Abstract
Objective
The aim of the study was to characterize magnetic resonance imaging findings in patients with recurrent ovarian adult granulosa cell tumors (AGCTs).
Methods
Clinical and magnetic resonance imaging manifestations of recurrent AGCTs were evaluated in 11 patients.
Results
Initial recurrences of AGCT were diagnosed between 13 months and 30 years (mean, 11.3 years). Recurrent tumors were located in the pelvic peritoneum, the abdominal peritoneum, the retroperitoneum, and bone. The number of recurrent tumors varied from 1 to 5. Tumors varied in morphology and all margins were well circumscribed. The internal structures noted were as follows: multilocular cystic and solid and cystic. Furthermore, internal hemorrhage and sponge-like multicystic components were identified.
Conclusions
Ovarian AGCTs recurred in the pelvic peritoneum, abdominal peritoneum, and the retroperitoneal lymph nodes. Large recurrent AGCTs were commonly well circumscribed, round or lobulated, and multilocular cystic or solid and cystic. Moreover, they frequently included internal hemorrhage and sponge-like multicystic components.
Key Words: adult granulosa cell tumor, ovary, recurrence, MR imaging
Adult granulosa cell tumors (AGCTs) of the ovary are low-grade malignant sex cord-stromal tumors. Clinically, they are well-known estrogen-producing tumors.1 They are usually diagnosed at stage I disease and have a relatively favorable prognosis with 10-year survival rates of 84% to 95% for stage I tumors.2–4 It should be noted, however, that AGCTs exhibit an unpredictable tendency to reoccur later in life, even in cases where the disease was identified and treated in the early stages.4 Patients often forget or misremember previous medical diagnoses, which makes it difficult for clinicians to make an accurate diagnosis before surgery.5 Therefore, imaging plays an important role in accurate diagnosis. To our knowledge, there are a limited number of studies that report the imaging of recurrent AGCTs6 and there are no previous articles that describe the magnetic resonance imaging (MRI) characteristics of recurrent tumors. In the present report, the MRI findings of recurrent AGCTs were reviewed, and characteristics were discussed.
MATERIALS AND METHODS
Eleven patients with a histologically recurrent ovarian AGCT diagnosed from September 2002 to October 2018 were reviewed. Each patient's medical records were reviewed and clinical characteristics, including the following: age, initial International Federation of Gynecology and Obstetrics (FIGO) stage, initial surgery, disease-free interval to first recurrence, clinical presentation at recurrence, total number of surgeries for recurrence, and current status after the surgery of first recurrence were noted. The study was approved by the local research ethics committee.
Abdominal and pelvic computed tomography (CT) scanning was conducted using Xvigor (Toshiba Medical Systems, Otawara, Japan), Aquilion PRIME (Canon Medical Systems, Otawara, Japan), or Discovery CT750 HD (GE Healthcare, Milwaukee, Wis). Unenhanced CT scans were examined in 5 patients and contrast-enhanced CT scans were examined in 6 patients. Contrast-enhanced CT scanning was performed after an intravenous injection of 100 to 150 mL of iodinated ionic contrast agents (Iopamidol [Iopamiron 300 mgI/mL, Bayer, Germany], Iomeprol [Iomeron 300 mgI/mL; Bracco-Eisai, Tokyo, Japan]). Each CT scan was reconstructed at a section thickness of 5 to 7 mm and at intervals of 5 to 7 mm.
Magnetic resonance imaging scanning was conducted using 1.5-T MR scanners (Signa HD xt; GE Healthcare, Milwaukee, Wis, or Intera Achieva, Philips Healthcare, Best, the Netherlands). T1-weighted spin-echo (SE) or fast SE (FSE) axial images and T2-weighted FSE images in axial and sagittal or coronal planes were acquired with phased-array coils. The scanning parameters for T1-weighted SE or FSE images were as follows: repetition time range/echo time range, 400 to 519/10 to 14 ms; slice thickness, 5 mm; interslice gap, 0 mm; matrix, 512 × 512; field of view, 25 to 42 cm. The scanning parameters for T2-weighted FSE images were as follows: repetition time range/echo time range, 3000 to 4918/80 to 108 ms; slice thickness, 5 mm; interslice gap, 0 mm; matrix, 384 × 384 to 512 × 512; field of view, 25 to 42 cm.
All CT and MRI images were retrospectively reviewed by 2 experienced abdominal radiologists (with 9 and 28 years of experience). Recurrent tumors were identified using both CT and MR images. The location and number of recurrent masses were examined, and the maximum diameter of the mass was measured on CT images. The locations were divided as follows: pelvic peritoneum, abdominal peritoneum, retroperitoneum and other.
The MRI findings of the largest recurrent masses (maximum diameter of 3 cm or above) were examined in individual location of each patient. The factors investigated included the following: morphologic appearance of the mass, the margin, internal structure, and the presence or absence of internal hemorrhage, and sponge-like multicystic components. Internal cysts with a signal intensity higher than that of skeletal muscle on T1-weighted images or fluid-fluid level in the cyst on T2-weighted images were defined as internal hemorrhage. Clusters of small cysts in the mass were defined as a sponge-like multicystic component.
RESULTS
The clinical data for 11 patients is summarized in Table 1. The age at the time of diagnosis of primary AGCT was 32 to 67 years (mean, 40.7 years). The initial FIGO stage was stage IA in 8 patients and IC in 3 patients. A unilateral salpingo-oophorectomy was performed in 9 patients, and total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed in 2 patients. The first recurrence of AGCT was diagnosed from 14 months to 30 years (mean, 11.3 years) after the initial surgery. At the time of recurrence, 4 patients presented with abdominal pain, 3 patients presented with irregular menstruation or amenorrhea, and 1 patient presented with buttock pain. In 2 patients, reoccurrence was discovered by imaging examination during follow-ups, and in 1 patient, reoccurrence was incidentally discovered on CT images of the lung.
TABLE 1.
Clinical Data for 11 Patients With Recurrent AGCT
| Case | Age | Initial FIGO Stage | Initial Surgery | Disease-Free Interval to First Recurrence | Clinical Presentation at Recurrence | Total No. Surgery for Recurrence | Follow-up Interval After the Surgery of First Recurrence and Current Status |
|---|---|---|---|---|---|---|---|
| 1 | 32 | IA | USO | 26 y | Abdominal pain | 1 | 14 y, NED |
| 2 | 32 | IC | USO | 4 y | Abdominal pain | 4 | 10 y, MR, PD, DOD |
| 3 | 33 | IA | USO | 4 y | Irregular menstruation | 1 | 4 y, MR, LTF |
| 4 | 34 | IA | USO | 19 y | Right buttock pain | 1 | 1 y, NED |
| 5 | 39 | IA | USO | 11 y | Abdominal pain | 1 | 2 y, MR, PD |
| 6 | 40 | IA | TAH BSO | 30 y | Incidentally discovered, on Chest CT images | 1 | 4 y, MR, LTF |
| 7 | 41 | IA | USO | 1 y | Discovered by follow-up imaging | 3 | 4 y, PD, DOD |
| 8 | 42 | IA | USO | 12 y | Discovered by follow-up imaging | 6 | 12 y, MR, PD, DOD |
| 9 | 42 | IC | USO | 5 y | Irregular menstruation | 1 | 5 y, MR, NED |
| 10 | 46 | IC | USO | 5 y | Amenorrhea | 4 | 17 y, PD, |
| 11 | 67 | IA | TAH BSO | 4 y | Abdominal pain | 1 | 3 y, MR, PD, DOD |
BSO, bilateral salpingo-oophorectomy; DOD, died of disease; LTF, lost to follow-up; MR, multiple recurrences; NED, no evidence of disease; PD, progressive disease; TAH, total abdominal hysterectomy; USO, unilateral salpingo-oophorectomy.
Seven patients experienced 1 episode of recurrence and the remaining 4 patients experienced 3 to 6 (mean, 2.2) episodes of recurrences, which required repeated surgical resections during the course of their disease. Four of the 11 patients died as a result of disease progression. The mean survival was 7.8 years in 4 alive patients. The remaining 2 patients were lost to follow-up.
The locations, number, and size of recurrent masses found through imaging are summarized in Table 2. The recurrent masses were located in the pelvic peritoneum only in 3 patients, in the abdominal and pelvic peritoneum in 3 patients, in the abdominal peritoneum only in 2 patients, in the retroperitoneum only in 1 patient, in the abdominal peritoneum, pelvic peritoneum, and retroperitoneum in 1 patient, and bone in 1 patient. The number of recurrent masses identified on the images at first recurrence was 1 in 4 patients, 2 in 1 patient, 3 in 3 patients, 4 in 1 patient, and more than 5 in 2 patients.
TABLE 2.
Locations, Numbers, and Sizes of Recurrent AGCT on Imaging
| Locations, Numbers, and Size of Recurrent AGCT on Imaging | ||||||
|---|---|---|---|---|---|---|
| Case | Pelvic Peritoneum | Abdominal Peritoneum | Retroperitoneum | Others | Total Number | Maximum Diameter of the recurrent tumors, cm |
| 1 | 0 | 0 | 1 | 0 | 1 | 10.4 |
| 2 | 0 | 2 | 0 | 0 | 2 | 5.9–7.4 |
| 3 | >5 | 5 | 0 | 0 | >5 | 0.7–3.7 |
| 4 | 0 | 0 | 0 | 1 (bone) | 1 | 5.8 |
| 5 | 2 | 1 | 0 | 0 | 3 | 1.0–6.7 |
| 6 | 0 | 4 | 0 | 0 | 4 | 1.9–6.2 |
| 7 | 1 | 0 | 0 | 0 | 1 | 5.5 |
| 8 | 3 | 0 | 0 | 0 | 3 | 1.8–4.7 |
| 9 | 4 | 1 | 1 | 0 | >5 | 2.0–15.4 |
| 10 | 1 | 0 | 0 | 0 | 1 | 3.7 |
| 11 | 2 | 1 | 0 | 0 | 3 | 0.5–6.8 |
The MRI characteristics of the 13 largest masses are summarized in Table 3. The morphologic appearance was round in 7 masses, lobulated in 4 masses, oval in 1 mass, and crescent in 1 mass (Figs. 1–4). The margins were all well circumscribed. The internal structure was multilocular cystic in 9 masses, solid and cystic in 3 masses, and solid in 1 mass. Internal hemorrhage and sponge-like multicystic components were found in 9 masses (69.2%; Figs. 1–4).
TABLE 3.
Magnetic Resonance Imaging Characteristics of Largest Recurrent AGCT
| Case | Location | Maximum Diameter, cm | MRI Characteristics | ||||
|---|---|---|---|---|---|---|---|
| Morphologic Appearance | Margin | Internal Structure | Internal Hemorrhage | Sponge-Like Multicystic Component | |||
| 1 | Retroperitoneum | 10.4 | Oval | Well circumscribed | Multilocular cystic | + | + |
| 2 | Abdominal peritoneum | 7.4 | Round | Well circumscribed | Multilocular cystic | + | + |
| 3 | Pelvic peritoneum | 3.7 | Round | Well circumscribed | Solid | − | − |
| 4 | Bone | 5.8 | Round | Well circumscribed | Solid and cystic | + | + |
| 5 | Pelvic peritoneum | 6.7 | Lobulated | Well circumscribed | Multilocular cystic | − | + |
| 6 | Abdominal peritoneum | 6.2 | Lobulated | Well circumscribed | Multilocular cystic | + | + |
| 7 | Pelvic peritoneum | 5.5 | Round | Well circumscribed | Multilocular cystic | + | − |
| 8 | Pelvic peritoneum | 4.7 | Round | Well circumscribed | Solid and cystic | + | − |
| 9 | Pelvic peritoneum | 5.8 | Round | Well circumscribed | Multilocular cystic | + | + |
| Retroperitoneum | 15.4 | Lobulated | Well circumscribed | Multilocular cystic | − | + | |
| Abdominal peritoneum | 9.8 | Crensent | Well circumscribed | Multilocular cystic | + | + | |
| 10 | Pelvic peritoneum | 3.7 | Round | Well circumscribed | Multilocular cystic | − | − |
| 11 | Pelvic peritoneum | 6.8 | Lobulated | Well circumscribed | Solid and cystic | + | + |
FIGURE 1.

Recurrent adult granulosa cell tumors in the pelvic peritoneum, abdominal peritoneum, and retroperitoneum of a 51-year-old woman who underwent a right salpingo-oophorectomy for a stage IC tumor 5 years ago (case 10). A, T2-weighted image shows a round tumor and a multilocular cystic mass (arrows) with a partial thickened wall (small allows) in the left pelvic peritoneum. A sponge-like cystic appearance is demonstrated by the mass. Moreover, 2 multilocular cystic masses (arrowheads) are demonstrated behind the previously mentioned mass and right pelvic peritoneum. B, T2-weighted image shows a lobulated and multilocular cystic mass (arrows) in the retroperitoneum. A sponge-like cystic appearance (arrowhead) is shown between the large cysts (asterisks). C, T2-weighted image shows a crescent-shaped mass and a multilocular cystic mass with a sponge-like cystic appearance (arrows) in right subphrenic space. D, T1-weighted image shows a region of high intensity (arrow), which is a suspected hemorrhagic focus. E, Histological sections of right subphrenic tumor, stained with hematoxylin and eosin (×40) show a multicystic lesion lined with granulosa cells.
FIGURE 4.

Recurrent adult granulosa cell tumor in the bone of a 53-year-old woman who underwent a right salpingo-oophorectomy for a stage IA tumor 19 years ago (case 4). A, Unenhanced CT shows a destructive expansile mass (arrows) in right ischium. B, T2-weighted image shows a round-shaped, solid, and cystic mass with sponge-like cystic appearance (arrows). C, T1-weighted image shows a region of high intensity (arrow) in the mass, which is a suspected internal hemorrhage.
FIGURE 2.

Recurrent adult granulosa cell tumors in the abdominal peritoneum of a 70-year-old woman who underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy for a stage IA tumor 30 years ago (case 6). A, T2-weighted image shows a lobulated and multilocular cystic mass with central low-signal intensity at the hepatic hilus, which accompanies a sponge-like multicystic appearance (arrows). B, T1-weighted image shows a region of high intensity (arrow) in the cysts, which is a suspected internal hemorrhage.
FIGURE 3.
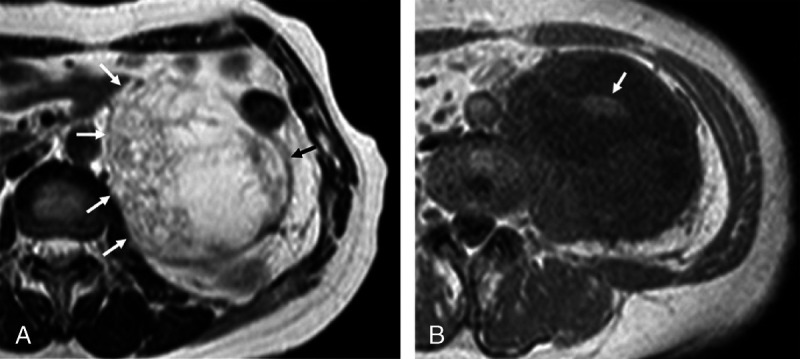
Recurrent adult granulosa cell tumor in the retroperitoneum of a 58-year-old woman who underwent a left salpingo-oophorectomy for a stage IA tumor 26 years ago (case 1). A, T2-weighted image shows an oval-shaped, multilocular cystic mass in the left retroperitoneum, which accompanies a sponge-like multicystic appearance (arrows). B, T1-weighted image shows a central region of high intensity (arrow) in the mass, which is suspected an internal hemorrhage.
DISCUSSION
Granulosa cell tumor of the ovary is a rare malignant tumor of the sex cord-stromal tumor, which accounts for 2% to 5% of all ovarian cancers.7 Granulosa cell tumors are divided into adult type and juvenile type, representing 95% and 5% of tumors, respectively.8 Adult granulosa cell tumors occur more often in middle-aged and postmenopausal women, with a peak incidence from 50 to 55 years of age.9 Adult granulosa cell tumors characteristically produce estradiol, inhibin B, and antimullerian hormone, which are used as diagnostic serum markers.10,11 Hyperestrogenism often causes irregular vaginal bleeding. Moreover, endometrial hyperplasia was found in 29.2% of the patients, and endometrial cancer occurred in 7.5% of the patients.1 Tumors are usually between 5 and 15 cm in size and more than 95% are unilateral.8 The gross appearance of these tumors is typically solid and cystic. The cysts are often blood filled, and some tumors are markedly hemorrhagic. These tumors are often entirely solid; however, they can also be entirely cystic.12 The histopathological characteristics include cells with a coffee bean–like longitudinal nuclear groove and a microfollicular structure termed the Call-Exner body. Immunohistochemical markers for AGCTs are α-inhibin and calretinin. The primary standard surgery carried out to treat these tumors is a hysterectomy and a bilateral salpingo-oophorectomy. Adjunctive chemotherapy is needed for advanced stages. Fertility-preserving surgery with unilateral salpingo-oophorectomy is an option in young patients with stage I AGCTs.13 They are usually diagnosed at stage I disease and have a relatively favorable prognosis with 10-year survival rates of 84% to 95% for stage I tumors.2–4 These tumors have a propensity for late recurrence; 20% to 25% of patients experienced long-term recurrence, with a median recurrence time of 4 to 6 years after the initial treatment; recurrence has been reported as much as 40 years after the initial treatment.4 High-risk features may include the following: large tumor size (>10 cm), poorly differentiated tumor, high mitotic index, tumor rupture, and stage IC as these factors are associated with a higher risk of recurrence.14 CD56, which is a pathogen recognition receptor on natural killer cells, is known to be expressed in primary and recurrent AGCTs.15 SMAD proteins transduce signals from transforming growth factor β superfamily ligands to regulate cell proliferation, differentiation, and death through the activation of receptor serine/threonine kinases. It was reported that a high expression of CD56 or SMAD3 showed a significant prediction of recurrence.16 It was recently reported that recurrence may be detected noninvasively from circulating plasma cell free DNA in patients with clinical disease via a liquid biopsy for the FOXL2 mutation.17
Adult granulosa cell tumors were found to reoccur locally in the pelvic peritoneum and metastasize in the abdominal peritoneum or in the retroperitoneal lymph nodes. Local pelvic recurrence is reported in 70% of cases, pelvic and abdominal reoccurrence is reported in 9% of cases, and retroperitoneal reoccurrence is reported in 6% of cases. In addition, reoccurrence was recorded in the pelvis and retroperitoneum in 6% of cases and in the pelvis, abdomen, and retroperitoneum in 3% of cases.18 In rare cases, AGCTs may reoccur in the lung, bone, and abdominal wall. Complete resection combined with postoperative adjuvant chemotherapy may improve the prognosis of recurrent tumors.
Imaging of primary AGCTs shows a spectrum of imaging manifestations because of various histologic appearances and various arrangements of tumor cells.19–23 They can show the appearance of entirely solid masses, masses with hemorrhagic or fibrotic changes, multilocular cystic masses, or entirely cystic masses.19–24 Magnetic resonance imaging characteristics are reported to be hemorrhagic foci or cysts19–24 and sponge-like multicyclic components in the mass.19,22,24
To our knowledge, few studies report the imaging features of recurrent AGCTs.6 Currently, there are no studies available that have investigated MRI imaging of recurrent AGCTs. On images, a large recurrent tumor can mimic a primary tumor originating from the liver, spleen and small intestine, or a primary retroperitoneal tumor.25–28 Rha et al6 reported that recurrent tumors are characterized as a relatively small number of discrete peritoneal or retroperitoneal tumors with an imaging appearance, which can vary from solid to cystic tumors. This study found that 7 of the 11 patients had 2 or more recurrent tumors. It was noteworthy in the article by Rha et al6 that all of 11 patients had large recurrent tumor measuring 3 cm or more. In this study, MRI findings of large recurrent tumors are commonly well circumscribed, round or lobulated, and multilocular cystic or solid and cystic in appearance. Moreover, they frequently included internal hemorrhage and sponge-like multicystic components, which are similar to those of primary AGCTs of the ovary.19–24
CONCLUSIONS
The possibility of a recurrent ovarian AGCT should be considered when multilocular or solid masses with internal hemorrhagic and sponge-like multicystic components are identified on MRI images of the peritoneum or retroperitoneum. The patient's past history should be thoroughly investigated along with the presence of other discrete disseminated tumors located in the pelvis and abdomen on the images.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Isao Numoto, Email: numoto.isao@hotmail.co.jp.
Ayako Suzuki, Email: i_am_sue_19861211_pinky@yahoo.co.jp.
Takefumi Hamakawa, Email: hamatake@outlook.jp.
Yuko Matsukubo, Email: moucocco_sheepdog@yahoo.co.jp.
Masakatsu Tsurusaki, Email: mtsuru@dk2.so-net.ne.jp.
Kazunari Ishii, Email: kishii@hbhc.jp.
Tomoyuki Otani, Email: tomoyu@gmail.com.
Noriomi Matsumura, Email: noriomi@med.kindai.ac.jp.
REFERENCES
- 1.Ottolina J Ferrandina G Gadducci A, et al. Is the endometrial evaluation routinely required in patients with adult granulosa cell tumors of the ovary? Gynecol Oncol. 2015;136:230–234. [DOI] [PubMed] [Google Scholar]
- 2.Pankratz E Boyes DA White GW, et al. Granulosa cell tumors. A clinical review of 61 cases. Obstet Gynecol. 1978;52:718–723. [PubMed] [Google Scholar]
- 3.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–1189. [DOI] [PubMed] [Google Scholar]
- 4.East N Alobaid A Goffin F, et al. Granulosa cell tumour: a recurrence 40 years after initial diagnosis. J Obstet Gynaecol Can. 2005;27:363–364. [DOI] [PubMed] [Google Scholar]
- 5.Chen YC Chang LC Soong RS, et al. A late recurring and easily forgotten tumor: ovarian granulosa cell tumor. World J Surg Oncol. 2012;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rha SE Oh SN Jung SE, et al. Recurrent ovarian granulosa cell tumors: clinical and imaging features. Abdom Imaging. 2008;33:119–125. [DOI] [PubMed] [Google Scholar]
- 7.Fox H, Agrawal K, Langley FA. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975;35:231–241. [DOI] [PubMed] [Google Scholar]
- 8.Colombo N Parma G Zanagnolo V, et al. Management of ovarian stromal cell tumors. J Clin Oncol. 2007;25:2944–2951. [DOI] [PubMed] [Google Scholar]
- 9.Stenwig JT, Hazekamp JT, Beecham JB. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol Oncol. 1979;7:136–152. [DOI] [PubMed] [Google Scholar]
- 10.Petraglia F Luisi S Pautier P, et al. Inhibin B is the major form of inhibin/activin family secreted by granulosa cell tumors. J Clin Endocrinol Metab. 1998;83:1029–1032. [DOI] [PubMed] [Google Scholar]
- 11.Rey RA Lhommé C Marcillac I, et al. Antimüllerian hormone as a serum marker of granulosa cell tumors of the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol. 1996;174:958–965. [DOI] [PubMed] [Google Scholar]
- 12.Young RH. Ovarian sex cord-stromal tumors: reflections on a 40-year experience with a fascinating group of tumors, including comments on the seminal observations of Robert E. Scully, MD. Arch Pathol Lab Med. 2018;142:1459–1484. [DOI] [PubMed] [Google Scholar]
- 13.Bergamini A Cormio G Ferrandina G, et al. Conservative surgery in stage I adult type granulosa cells tumors of the ovary: results from the MITO-9 study. Gynecol Oncol. 2019;154:323–327. [DOI] [PubMed] [Google Scholar]
- 14.Khosla D Dimri K Pandey AK, et al. Ovarian granulose cell tumor: clinical features, treatment, outcome, and prognostic factors. N Am J Med Sci. 2014;6:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volker HU Engert S Cramer A, et al. Expression of CD56 isoforms in primary and relapsed adult granulosa cell tumors of the ovary. Diagn Pathol. 2008;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakr S Abdulfatah E Thomas S, et al. Granulosa cell tumors: novel predictors of recurrence in early-stage patients. Int J Gynecol Pathol. 2017;36:240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanagida S Anglesio MS Nazeran TM, et al. Clinical and genetic analysis of recurrent adult-type granulosa cell tumor of the ovary: persistent preservation of heterozygous c.402C>G FOXL2 mutation. PLoS One. 2017;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Rustum NR Restivo A Ivy J, et al. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumors of the ovary. Gynecol Oncol. 2006;103:31–34. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa K Hatabu H Togashi K, et al. Granulosa cell tumor of the ovary: MR findings. J Comput Assist Tomogr. 1997;21:1001–1004. [DOI] [PubMed] [Google Scholar]
- 20.Ko SF Wan YL Ng SH, et al. Adult ovarian granulosa cell tumors: spectrum of sonographic and CT findings with pathologic correlation. AJR. 1999;172:1227–1233. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Kim SH. Granulosa cell tumor of the ovary: common findings and unusual appearances on CT and MR. J Comput Assist Tomogr. 2002;26:756–761. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka YO Tsunoda H Kitagawa Y, et al. Functioning ovarian tumors: direct and indirect findings at MR imaging. Radiographics. 2004;24:147–166. [DOI] [PubMed] [Google Scholar]
- 23.Jung SE Rha SE Lee JM, et al. CT and MRI findings of sex cord-stromal tumor of the ovary. AJR Am J Roentgenol. 2005;185:207–215. [DOI] [PubMed] [Google Scholar]
- 24.Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu S Zhou X Hou B, et al. Metastasis of the liver with a granulosa cell tumor of the ovary: a case report. Oncol Lett. 2015;9:816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledinsky I Ulamec M Jukić Z, et al. Late metastasis of granulosa cell tumor mimicking primary angiosarcoma of the spleen; case report and literature review. MOJ Tumor Res. 2018;1:89–91. [Google Scholar]
- 27.Singh-Ranger G, Sharp A, Crinnion JN. Recurrence of granulosa cell tumour after thirty years with small bowel obstruction. Int Semin Surg Oncol. 2004;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abaid LN Mosquera-Caro M Kankus RC, et al. Extraordinarily prolonged disease recurrence in a granulosa cell tumor patient. Case Rep Oncol. 2010;3:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]


