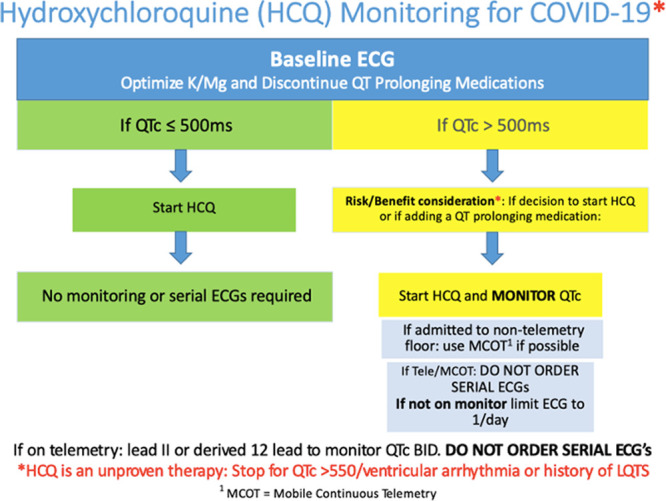
The simplified approach to monitoring QT interval and arrythmia in hospitalized patients with coronavirus disease 2019 (COVID-19) receiving hydroxychloroquine (HCQ). Flow chart demonstrating simplified monitoring. MCOT indicates mobile continuous telemetry; and QTc, corrected QT interval.
A customized ClinPhen python package was used to identify any mention of QT prolongation, TdP, nonsustained ventricular tachycardia (VT), VT, ventricular fibrillation, polymorphic VT, wide complex tachycardia, and cardiac arrest within the electronic medical records. Different variations of the above terms were used to ensure that the search captured all of the events. The ClinPhen python package has been approved as an efficient tool to extract and prioritize patient phenotypes directly from medical records.6 The box connected the free text content in the electronic medical record notes and broke it into sentences, sub-sentences, and words. The package used a custom rule-based heuristic to match sub-sentences against arrhythmias names and synonyms as defined above. As an example, prolonged QTc would be a valid mention of the words QT prolongation. After the processing with the package, a spreadsheet displaying the key findings, the sub-sentences, essential findings, and original text was created. A subset of these search results was manually validated using the cohort of patients treated with hydroxychloroquine±azithromycin from March 1 to 23 in our previous study.5
An in-depth chart review of all identified arrhythmic events and every case of cardiac arrest was performed. Demographics, comorbidities, duration of therapy with hydroxychloroquine±azithromycin, concomitant use of other QT prolonging agents, electrolyte abnormalities, baseline ECG values including QRS duration, QRS morphology, QT interval duration as well as QT interval duration from the day of the event were collected. Comorbidities were identified from International Classification of Diseases Ninth or Tenth Revision codes documented on admission. All telemetry strips, ECGs, and MCOT strips were reviewed for patients with arrhythmias or QTc prolongations. All QT intervals were manually measured regardless of source. Bazett’s formula was used to calculate the QTc. QT interval measurement methodology was previously described.5 Arrhythmic event findings were adjudicated by a senior board certified cardiac electrophysiologist and a cardiac electrophysiology fellow board certified in cardiovascular disease blinded to duration or course of therapy.
All ECGs performed at the Northwell Heart Hospital (Northshore University Hospital) were tabulated for the 10 days before April 5, 2020, and the 10 days after as a marker of HCP exposure and PPE usage. The baseline ECGs for each patient performed in the emergency department were excluded for both time periods.
Outcome Measures
The primary clinical outcome of the study was QT prolongation resulting in TdP. Secondary outcomes included QT prolongation resulting in premature discontinuation of hydroxychloroquine and azithromycin, incidence of ventricular arrhythmias, and arrhythmogenic death.
Statistical Analysis
Continuous variables are reported a mean±SD, and categorical variables are reported a number (percentage). SAS Version 9.4 (Cary, NC) was used to compute descriptive statistics and 95% exact binomial CIs.
Results
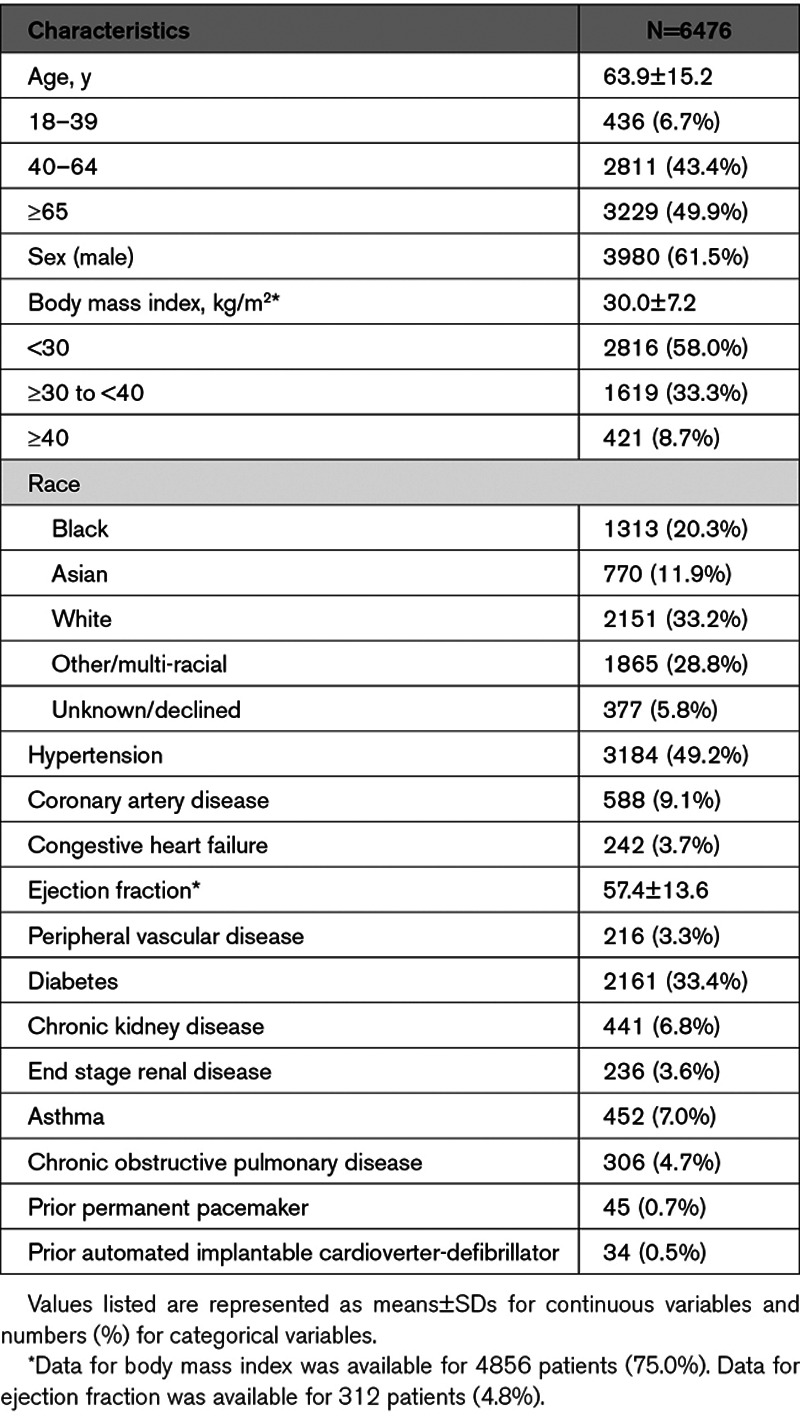
Between March 1 and April 15, there were 6476 hospitalized patients who were treated for COVID-19 with hydroxychloroquine±azithromycin across 13 hospitals in the Northwell Health system. Three thousand six hundred twenty-nine patients (56%) received azithromycin in addition to hydroxychloroquine. The average age of the population was 63.9±15.2 years and 3980 (61.5%) were men. Complete demographics are displayed in Table 1. The treatment regimens for these medications were as follows: hydroxychloroquine: either 400 mg by mouth twice daily or 800 mg by mouth once daily for 1 day followed by 200 mg by mouth twice daily or 400 mg by mouth once daily for 4 days. Azithromycin: 500 mg by mouth or intravenous daily for up to 5 days. Four thousand one hundred and fourteen patients were treated before the institution of the simplified monitoring approach and 2362 patients were treated after.
Table 1.
Patient Demographics
The primary end point of QTc prolongation resulting in TdP occurred in 1 patient (0.015% [exact 95% CI, 0.0%–0.09%]). The patient’s baseline QTc was 514 ms. After receiving two 400 mg doses of hydroxychloroquine and one dose of azithromycin, the QTc increased to 538 ms and a nonsustained, asymptomatic episode of TdP was observed. This patient had also received albuterol and furosemide, which are both known to cause QT prolongation. There were no electrolyte abnormalities associated with the episode. Hydroxychloroquine and azithromycin were both discontinued and the QTc shortened back to 518 ms. The patient was subsequently discharged home with no further episodes of TdP noted during the hospitalization.
QTc prolongation occurred in 67 patients monitored on hydroxychloroquine±azithromycin. Of these patients, 30 were receiving monotherapy with hydroxychloroquine and 37 were receiving both hydroxychloroquine and azithromycin. Of the 67 patients with a significant prolongation in the QTc, 45 (67.2%) patients had their treatment initiated before April 5 and the remaining 22 (32.8%) were treated with hydroxychloroquine±azithromycin after the simplified monitoring plan was implemented. The average number of other QT prolonging agents received in this subset of patients during the hospitalization was 3.0±1.9. The average baseline QTc for these patients was 473.7±35.9 ms, and the average maximum QTc was 532.6±31.6 ms. The average change in the QTc interval from baseline was 60.5±40.5 ms. Of the 67 patients, a majority of patients (n=58, 86.6%) had hydroxychloroquine±azithromycin discontinued as a result of the QT prolongation. The remaining 9 patients (13.4%) had one or more doses of these medications held, but ultimately were able to finish the course of hydroxychloroquine±azithromycin. Of the 67 patients whose QT prolongation necessitated a change in management, one additional patient (1.5%) was noted to have a ventricular arrhythmia. This patient had 10 seconds of monomorphic nonsustained VT at a rate of 190 beats per minute that was not associated with symptoms.
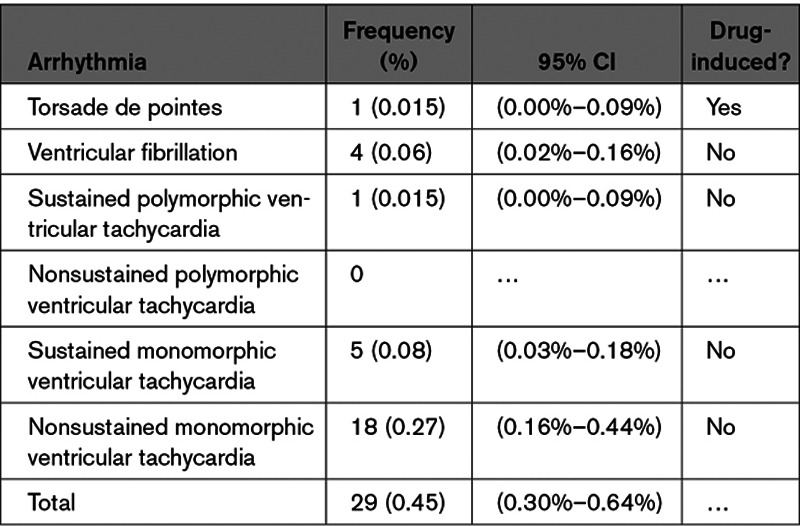
One episode of sustained polymorphic VT in the setting of sustained hypotension occurred in a patient who was made comfort care per the patient’s wishes. Four other patients (0.06%) had ventricular fibrillation in the setting of myocardial ischemia (n=2) and hyperkalemia and severe acidosis (n=2). These rhythms were rapidly identified, and advanced cardiovascular life support was quickly initiated. Five additional patients (0.08%) had sustained monomorphic VT. These episodes were managed with intravenous amiodarone and synchronized direct current cardioversion. Eighteen patients (0.27%) had monomorphic nonsustained VT. A list of the 29 patients (0.45%) who were found to have ventricular arrhythmias while receiving therapy is displayed in Table 2. There were no deaths related to the use of hydroxychloroquine±azithromycin in the entire population.
Table 2.
Ventricular Arrhythmias Observed in 6476 Patients
For the 10-day period before instituting the simplified monitoring approach, there were 1475 ECGs performed (147.5 per day) at the largest hospital in the health system. In the 10-day period following the institution of the simplified approach, 510 ECGs were performed (51 per day). Eliminating the need for serial ECG monitoring in select patients lead to an absolute reduction of 965 ECGs (96.5 per day) and a 65% relative reduction compared with serial monitoring.
Discussion
In this study, involving a large cohort of patients taken from a network of hospitals at the epicenter of the US COVID–19 crisis, we demonstrated that (1) a simplified approach to monitoring patients receiving hydroxychloroquine±azithromycin for the treatment of COVID-19 designed to reduce HCP exposure and PPE use was safe; (2) the incidence of life threatening tachy-arrhythmias associated with the inpatient use of hydroxychloroquine±azithromycin, with baseline screening, was exceedingly low.
The SARs-CoV-2 virus binds and enters human upper and lower respiratory cells through the viral type I membrane spike glycoprotein and the human ACE (angiotensin-converting enzyme)-2 receptors.7,8 Small studies in-vitro have shown to successfully inhibit viral infection and replication by affecting endosomal dependent fusion and interfering with the glycosylation of cellular receptors of the virus.2,3 Incidences of TdP with the use of chloroquine and hydroxychloroquine in patients with COVID-19 have been reported, though remain low.9,10 The efficacy of the therapy on morbidity and mortality continues to be controversial, with conflicting and at times unsubstantiated claims being made. A French study involving 36 patients, which ignited the world’s use of hydroxychloroquine and azithromycin, has since been redacted (https://www.isac.world/news-and-publications/official-isac-statement). The unprecedented response by some news outlets, social media, politicians and ultimately the Food and Drug Administration led to the widespread use of these agents. Subsequent studies showed lack of efficacy and potential harm. A double-blinded, randomized, phase IIb clinical trial, looking at the use of high versus low dose chloroquine, a sister drug of hydroxychloroquine, for 10 days in patients with COVID-19 reported increased incidence of QT prolongation and overall death in those receiving the high dose, requiring early termination of that study arm. Two episodes of VT resulting in death were reported, though neither episode was related to TdP.11 A preprint retrospective study of 368 patients with COVID-19 from the Veterans Administration showed no benefit to the use of hydroxychloroquine with or without azithromycin in reducing the need for mechanical ventilation and suggested a possible overall increase in mortality with the use of hydroxychloroquine.12 It is important to note that the mechanism of death in the study was not reported, leaving room for debate whether it was arrhythmic or related to some other mechanism. A large multinational COVID-19 registry analysis of the use of hydroxychloroquine or chloroquine±a macrolide showed no benefit of either monotherapy or combination therapy on in-hospital outcomes. Moreover, the regiments were associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias.13 However, the study did not report a breakdown of arrythmias and did not report the incidence of arrhythmic death. Therefore, based on the findings of this study, the excess mortality may be due to a nonarrhythmic cause. The results of this study, however, have come under great scrutiny and were subsequently retracted after the authors were unable to verify the data set.14 The Food and Drug Administration has issued a warning against the use of hydroxychloroquine±azithromycin in the outpatient setting or outside of a clinical trial for inpatient use. On April 28, following the Food and Drug Administration recommendation, the Northwell Health system issued an updated guidance statement recommending against the use of hydroxychloroquine outside of a clinical trial.
The initial widespread use of hydroxychloroquine±azithromycin occurred at a time when hospitals were overwhelmed with patients with COVID-19-positive and needed to expand the capacity of both intensive care units and general medical wards. In addition, there was a need to minimize HCP exposure and PPE use. Classical approaches to inpatient monitoring during the initiation of QT prolonging drugs were not possible. There were far too few telemetry beds and serial ECGs required repeated HCP exposure and use of PPE. The hallmark of managing during the COVID-19 pandemic is innovation and flexibility. To reduce the need for serial ECGs, MCOT patches were used to monitor patients in nontelemetry beds.4 In a prospective observational study of 201 patients treated on average for 5 days with chloroquine/hydroxychloroquine±azithromycin, no incidence of TdP or arrhythmogenic death was observed despite a statistically significant increase in QTc.5 This, along with our health system’s decision to no longer use azithromycin in patients with COVID-19, led us to adopt a simplified approach to monitoring across the health system beginning April 5 (Figure).
This study showed that our simplified approach did not result in increased risk of sustained TdP or arrhythmic death related to hydroxychloroquine±azithromycin in a cohort of 6476 patients. The arrhythmic risk of the therapy was low, with only one episode of nonsustained TdP occurring before our simplified approach to monitoring had been implemented. Had that patient presented after the time when we were using the approach outlined in Figure, the patient would still have received monitoring per our simplified approach, given that the baseline QTc was >500 ms. Our algorithm, therefore, would still have captured the single episode of nonsustained TdP that occurred in the entire cohort, regardless of when that patient was treated. It is important to note that the number of QT prolongation events, and therefore, potential TdP episodes, in the study may underestimate the true incidence due to the change in our monitoring approach. QT prolongation occurring in patients not monitored would have not been captured. However, we believe this did not lead to any increased incidence of tachy-arrhythmic death. All in-hospital cardiac arrests in patients receiving hydroxychloroquine±azithromycin were individually reviewed, and no instances of TdP were noted in those patients.
Historically, antiarrhythmics have the highest risk of TdP with an incidence of 1% to 5%. The risk with noncardiovascular drugs is much lower at 0.001%.15 Though the risk of developing TdP in this study is tenfold higher compared with historical data (0.015% versus 0.001%), it remains exceptionally low, especially considering that the treatment duration for these medications is 5 days. The single episode of nonsustained TdP that we observed is distinctly different than what was reported by Mecuro et al9 in their cohort of 90 patients. As opposed to our patient, who was actively being treated with hydroxychloroquine and azithromycin at the time the event occurred, their case of TdP was seen in a patient that had discontinued hydroxychloroquine and azithromycin 3 days before the event for a QTc of 499 ms. At the time of their patient’s TdP event, multiple other concomitant conditions known to prolong the QT and cause TdP were present. Therefore, it is difficult, especially since therapy had been discontinued, to determine how much, if at all, the use of hydroxychloroquine or azithromycin contributed to the episode. The results of our study demonstrate that despite the change in our monitoring practice patterns, sudden cardiac death did not increase with the use of this unproven therapy for COVID-19. What is of utmost importance is that by using this simplified approach, we were able to safely deliver this therapy while successfully reducing HCP exposure and PPE use by 65%.
Limitations
Limitations of this study include its retrospective nature and the lack of a control group. Given that a search of the electronic medical record was performed to identify arrhythmic events, the data is also subject to reporting bias. During this pandemic, omissions in documentation due to the surge in volume across the health system may have contributed to a reporting bias. Furthermore, although we validated a subset of patients from a previous study in which a manual chart review was performed, it is possible that our search algorithm failed to identify every arrhythmia in this population. However, as opposed to other events, significant ventricular arrhythmias and cardiac arrests would be considered noteworthy and would most likely result in documentation at some level Similarly, due to omissions in documentation and the way comorbidities were collected, the study may underrepresent the true prevalence of certain comorbidities in the cohort. The true number of QT prolongation events after the simplified approach was initiated is larger than reported as only those with a baseline QTc >500 ms would have been captured. It is important to note, despite this we did not see a significant incidence of TdP. Lastly, given that clinicians may have withheld hydroxychloroquine±azithromycin due to a patient having a prolonged QTc at baseline, and that we did not include patients treated with chloroquine, our results can only be applied to the incidence of TdP and QT prolongation in patients with COVID-19 positive who received hydroxychloroquine±azithromycin.
Conclusions
Despite a known increased incidence of QT prolongation in patients with COVID-19 receiving hydroxychloroquine±azithromycin, the risk of TdP and arrhythmogenic death with our simplified approach to monitoring the QTc remained exceedingly low. This simplified approach allowed for less HCP exposure and decreased use of PPE. Though the arrhythmic risk appears to be low, no meaningful clinical benefit for the use of hydroxychloroquine±azithromycin in patients with COVID-19 has been shown. Given that there is data to suggest increased overall death, unrelated to TdP, caution is advised in their use. Further studies are needed to better evaluate the risk versus benefit of using hydroxychloroquine±azithromycin for the treatment of COVID-19.
Sources of Funding
None.
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- HCP
- health care provider
- MCOT
- mobile continuous telemetry
- PPE
- personal protective equipment
- QTc
- corrected QT interval
- SARs-
- severe acute respiratory syndrome CoV-2 coronavirus 2
- TdP
- torsade de pointes
- VT
- ventricular tachycardia
A list of all Northwell COVID-19 Research Consortium members is given in the Data Supplement.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.120.008937.
Contributor Information
James Gabriels, Email: jgabriel13@northwell.edu.
David Chang, Email: davidchang7787@gmail.com.
Joanna Fishbein, Email: jfishbein1@northwell.edu.
Michael Qiu, Email: gqiu@northwell.edu.
Stavros E. Mountantonakis, Email: Smountanto@northwell.edu.
Laurence M. Epstein, Email: lmepstein@partners.org.
References
- 1.World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report 91. 2020. Geneva, Switzerland: WHO [Google Scholar]
- 2.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang D, Saleh M, Gabriels J, Ismail H, Goldner B, Willner J, Beldner S, Mitra R, John R, Epstein LM. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75:2992–2993. doi: 10.1016/j.jacc.2020.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleh M, Gabriels J, Chang D, Soo Kim B, Mansoor A, Mahmood E, Makker P, Ismail H, Goldner B, Willner J, et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020;13:e008662. doi: 10.1161/CIRCEP.120.008662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deisseroth CA, Birgmeier J, Bodle EE, Kohler JN, Matalon DR, Nazarenko Y, Genetti CA, Brownstein CA, Schmitz-Abe K, Schoch K, et al. ClinPhen extracts and prioritizes patient phenotypes directly from medical records to expedite genetic disease diagnosis. Genet Med. 2019;21:1585–1593. doi: 10.1038/s41436-018-0381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;94:e00127-20. doi: 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci. 2004;61:2738–2743. doi: 10.1007/s00018-004-4242-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mecuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, Nadeau-Routhier C, Knotts R, Bar-Cohen R, Kogan E, Barbhaiya C, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425–1433. doi: 10.1016/j.hrthm.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, et al. ; CloroCovid-19 Team. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [published online June 5, 2020]. Med (NY). doi: 10.1016/j.medj.2020.06.001. https://www.sciencedirect.com/science/article/pii/S2666634020300064?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra M, Desai S, Ruschitzka F, Patel A. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020doi: 10.1016/s0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Mehra M, Desai S, Ruschitzka F, Patel AN. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis [published online May 22, 2020. Published correction appears in Lancet. May 30, 2020. doi: 10.1016/S0140-6736(20)31249-6. Published retraction appears in Lancet. June 5, 2020. doi: 10.1016/S0140-6736(20)31324-6]. Lancet. doi: 10.1016/S0140-6736(20)31180-6. https://www.sciencedirect.com/science/article/pii/S0140673620311806?via%3Dihub [Google Scholar]
- 15.Roden DM. Predicting drug-induced QT prolongation and torsades de pointes. J Physiol. 2016;594:2459–2468. doi: 10.1113/JP270526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


