Supplemental Digital Content is available in the text.
Keywords: arrhythmias, cardiac; atrioventricular block; coronavirus; myocardial infarction; ventricular fibrillation
Abstract
Background:
Patients with coronavirus disease 2019 (COVID-19) who develop cardiac injury are reported to experience higher rates of malignant cardiac arrhythmias. However, little is known about these arrhythmias—their frequency, the underlying mechanisms, and their impact on mortality.
Methods:
We extracted data from a registry (NCT04358029) regarding consecutive inpatients with confirmed COVID-19 who were receiving continuous telemetric ECG monitoring and had a definitive disposition of hospital discharge or death. Between patients who died versus discharged, we compared a primary composite end point of cardiac arrest from ventricular tachycardia/fibrillation or bradyarrhythmias such as atrioventricular block.
Results:
Among 800 patients with COVID-19 at Mount Sinai Hospital with definitive dispositions, 140 patients had telemetric monitoring, and either died (52) or were discharged (88). The median (interquartile range) age was 61 years (48–74); 73% men; and ethnicity was White in 34%. Comorbidities included hypertension in 61%, coronary artery disease in 25%, ventricular arrhythmia history in 1.4%, and no significant comorbidities in 16%. Compared with discharged patients, those who died had elevated peak troponin I levels (0.27 versus 0.02 ng/mL) and more primary end point events (17% versus 4%, P=0.01)—a difference driven by tachyarrhythmias. Fatal tachyarrhythmias invariably occurred in the presence of severe metabolic imbalance, while atrioventricular block was largely an independent primary event.
Conclusions:
Hospitalized patients with COVID-19 who die experience malignant cardiac arrhythmias more often than those surviving to discharge. However, these events represent a minority of cardiovascular deaths, and ventricular tachyarrhythmias are mainly associated with severe metabolic derangement.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04358029.
What Is Known?
A significant elevation in cardiac troponin—a marker of myocardial injury—is present in ≈20% of hospitalized patients with coronavirus disease 2019 (COVID-19) and portends a poor clinical prognosis particularly in the presence of coexisting cardiovascular disease.
There is a reported interaction between disease severity and cardiac arrhythmias, particularly in critically ill patients with COVID-19. However, the exact nature and frequency of malignant arrhythmias have not been well characterized and more importantly, whether they contribute to death.
What the Study Adds?
Hospitalized patients with COVID-19 who die experience acute malignant cardiac arrhythmias more often than those surviving to discharge.
Acute malignant cardiac arrhythmias do contribute to the ultimate demise of a small proportion of patients with COVID-19, some involving specific mechanisms potentially amenable to targeted interventions such as pacemaker implantation or revascularization. However, the majority appear to occur as a generalized response to acute critical illness and may not prove amenable to antiarrhythmic interventions.
Coronavirus disease 2019 (COVID-19) is a pandemic caused by a novel enveloped RNA betacoronavirus named severe acute respiratory syndrome coronavirus-2.1–3 As of May 23, 2020, the infection has been confirmed in ≈5 million individuals worldwide, and the United States was most affected, with over 1.5 million cases.4
In addition to severe clinical symptoms, COVID-19 is associated with a substantial risk for death. The worldwide case-fatality ratio is currently estimated at 6.5% and 6% in the United States.4 Indeed, as of May 23, 2020, over 340 000 individuals have died worldwide, of whom ≈97 000 have died in the United States, in turn of whom nearly one-fifth are from New York City alone.4
These staggering numbers obligate a better understanding of how COVID-19 culminates in death. Early reports identified demographic characteristics predicting poor outcomes, including older age and comorbidities such as hypertension, diabetes, and cardiovascular disease.5–9 While these general risk factors do not provide much mechanistic information, a relationship has been observed between elevated levels of certain biomarkers and COVID-19–related mortality.5–10 These observations have provoked intense speculation as to the nature and significance of this cardiac injury.9,11
Intriguingly, there is a reported interaction between disease severity and cardiac arrhythmias, with the latter reportedly ranging in frequency from 16.7% to 30.3% and even higher rates (44.4%–74.6%) reported in critically ill patients with COVID-19. Indeed, malignant ventricular arrhythmias were reported in 17.3% of COVID-19 patients with abnormal troponin levels.5,12 However, the exact nature and frequency of these malignant arrhythmias have not been well characterized.13 Accordingly, we conducted a rigorous patient-level analysis to determine whether acute malignant cardiac arrhythmias, such as tachy- or bradyarrhythmias, are major contributors to the demise of hospitalized patients with COVID-19. Furthermore, we characterized whether these arrhythmic events were the primary incitements or merely epiphenomena of the severe hypoxic and metabolic stress of this critical illness.
Methods
Study Population and Data Collection
The data that support the findings of this study are available from the corresponding author upon reasonable request. This single-center retrospective cohort study included consecutive adult patients (≥18 years of age) with laboratory-confirmed COVID-19 infection admitted to the Mount Sinai Hospital (New York, NY) between March 7 and April 12, 2020, as part of a COVID-19 registry (NCT04358029). Patients were diagnosed with COVID-19 infection based on a reverse-transcriptase polymerase chain reaction assay of a nasal or pharyngeal swab specimen.
Laboratory-confirmed COVID-19 patients were included provided they received (1) at least 24 hours of continuous inpatient telemetric electrocardiographic monitoring and (2) reached a final disposition—death or hospital discharge. The decision to receive telemetry was partially based on the physician assessment of acuity of illness but mostly on the availability of telemetry in a resource-constrained environment (ie, allocation based on first available bed). Patients who remained hospitalized for ongoing treatment, pregnant women, and children (age <18 years) were excluded. Patients readmitted during the study period were also excluded from analysis. A successful hospital discharge comprised of near-complete resolution of clinical symptoms with resolution of fever and improvement in blood inflammatory markers.
We extracted patient demographics, laboratory findings, imaging results, EKG/telemetry data, treatments received, and clinical outcomes on admission and during hospitalization from the electronic medical records. All available imaging scans, electrocardiographic, and telemetry data were reviewed by experienced cardiologists, and differences in interpretation were reconciled by consultation with a senior electrophysiologist. Telemetry data were obtained by General Electric monitoring systems (GE Healthcare, Waukesha, WI) from 191 inpatient beds and 101 critical care beds and stored at 240 hz with 12-bit magnitude using BedMasterEx V5.1.2 (Excel Medical Electronics LLC, Jupiter, FL). Allocation to these units was based on acuity of illness, physician discretion, and bed availability. During high patient volumes, intensive care unit rooms housed 2 patients in which case, 1 patient’s data was not recorded. Available autopsy reports were also examined.
The study was approved by the institutional review board governing research in human subjects at the Icahn School of Medicine at Mount Sinai, which waived informed consent. Extracted data were secured in a computerized database and missing data clarified by revisiting the electronic medical records. Patient-level data were deidentified before analysis. The authors had full access to the data and take responsibility for the integrity of the data. All authors have read and agree to the findings in the article as written.
Study Outcomes
The composite primary end point was acute malignant cardiac arrhythmias as defined by either ventricular tachycardia (VT) or ventricular fibrillation (VF) or bradyarrhythmias such as atrioventricular block causing hemodynamic compromise or cardiac arrest. The secondary end point was acute ST-segment–elevation myocardial infarction (MI).
Study Definitions
Cardiac arrest from VT/VF or atrioventricular block was defined as sudden loss of consciousness with no signs of systemic circulation. Acute MI was defined by chest pain (if conscious) and new ST-segment elevation in ≥2 contiguous electrocardiographic leads (when available).14 Similarly, myocardial injury was defined by a rise or fall of blood troponin values with at least 1 value above the 99th-percentile upper reference limit. Pulseless electrical activity was defined by organized or semiorganized cardiac electrical activity other than VT or VF resulting in hemodynamic compromise or death.
Acute respiratory distress syndrome,15 acute kidney injury, and septic shock were defined according to the standard guidelines.16 Cardiovascular mortality was defined as death attributable to acute MI, decompensated heart failure, cerebrovascular accident, or cardiac arrest from primary VT or VF. Mortality due to other causes was categorized as noncardiovascular deaths.
Laboratory Confirmation
Clinical specimens were obtained and diagnostic testing for COVID-19 performed as recommended by the Centers for Disease Control and Prevention in patients who met clinical and epidemiological criteria. The reverse-transcriptase polymerase chain reaction tests were performed using the Roche cobas 6800 System (Basel, Switzerland), which targets the ORF1 gene (target 1) and SARS-CoV-2 E gene (target 2).17 If both targets were detected, the assay was reported as positive; detection of target 2 without target 1 was interpreted as a presumptively positive.
Statistical Analyses
Based on previous reports demonstrating a high prevalence of arrhythmias, as well as elevated troponin levels in 1 of 5 patients with COVID-19, we hypothesized that patients with COVID-19 who die would have a substantially higher incidence of acute malignant cardiac arrhythmias (VT/VF or AV block) than those surviving to discharge. Because the relative rate of accrual of consecutive telemetry patients varied between groups, a sample size curve was derived based on an 80% chance of detecting, as significant at the 5% level, an increase in the primary outcome from 3% in the discharged group to 17% in the mortality group.5 Based on the ratio of discharged:mortality subjects, between 139 and 160 total patients were required (see the Data Supplement for details). At the manifest discharge:mortality ratio of 1.7, the sample size of 140 patients provided sufficient power for the primary end point.
Continuous variables were summarized as median and interquartile range or means and SDs, as appropriate. Categorical variables were summarized as counts or percentages. No imputation was made for missing data. Mann-Whitney U test, Fisher exact test, or χ2 test was used to compare data between dead and discharged patients where appropriate. Poisson linear regression model was used to analyze the composite primary outcomes and calculate the incidence rate ratios between the patients who died or survived hospitalization. A multivariable binary logistic regression analysis was performed to calculate the odds ratio to estimate the association of risk between mortality and various covariates. A model was created including various risk factors that were significant on univariable analysis and had ≥10 events. P≤0.05 (2 tailed) was considered statistically significant. Statistical analysis was performed using SPSS, version 25.0 (IBM Corp, Armonk, NY).
Results
Demographics and Clinical Characteristics
A total of 1354 patients were screened using electronic medical records from March 7, 2020, to April 12, 2020, of whom 800 patients were hospitalized for treatment of COVID-19. Among these, 670 were discharged and 130 died. No telemetry data were available from 78 patients who died and 582 patients who were discharged, leaving 140 consecutive patients (mortality group, 52; discharge group, 88) for the final analysis (Figure I in the Data Supplement).
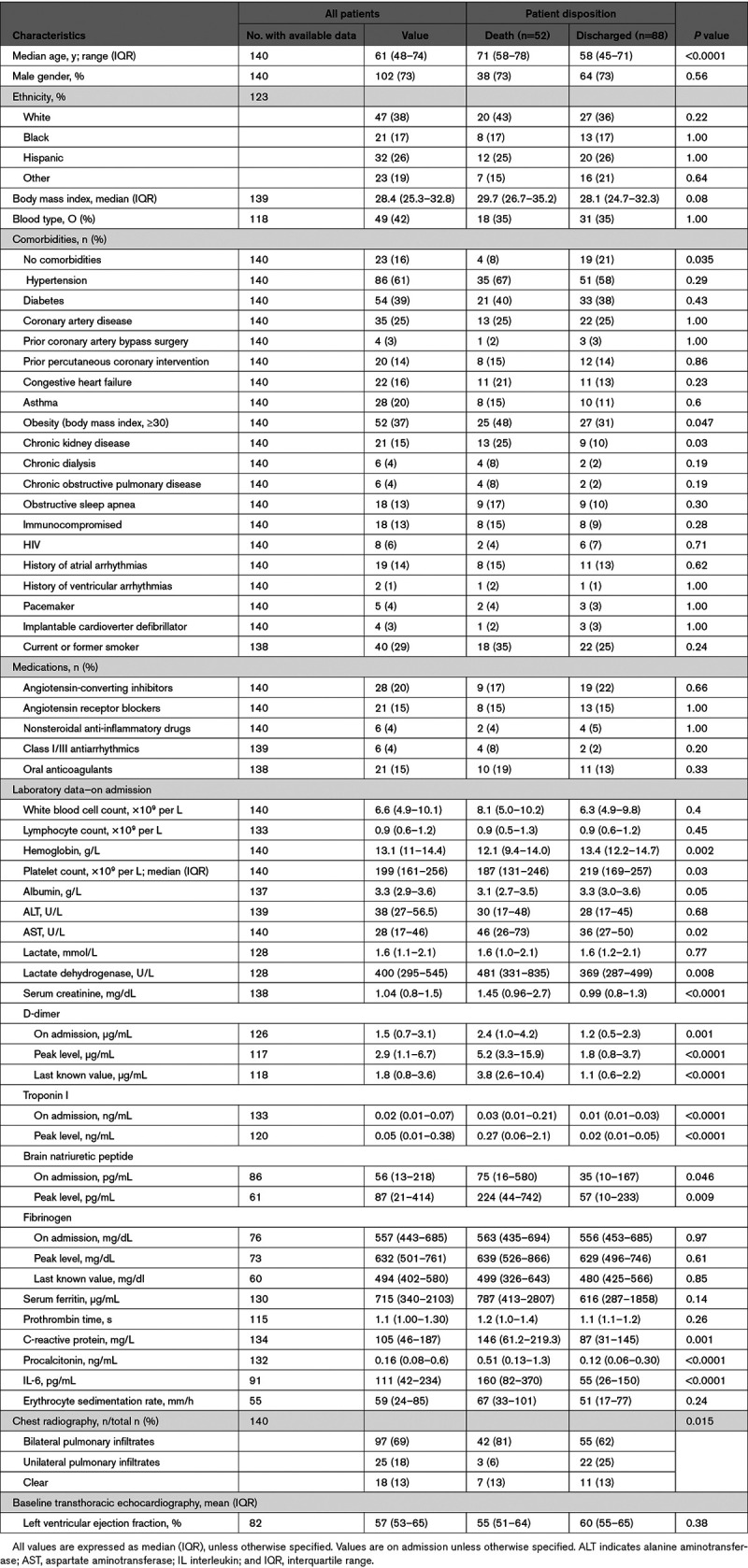
As shown in Table 1, the median age of the patient cohort was 61 years (range, 23–97 years), 102 (73%) patients were men, and only 47 (38%) were White. Among the overall population, 84% had at least 1 coexisting illness. Compared with patients in the discharge group, patients in the mortality group were older (median [interquartile range], 71 [58–78] versus 58 [45–71] years; P<0.0001) and more likely to have chronic kidney disease (25% versus 10%; P=0.03) and obesity (body mass index, ≥30). There were no significant differences between groups in the frequency of other chronic comorbidities including hypertension, diabetes, coronary artery disease, chronic obstructive airway disease, or heart failure (Table 1).
Table 1.
Demographics, Clinical Characteristics, and Laboratory Data
Laboratory and Radiological Findings
Table 1 details the laboratory and radiological findings upon admission and during hospitalization. On admission, when compared with the discharge group, patients in the mortality group presented with significantly elevated inflammatory markers such as C-reactive protein, procalcitonin, and IL (interleukin)-6.
Patients in the mortality group also had significantly higher d-dimer levels at admission (median, 2.4 versus 1.2; P=0.001), peak (5.2 versus 1.8; P<0.0001), and last known (3.8 versus 1.1; P<0.0001) time points compared with the discharge group. Furthermore, both admission (0.03 versus 0.01 ng/mL; P<0.0001) and peak (0.27 versus 0.02 ng/mL; P<0.0001) troponin I levels were significantly elevated in the mortality group compared with the discharge group.
Treatments and Complications
Admission to the intensive care unit (42% versus 20%) and initiation of invasive mechanical ventilation (40% versus 7%) during admission occurred more often in the mortality group than in the discharge group (Table 2).
Table 2.
Treatments and Complications
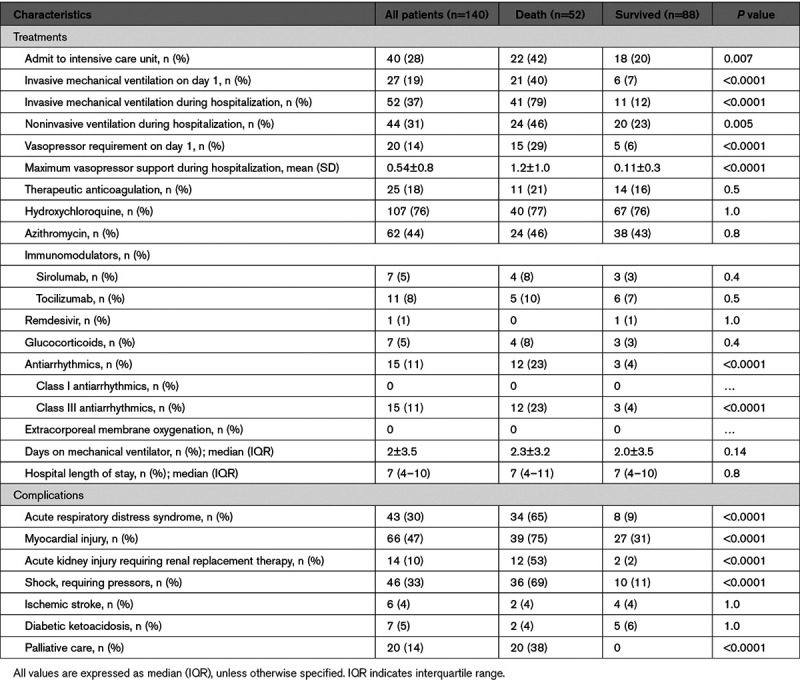
Twenty patients (14%) were admitted with severe hypotension requiring vasopressors, occurring more often in the mortality group (15 patients, 29%) than the discharge group (5 patients, 6%). A higher percentage of patients who died received class III antiarrhythmic drugs than those who survived (23% versus 4%). There were no significant differences in the proportions of patients who received hydroxychloroquine, tocilizumab, sirolumab, and remdesivir in the 2 groups.
During hospitalization, 47% had a diagnosis of myocardial injury, followed by shock (33%) and acute respiratory distress syndrome (30%)—all significantly higher among patients in the mortality group.
Clinical Outcomes
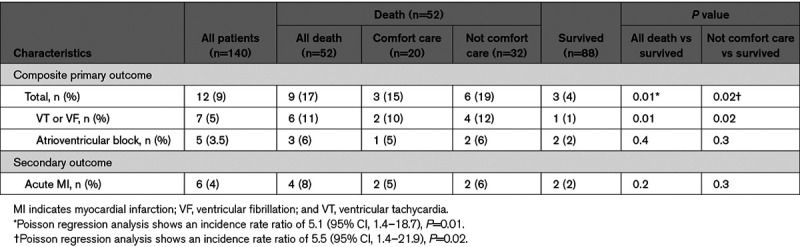
In the overall cohort, primary end point events occurred in 12 patients (9%)—7 patients (5%) with VT/VF and 5 patients (3.5%) with AV block (Table 3). This primary composite end point occurred more frequently in the mortality versus discharge group (17% versus 4%; incidence rate ratio, 5.1 [95% CI, 1.4–18.7]; P=0.01). The difference was mainly driven by the higher incidence of VT or VF in the mortality group (11%) compared with the discharge group (1%; P=0.01). The secondary outcome of acute ST-segment–elevation MI occurred in 6 patients (4%); there was no significant difference between groups.
Table 3.
Primary Composite Outcome
Among the 52 deaths in the mortality group, only 6 (12%) were categorized as cardiovascular deaths, of which 4 (8%) were attributed to MI and 2 (4%) to decompensated heart failure. None of the deaths were directly attributed to either VT/VF or AV block. The rest of the 48 (88%) deaths were categorized as noncardiovascular deaths. The arrhythmia at the time of death in these patients was pulseless electrical activity in 46 (89%) patients and VT/VF in 6 (11%) patients, respectively.
In the mortality cohort, 20 patients (38%) received care focusing on palliative/comfort measures. The median time to death after withdrawing aggressive treatments was only 19 hours (interquartile range, 6–84 hours). The higher rate of primary composite end point event in the mortality group was also observed (19% versus 4%; P=0.02) when comparing the 32 patients who died unexpectedly (without a decision to implement palliative care) with those patients who survived, again mainly driven by VT or VF. However, among these 32 patients, only 4 (12.5%) deaths were categorized as cardiovascular deaths, 3 deaths due to MI, and 1 death due to congestive heart failure.
To reduce potential bias related to selective application of telemetry monitoring, we investigated primary outcome events occurring in the 78 patients who died but without telemetric monitoring (Table I in the Data Supplement). In that population, there was only 1 (1%) primary outcome event and only 2 (3%) cardiovascular deaths, respectively.
Relationship of Malignant Cardiac Arrhythmias and Mortality
To better distinguish whether the cause of mortality was cardiovascular or noncardiovascular death, we explored associated clinical conditions (Table II in the Data Supplement). All 6 patients with ventricular tachyarrhythmias in the mortality group had VF (Figure [A]), and 5 of them had either metabolic or hypoxic abnormalities or extremely high vasopressor requirements at the time of death. None of the 6 deaths were categorized as cardiovascular deaths. Furthermore, autopsy data were available for 2 patients, and both demonstrated large lobar pulmonary emboli. Only 1 other patient, in the discharge group, with a history of nonischemic cardiomyopathy (left ventricular ejection fraction, 35%) and a biventricular implantable cardioverter pacemaker had monomorphic VT, apparently of outflow tract origin, which resolved with medications.
Figure.
Cardiac arrhythmias. COVID-19 indicates coronavirus disease 2019; and PCR, polymerase chain reaction.
Among the 5 patients manifesting atrioventricular block, 2 were associated with MI (Figure [B]), 2 had either metabolic abnormalities or high pressor requirements, suggesting that refractory shock was primarily responsible for conduction block, and 1 patient had AV block in the setting of non–ST-segment–elevation myocardial infarction and newly depressed left ventricular ejection fraction (Table III in the Data Supplement).
Univariable analysis demonstrated age >65 years, obesity, myocardial injury, admission IL-6 >100 pg/mL, vasopressors during hospitalization, acute respiratory distress syndrome, and composite primary outcomes were significantly different between the death and discharged groups (Table IV in the Data Supplement).
Predictors of mortality by multivariable binary logistic regression analysis were age >65 years (odds ratio, 3.10 [1.10–9.37]; P=0.05), vasopressor during hospitalization (odds ratio, 4.97 [1.44–17.10]; P=0.01), and acute respiratory distress syndrome (odds ratio, 12.93 [3.20–52.17]; P<0.0001) but not myocardial injury, obesity, or admission IL-6 >100 pg/mL (Table V in the Data Supplement).
12-Lead Electrocardiographic and Telemetric Monitoring
The 12-lead electrocardiographic findings during admission and before death of discharge were benign. Overall, the electrocardiographic intervals, including the QTc interval, remained within normal limits, and there were no significant differences between groups (Table VI in the Data Supplement).
The most common rhythm at the time of death/demise was pulseless electrical activity, which occurred in 46 patients (88%), followed by VF in 6 patients (12%). Importantly, none of these episodes of VF were preceded by other nonsustained ventricular arrhythmias. There were also no instances of QT prolongation culminating in Torsades de pointes.
Discussion
In this study, patients who died experienced more primary end point events of acute malignant arrhythmias including VT/VF or AV block (17% versus 4%; P=0.01) than compared with those discharged. There was no significant difference in the secondary end point of ST-segment–elevation MI (8% versus 2%; P=0.2). Only a small proportion (12%) of deaths was categorized as cardiovascular deaths, and most of these deaths (67%) occurred in the setting of ST-segment–elevation MI. Tachyarrhythmias such as VT/VF invariably occurred in the setting of severe metabolic stress, while bradyarrhythmias were not necessarily related to metabolic derangements but could instead be primary inciting events contributing to mortality.
Cardiovascular complications have been reported with the 2 other major coronaviruses that have caused major epidemics, severe acute respiratory syndrome and Middle East respiratory syndrome, including reports of left ventricular dysfunction, acute myocarditis, and cardiac arrest.18 However, the data pale next to the plethora of reports purporting various cardiac arrhythmias in COVID-19. Arrhythmia was poorly defined in most of these studies, but one clearly defined malignant arrhythmia as rapid VT lasting >30 seconds, inducing hemodynamic instability or VF.5 They reported malignant arrhythmias in 17.3% of COVID-19 patients with abnormal troponin values, versus only 1.5% for patients with low troponin values.5 A recent review postulated that this propensity for developing malignant ventricular arrhythmias was related to the hyperabnormal systemic immune-inflammatory response elicited by the severe acute respiratory syndrome coronavirus-2 virus.19
Indeed, in the mortality group, we observed significantly higher levels of both the cardiac injury biomarker, troponin I, and the inflammation-related biomarkers, C-reactive protein, procalcitonin, and IL-6. And using a strict definition of malignant ventricular arrhythmias, and a chart review including a comprehensive review of continuous ECG telemetry, we identified ventricular tachyarrhythmias as the terminal event in 11% of the mortality cohort. But it is important to recognize that these arrhythmias were not preceded by nonsustained ventricular tachyarrhythmia episodes, and all these events were VF, not VT. This is more consistent with a nonspecific arrhythmia in the context of a toxic milieu of hypoxemia and metabolic disarray atop a proarrhythmogenic environment of catecholamine and inflammatory stress.
Furthermore, careful review of the 12-lead electrocardiograms failed to identify other critical proarrhythmic factors like prolonged QT intervals. Recent data have suggested that 30% of patients treated with hydroxychloroquine/azithromycin for COVID-19 exhibited QT interval prolongation by 40 ms, with 11% increasing to >500 ms.20 In our study, hydroxychloroquine was used in 76% of our patients for a median duration of 4 days (interquartile range, 1–5 days), and a QTc increase by ≥50 ms occurred in 9% of patients. Together, these data suggest that while ventricular arrhythmias are more common with severe COVID-19, it is likely that the mechanism is not a specific myocardial inflammatory or coronary vascular process related to severe acute respiratory syndrome coronavirus-2 but rather a generalized response to a pulmonary and metabolic catastrophe.
On the other hand, while some instances of atrioventricular block were similarly related to metabolic disarray, it appeared to be the primary cause of cardiac arrest in other patients, including some that survived hospitalization. Similarly, ST-segment–elevation MI was also a primary inciting factor in the cardiac arrest. An association of acute coronary syndrome with COVID-19 has been reported.21,22 However, this connection would not be surprising given the hyperintense inflammatory response attendant with COVID-19 and associated thrombogenicity. Indeed, the mortality cohort did exhibit markedly elevated levels of the various inflammatory biomarkers. Furthermore, acute MI has been frequent during other respiratory infections, particularly H1N1 influenza.23
Taken together, these data indicate that acute malignant cardiac arrhythmias do contribute to the ultimate demise of a small proportion of patients with COVID-19, some involving specific mechanisms potentially amenable to targeted interventions such as pacemaker implantation or revascularization. But the majority may occur as a generalized response to acute critical illness and may not prove amenable to antiarrhythmic interventions.
Limitations
First, this is a retrospective, nonrandomized analysis of hospitalized patients with no long-term follow-up data. However, in contrast with many previous COVID-19 studies, all patients in our analysis had a definitive disposition of either hospital discharge or death, and the chart and data review were rigorous. Second, while the power analysis indicated sufficient sample size to test the study hypothesis, the study was not powered to assess for differences in the individual components of the composite end point. However, it is unlikely that including more patients would appreciably change any important conclusions. Third, not all hospitalized patients with COVID-19 received telemetry, as allocation of telemetry beds was based partially on medical acuity and largely upon bed availability at a time of constrained resources, rather than chronic comorbidities. Therefore, we cannot verify the frequency of arrhythmic events in patients who did not receive telemetry monitoring. Fourth, systematic echocardiography and other cardiovascular imaging data are lacking due to the logistic challenges posed by isolation units. But we did have echocardiography results on 9 of our patients, revealing normal ventricular function in most patients (7 of 9; Table VII in the Data Supplement). Fifth, the COVID-19 patients included in the study were admitted earlier during this epidemic in New York City; hence there is some variation in treatments received during hospitalization; however, the efficacy of these treatments is uncertain. Finally, virtually all the patients we studied had normal ventricular function preceding hospitalization. It is possible, indeed likely, that monomorphic VT would have occurred with greater frequency in patients who had preexisting structural heart disease and ventricular scarring.
Conclusions
As COVID-19 rages across the world, there is a pressing need to better understand the mechanisms of mortality in this deadly disease. Our data indicate that malignant arrhythmic events contribute to a minority of deaths in these patients. While ventricular tachyarrhythmias appear largely secondary to metabolic derangement, there are some patients who sustain acute MI or atrioventricular block that may be amenable to treatment.
Sources of Funding
None.
Disclosures
The authors report no conflicts of interest related to this article. A comprehensive list of the conflicts with other companies is in the Data Supplement.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- IL
- interleukin
- MI
- myocardial infarction
- VF
- ventricular fibrillation
- VT
- ventricular tachycardia
Drs Turagam and Musikantow contributed equally to this work as co-first authors.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.120.008920.
For Sources of Funding and Disclosures, see page 1329.
Contributor Information
Mohit K. Turagam, Email: mohitkturagam@gmail.com.
Daniel Musikantow, Email: daniel.musikantow@mountsinai.org.
Martin E. Goldman, Email: martin.goldman@mssm.edu.
Adel Bassily-Marcus, Email: adel.bassily-marcus@mountsinai.org.
Edward Chu, Email: edward.chu@mountsinai.org.
Poojita Shivamurthy, Email: poojita.shivamurthy@mountsinai.org.
Joshua Lampert, Email: joshuamlampert@gmail.com.
Iwanari Kawamura, Email: iwanari.kawamura@mountsinai.org.
Mahmoud Bokhari, Email: mbokhari2@gmail.com.
William Whang, Email: william.whang@mountsinai.org.
Benjamin Aaron Bier, Email: benjamin.bier@mountsinai.org.
Waqas Malick, Email: waqas.malick@mountsinai.org.
Helen Hashemi, Email: helen.hashemi@mountsinai.org.
Marc A. Miller, Email: marc.miller@mssm.edu.
Subbarao Choudry, Email: subbarao.choudry@mountsinai.org.
Christopher Pumill, Email: chris.pumill@gmail.com.
Tania Ruiz-Maya, Email: tania.ruizmaya@mountsinai.org.
Michael Hadley, Email: michael.hadley@mountsinai.org.
Gennaro Giustino, Email: gennaro.giustino@mountsinai.org.
Jacob S. Koruth, Email: jacob.koruth@mountsinai.org.
Noelle Langan, Email: marie-noelle.langan@mountsinai.org.
Aamir Sofi, Email: aamir.sofi@mountsinai.org.
Srinivas R. Dukkipati, Email: srinivas.dukkipati@mountsinai.org.
Jonathan L. Halperin, Email: Jonathan.halperin@mssm.edu.
Valentin Fuster, Email: valentin.fuster@mountsinai.org.
Roopa Kohli-Seth, Email: roopa.kohli-seth@mountsinai.org.
References
- 1.World Health Organization. Novel coronavirus – China. January 12, 2020Accessed May 23, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=CjwKCAjwoc_8BRAcEiwAzJevteLNvVcwNtRgXWUN6ZUfEVR-4qz-fDeV7pY_nHISb3_nn-Gj-qdhPBoCnCwQAvD_BwE
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns Hopkins University of Medicine Coronavirus Resource Center. 2020. Accessed May 23, 2020. https://coronavirus.jhu.edu/map.html.
- 5.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Retraction: cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2007621. N Engl J Med. 2020;382:2582. doi: 10.1056/NEJMc2021225 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Chapman AR, Bularga A, Mills NL. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141:1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008 [DOI] [PubMed] [Google Scholar]
- 12.Hu L, Chen S, Fu Y, Gao Z, Long H, Ren H-W, Zuo Y, Li H, Wang J, Xv Q-B, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. Clin Infect Dis. 2020;ciaa539. doi: 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam M, Cheung J, Patel P, Sotomonte J, Lampert R, et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020;141:e823–e831. doi: 10.1161/CIRCULATIONAHA.120.047063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 15.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 16.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M; Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, arrhythmic risk, and inflammation: mind the gap! Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293 [DOI] [PubMed] [Google Scholar]
- 20.Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, Nadeau-Routhier C, Knotts R, Bar-Cohen R, Kogan E, Barbhaiya C, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425–1433. doi: 10.1016/j.hrthm.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, et al. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, Katz K, Ko DT, McGeer AJ, McNally D, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


