Transmission modeling suggests that screening guidelines focused on men who have sex with men are likely insufficient for syphilis control in contexts with substantial infection burden in heterosexual populations.
Supplemental digital content is available in the text.
Background
The current syphilis epidemic in the United States is concentrated in gay, bisexual, and other men who have sex with men (MSM), but substantial heterosexual transmission is reported in some parts of the country. Using the US states of Louisiana and Massachusetts as case studies, we investigated how epidemic context influences the impact of population screening approaches for syphilis control.
Methods
We constructed a compartmental metapopulation model parameterized to describe observed patterns of syphilis transmission. We estimated the impact of different approaches to screening, including perfect adherence to current US screening guidelines in MSM.
Results
In Louisiana, where syphilis cases are more evenly distributed among MSM and heterosexual populations, we projected that screening according to guidelines would contribute to no change or an increase in syphilis burden, compared with burden with current estimated screening coverage. In Massachusetts, which has a more MSM-focused outbreak, we projected that screening according to guidelines would be as or more effective than current screening coverage in most population groups.
Conclusions
Men who have sex with men–focused approaches to screening may be insufficient for control when there is substantial transmission in heterosexual populations. Epidemic characteristics may be useful when identifying at-risk groups for syphilis screening.
Syphilis is resurgent in the United States. The most striking epidemiological feature of the current epidemic is the disproportionate representation of gay, bisexual, and other men who have sex with men (MSM) among cases and high rates of infection among people living with HIV.1 After a nadir in the mid-1990s, many jurisdictions have observed increasing syphilis rates since the late 1990s or early 2000s. Although the current syphilis epidemic is concentrated in MSM, reported cases in women have been increasing in recent years, as have rates of congenital syphilis.1,2 At present, heterosexual and congenital syphilis cases remain geographically concentrated, with 44% of primary and secondary cases in women and 65% of congenital syphilis cases reported from 4 states in 2018: California, Florida, Louisiana, and Texas.1
Current public health efforts are not having the desired effect on syphilis control. US screening guidelines recommend screening for syphilis in sexually active MSM, persons living with HIV, and pregnant women.3–6 At least annual screening is recommended in sexually active MSM and persons living with HIV, with more frequent screening recommended if individual risk behaviors and local epidemiology warrant it.3,6 The guidelines provide screening recommendations for pregnant women but do not otherwise recommend routine screening in non–HIV-infected heterosexual populations.
Predicting the future trajectory of the syphilis epidemic is difficult. As historical epidemiological data show, social change, the shifting focus of public health investment, and the evolving HIV epidemic may contribute to upsurges or declines in infection rates.7–9 The complex natural history of syphilis in humans further complicates projection of intervention impacts.10 Despite these challenges, mathematical models are useful for understanding the potential effects of public health interventions on epidemic dynamics. Mathematical modeling studies have suggested that frequent screening may be an effective and cost-effective approach to syphilis control among populations with high rates of syphilis incidence.11–14 Based on past experience with syphilis resurgence after apparent control,15 it is also important to consider how to implement screening programs among those most at risk, to maximize and sustain impact.
Because of the concentration of outbreaks among MSM in many high-income countries, many mathematical models examining approaches to improving syphilis control have focused on MSM populations.16–19 Given the changing nature of the epidemic in the United States, we sought to evaluate how different approaches to screening would influence syphilis epidemiology, using a transmission model describing syphilis transmission in both MSM and heterosexual populations. We hypothesized that local epidemic characteristics would impact the effectiveness of current screening recommendations, as well as possible alternate approaches to screening. To examine this hypothesis, we fit a model separately to data from Massachusetts and Louisiana, 2 US states experiencing a significant burden of infection, but with different epidemic characteristics in terms of the affected population groups.
METHODS
Model Overview
We developed a dynamic compartmental metapopulation mathematical model that described syphilis transmission in MSM and heterosexual populations of different racial/ethnic groups (Fig. 1). In the model, bridging between heterosexual and MSM populations occurred between men in the MSM compartments and women. We modeled non-Hispanic Black, Hispanic, and non-Hispanic non-Black heterosexual subpopulations. Although we did not model HIV cotransmission, the model stratified the MSM population by HIV status, given the high rates of syphilis infection among HIV-infected individuals and the potential for targeted inventions in this group.20–23 The population was further stratified by age group: 20–44 and 45–64 years, and by sexual activity level: low and high. Sexual activity levels were defined by rates of partner change. A more complete description of the model is provided in the Technical Appendix, http://links.lww.com/OLQ/A529.
Figure 1.
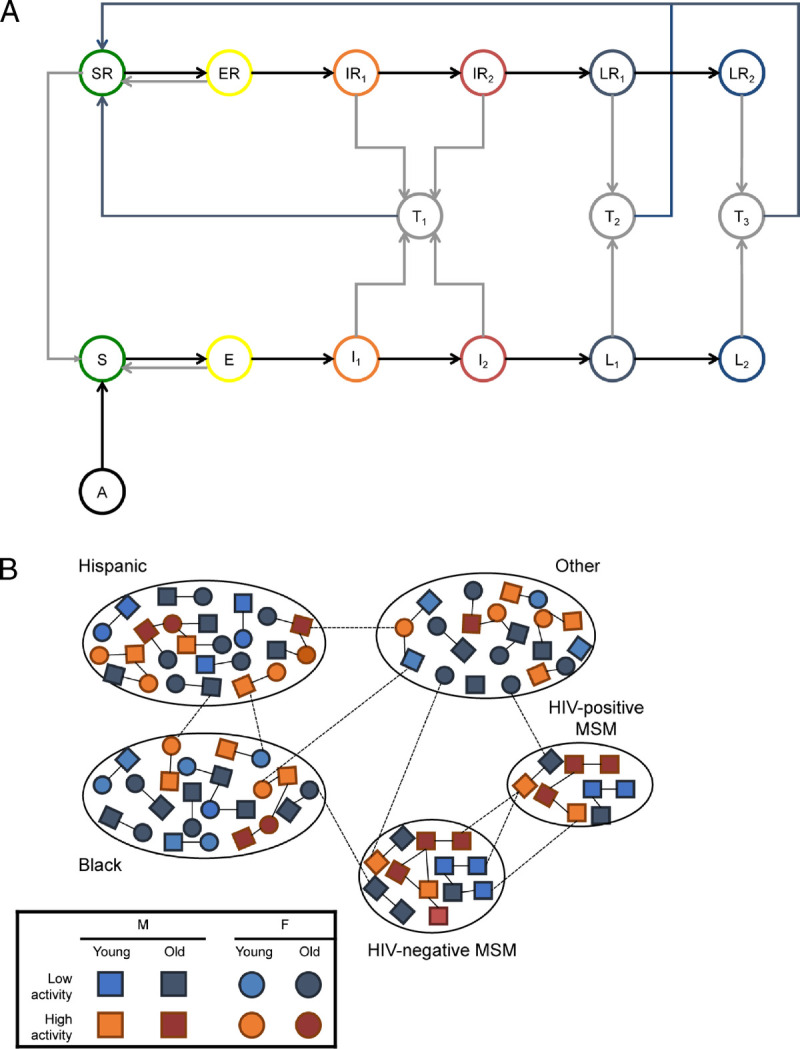
Overview of syphilis transmission model. A, Syphilis natural history is described by the following states: not sexually active (A), susceptible (S, SR), exposed (E, ER), primary syphilis (I1, IR1), secondary syphilis (I2, IR2), early latent syphilis (L1, LR1), and late latent syphilis (L2, LR2). The states followed by “R” indicate a separate set of compartments for those with a prior treated infection. T1–T3 are treated states during which an individual is protected from reinfection with time spent in these states dependent on infection stage at treatment. B, Mixing within and between subpopulations is dependent on age group (young: 20–44 years: 45–64 years old), sex, and sexual activity level (based on annual rate of partner change). Lines representing partnerships are illustrative only and do not represent all possible combinations of sexual partnerships. Additional details, including model equations, are provided in the Technical Appendix, http://links.lww.com/OLQ/A529.
The natural history of syphilis was modeled using a previously described approach10 and included the following health states: “susceptible” (S), “incubating” (E), “primary syphilis” (I1), “secondary syphilis” (I2), “early latent syphilis,” (L1), and “late latent syphilis” (L2). The primary and secondary stages were assumed to be infectious. Although there is some evidence that those with early latent syphilis can relapse to secondary syphilis,24 for this analysis, we assumed that the early latent stage of infection was not infectious. Without treatment, infected individuals progressed through the different stages of syphilis and remained in the late latent state for the duration of their time in the model. With treatment, individuals entered a “treated” (T1–T3) state before returning to the “susceptible, prior infection” (SR) state. Consistent with data suggesting that there may be a period of transient protection from reinfection after treatment that increases with stage of infection before receipt of treatment,10,25 time spent in the treated state varied with infection stage at treatment. Individuals with prior treated infections were modeled separately from those with no prior infections, to investigate interventions in individuals with a recorded history of treated syphilis infection.19 Syphilis natural history was not assumed to differ in those experiencing multiple infections.
We included a 100-year burn-in period before calibration. Calibration was conducted for a 5-year period, representing the years 2012 to 2016. We began tracking individuals with a prior infection 5 years before the start of the calibration period. Susceptible individuals with a prior treated infection returned to the “no prior infection” component of the model after 2 years on average, an amount of time considered feasible for posttreatment follow-up for enhanced surveillance by a local public health department.
Testing and Treatment
Syphilis infections could be diagnosed and treated either by (i) individuals actively seeking medical care (either because of symptoms or partner notification) or (ii) opportunistic screening. There was an associated probability that a diagnosed and treated case was reported and included in syphilis surveillance data. Reporting probabilities were allowed to differ by mechanism of case detection (actively seeking care vs. screening). We also included a background antibiotic treatment rate, allowing for treatment of syphilis without testing and diagnosis when individuals received treatment for another medical indication.14 This rate was assumed to be 0 for the first 45 years of the precalibration burn-in period, was increased to 10% per year for 35 years, and was then reduced to a low level (1%) after this initial introductory period (and 20 years before the start of the model calibration period).
Model Fitting
We developed separate models for Louisiana and Massachusetts. Population sizes and population distributions by race/ethnicity were based on estimates from the 2015 census.26 The proportion of male individuals allocated to the MSM compartment was based on a recent study.27 Estimates of HIV prevalence in MSM were provided by the Louisiana and Massachusetts Departments of Public Health and were not adjusted for underreporting.
For model calibration, we used data on reported syphilis cases aged 20 to 64 years in Louisiana and Massachusetts for the period 2012 to 2016. Available information included state-level reported diagnosis rates of early (primary, secondary, and early latent) syphilis by sex, race/ethnicity, and age group. We also used data on the proportion of male cases identified as MSM and the proportion of syphilis cases in MSM with a diagnosed HIV coinfection (available for the years 2014–2016 only for Louisiana). Given data limitations, the MSM populations were not further stratified by race/ethnicity.
We used an adaptive Metropolis-Hastings Markov Chain Monte Carlo algorithm28 for model calibration of parameters describing syphilis natural history, sexual mixing, and testing and treatment processes. Prior distributions were based on estimates from the biomedical literature, where available, or expert opinion and assumption otherwise (Tables 1 and 2). When state-specific estimates for parameters were unavailable (e.g., those describing number of sexual partners and screening rates), we used national estimates. Parameters describing syphilis natural history were assumed to be the same in Louisiana and Massachusetts, whereas behavioral, demographic, and screening and treatment parameters were allowed to vary between the 2 states. We did not model screening test characteristics and assumed perfect test sensitivity and specificity.
TABLE 1.
Population Structure, Sexual Behavior, and Mixing Parameters
| Parameter | Details | Symbol* | Prior Distribution† | Value, Mean and 95% Interval | Source |
|---|---|---|---|---|---|
| Total population aged 20–64 y | Louisiana (LA) | N | Fixed | 2.8 × 106 | US Census Bureau26 |
| Massachusetts (MA) | 4.2 × 106 | ||||
| Average time in each age group, y | 20–44 | 1/μ | Fixed | 25 | Assumption |
| 45–64 | Fixed | 20 | |||
| Proportion of men in each subpopulation | Black, LA | popij | Fixed | 0.310 | US Census Bureau26; Grey et al.27 |
| Black, LA | Fixed | 0.070 | |||
| Hispanic, LA | Fixed | 0.048 | |||
| Hispanic, MA | Fixed | 0.102 | |||
| Men who have sex with men, LA | Fixed | 0.025 | |||
| Men who have sex with men, MA | Fixed | 0.045 | |||
| Other, LA | Fixed | 0.616 | |||
| Other, MA | Fixed | 0.783 | |||
| Proportion of females in each subpopulation | Black, LA | popij | Fixed | 0.318 | US Census Bureau26 |
| Black, MA | Fixed | 0.073 | |||
| Hispanic, LA | Fixed | 0.050 | |||
| Hispanic, MA | Fixed | 0.107 | |||
| Other, LA | Fixed | 0.632 | |||
| Other, MA | Fixed | 0.820 | |||
| Proportion of MSM with HIV infection | 20–44 y, LA | pHIV | Fixed | 0.22 | Louisiana Department of Public Health, Massachusetts Department of Public Health (2015) |
| 20–44 y, MA | Fixed | 0.05 | |||
| 45–64 y, LA | Fixed | 0.28 | |||
| 45–64 y, MA | Fixed | 0.12 | |||
| Proportion ever had sex, male | Black, 20–44 y | PS,ijkl | Fixed | 0.98 | Ref. 40s and assumption |
| Hispanic, 20–44 y | Fixed | 0.94 | |||
| Other, 20–44 y | Fixed | 0.95 | |||
| HIV− MSM, 20–44 y | Fixed | 0.94 (male average) | |||
| HIV+ MSM, 20–44 y | Fixed | 1 | |||
| HIV+ MSM, 45–64 y | Fixed | 1 | |||
| All other men, 45–64 y | Fixed | 0.99 | |||
| Proportion ever had sex, female | PS,ijkl | Ref. 40s and assumption | |||
| All, 20–44 y | Fixed | 0.96 | |||
| All, 45–64 y | Fixed | 0.99 | |||
| Proportion of population in each sexual activity group | Pk | Assumption | |||
| MSM, low | Fixed | 0.80 | |||
| MSM, high | Fixed | 0.20 | |||
| All others, low | Fixed | 0.90 | |||
| All others, high | Fixed | 0.10 | |||
| Minimum rate of partner acquisition (per year) | cmin,jl | Assumption | |||
| Male, 20–44 y | Gamma (5) | 1 (0.32–2.0) | |||
| Male, 20–64 y | Gamma (5) | 1 (0.32–2.0) | |||
| Female, 20–44 y | Gamma (5) | 1 (0.32–2.0) | |||
| Female, 45–64 y | Gamma (5) | 1 (0.32–2.0) | |||
| MSM, 20–44 y | Gamma (5) | 1 (0.32–2.0) | |||
| MSM, 45–64 y | Gamma (5) | 1 (0.32–2.0) | |||
| Relative rate of partner acquisition, heterosexual men aged 20–44 y‡ | rpijkl | Ref. 40s; assumption | |||
| Black, low | Gamma (2.2, 0.6) | 3.7 (0.5–10.0) | |||
| Black, high | Normal (32.5, 8.9) | 32.5 (15.0–50.0) | |||
| Hispanic, low | Fixed | 1.0 | |||
| Hispanic, high | Gamma (4.3, 0.6) | 7.0 (2.0–15.0) | |||
| Other, low | Gamma (2.2, 0.6) | 3.7 (0.5–10.0) | |||
| Other, high | Normal (27.5, 11.5) | 27.5 (5.0–50.0) | |||
| Relative rate of partner acquisition, MSM aged 20–44 y‡ | rpijkl | Assumption | |||
| HIV− MSM, low | Fixed | 1 | |||
| HIV− MSM, high | Normal (45, 15.3) | 45.0 (15.0–75.0) | |||
| HIV+ MSM, low | Gamma (2.2, 0.6) | 3.7 (0.5–10.0) | |||
| HIV+ MSM, high | Normal (27.5, 11.5) | 45.0 (15.0–75.0) | |||
| Relative rate of partner acquisition, heterosexual men aged 45–64 y‡ | rpijkl | Assumption | |||
| Black, low | Gamma (3.4, 1.6) | 2.2 (0.5–5.0) | |||
| Black, high | Normal (45, 15.3) | 45.0 (15.0–75.0) | |||
| Hispanic, low | Fixed | 1 | |||
| Hispanic, high | Gamma (5.3,0.4) | 7.0 (5.0, 30.0) | |||
| Other, low | Gamma (3.4, 1.6) | 2.2 (0.5–5.0) | |||
| Other, high | Normal (45, 15.3) | 45.0 (15.0–75.0) | |||
| Relative rate of partner acquisition, MSM aged 45–64 y‡ | rpijkl | Assumption | |||
| HIV− MSM, low | Fixed | 1 | |||
| HIV− MSM, high | Normal (45.0, 15.3) | 45.0 (15.0–75.0) | |||
| HIV+ MSM, low | Gamma (3.4, 1.6) | 2.2 (0.5–5.0) | |||
| HIV+ MSM, high | Normal (45, 15.3) | 45.0 (15.0–75.0) | |||
| Relative rate of partner acquisition, women aged 20–44 y‡ | rpijkl | Ref. 40s; assumption | |||
| Black, low | Gamma (2.2, 0.6) | 3.7 (0.5–10.0) | |||
| Black, high | Gamma (1.9, 0.1) | 17.7 (2.0–50.0) | |||
| Hispanic, low | Fixed | 1 | |||
| Hispanic, high | Gamma (4.3, 0.6) | 7.0 (2.0, 15.0) | |||
| Other, low | Gamma (2.2, 0.6) | 3.7 (0.5–10.0) | |||
| Other, high | Gamma (5.3, 0.4) | 14.9 (5.0–30.0) | |||
| Relative rate of partner acquisition, women aged 45–64 y‡ | rpijkl | Assumption | |||
| Black, low | Gamma (3.4, 1.6) | 2.2 (0.5–5.0) | |||
| Black, high | Gamma (8.5, 0.8) | 11.3 (5.0–20.0) | |||
| Hispanic, low | Fixed | 1 | |||
| Hispanic, high | Gamma (5.3,0.4) | 14.9 (5.0, 30.0) | |||
| Other, low | Gamma (3.4,1.6) | 2.2 (0.5–5.0) | |||
| Other, high | Gamma (5.3,0.4) | 14.9 (5.0–30.0) | |||
| Mixing between sexual activity groups | ε1,i | Assumption | |||
| Black | Beta (1.1, 1.1) | 0.5 (0.032–0.97) | |||
| Hispanic | Beta (1.1, 1.1) | 0.5 (0.032–0.97) | |||
| Other | Beta (1.1, 1.1) | 0.5 (0.032–0.97) | |||
| HIV− MSM | Beta (1.1, 1.1) | 0.5 (0.032–0.97) | |||
| HIV+ MSM | Beta (1.1, 1.1) | 0.5 (0.032–0.97) | |||
| Mixing with same age group | ε2,ijl | Ref. 40s; assumption | |||
| Men aged 20–44 y and Women aged 45–64 y |
Beta (9, 2.7) | 0.86 (0.5–0.95) | |||
| Men aged 45–65 y y and Women aged 20–44 y |
Beta (6.1, 2.3) | 0.81 (0.4–0.95) | |||
| MSM | Beta (8.0, 3.8) | 0.68 (0.40–0.90) | |||
| Mixing within subpopulation | ε3,ij | Refs, 40s,41s; assumption | |||
| Black male | Beta (172.7, 52.4) | 0.77 (0.71–0.82) | |||
| Hispanic male | Beta (183.7, 72.6) | 0.72 (0.66–0.77) | |||
| Other male | Beta (547.2, 70.3) | 0.89 (0.86–0.91) | |||
| HIV− MSM | Beta (47.5, 2.5) | 0.95 (0.88–0.99) | |||
| HIV+ MSM | Beta (47.5, 2.5) | 0.95 (0.88–0.99) | |||
| Black female | Beta (217.0, 28.8) | 0.88 (0.84–0.92) | |||
| Hispanic female | Beta (99.1, 59.1) | 0.63 (0.55–0.70) | |||
| Other female | Beta (437.1, 70.4) | 0.86 (0.83–0.89) | |||
| Mixing with MSM of same HIV status | θHIV | Beta (9.0, 2.7) | 0.77 (0.50–0.95) | 42s |
*Subscripts i, j, k, and l indicate subpopulation, sex, sexual activity group, and age group, respectively.
†Gamma distributions are described by shape (α) and rate (β) parameters; beta distributions are described by shape parameters (α and β).
‡Relative rates are expressed in reference to the group with the lowest level in age/sex category, which has a value fixed at 1.
TABLE 2.
Syphilis Natural History, Screening, and Treatment Parameters
| Parameter | Details | Symbol* | Prior Distribution† | Value (Mean and 95% Interval) | Source |
|---|---|---|---|---|---|
| Probability of transmission (during primary and secondary infection) | βji | Garnett et al.10 | |||
| Female to male | Beta (14.3, 9.7) | 0.60 (0.40–0.78) | |||
| Male to female | Beta (14.3, 9.7) | 0.60 (0.40–0.78) | |||
| Male to male | Beta (14.3, 9.7) | 0.60 (0.40–0.78) | |||
| Average duration of infection stage, d | Garnett et al.10 | ||||
| Incubating | 1/δ | Normal (25, 2.23) | 25.0 (20.6–29.4) | ||
| Primary | 1/γp | Normal (45, 7.74) | 45.0 (29.8–60.2) | ||
| Secondary | 1/γs | Normal (108, 16) | 108.0 (80.6–139.3) | ||
| Early latent | 1/γe | 365–(duration primary + duration secondary) | |||
| Average duration of protection from reinfection after treatment, d‡ | Garnett et al.10; Magnuson et al. | ||||
| Primary and secondary syphilis | 1/ξps | Normal (14.0, 3.6) | 14.0 (7.0–21.0) | ||
| Late latent syphilis | 1/ξe | Normal (927.5, 457.9) | 927.5 (30.0–1825.0) | ||
| Multiplier for duration of protection from reinfection if treated during early latent stage | Relative to primary and secondary infection | rrimm | Uniform (2,5) | 3.5 (2.1–4.9) | Garnett et al.10; assumption |
| Background antibiotic treatment rate (per year) | Φ | Assumption | |||
| For 35-y period ending 20 y before calibration start | 0.1 | ||||
| For remainder of model time | 0.01 | ||||
| Rate of transition from “susceptible, previously treated” to “susceptible” compartment (per year) | Used to track population eligible for interventions in individuals with previously diagnosed infection | ζ | 0.5 | Assumption | |
| Symptomatic treatment rate, primary syphilis (per year) | τp | Assumption | |||
| Male | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| HIV− MSM | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| HIV+ MSM | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| Female | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| Symptomatic treatment rate, secondary syphilis (per year) | τs | Assumption | |||
| Male | Beta (9.2,13.6) | 0.40 (0.22–0.61) | |||
| HIV− MSM | Beta (9.2,13.6) | 0.40 (0.22–0.61) | |||
| HIV+ MSM | Beta (9.2,13.6) | 0.40 (0.22–0.61) | |||
| Female | Beta (9.2,13.6) | 0.40 (0.22–0.61) | |||
| Treatment rate, early latent syphilis (per year) | τe | Assumption | |||
| Male | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| HIV− MSM | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| HIV+ MSM | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| Female | Beta (3.4,14.2) | 0.19 (0.05–0.40) | |||
| Treatment rate, late latent syphilis (per year) | τl | Assumption | |||
| Male | Beta (1.9, 21.5) | 0.08 (0.01–0.22) | |||
| HIV− MSM | Beta (1.9, 21.5) | 0.08 (0.01–0.22) | |||
| HIV+ MSM | Beta (1.9, 21.5) | 0.08 (0.01–0.22) | |||
| Female | Beta (1.9, 21.5) | 0.08 (0.01–0.22) | |||
| Annual asymptomatic screen and treat rate, low sexual activity group§ | Start = 2010 End = 2016 |
ψij | Bezier curve | Ref. 40s; assumption | |
| Male | Start: Beta (2.6, 22.3) End: Beta (2.6, 22.3) |
0.11 (0.02–0.25) 0.11 (0.02–0.25) |
|||
| MSM | Start: Beta (5.7, 10.2) End: Beta (7.0, 9.9) |
0.36 (0.15–0.60) 0.42 (0.20–0.65) |
|||
| Female | Start: Beta (2.6, 22.3) End: Beta (2.6, 22.3) |
0.11 (0.02–0.25) 0.11 (0.02–0.25) |
|||
| Relative risk of screening, by subpopulation and sex‡ | rr_popij | Ref. 40s; assumption | |||
| Black male | Gamma (9.3, 5.4) | 1.7 (0.8–3.0) | |||
| Hispanic male | Gamma (8.5, 7.5) | 1.1 (0.5–2.0) | |||
| Other male | Fixed | 1 | |||
| HIV− MSM | Fixed | 1 | |||
| HIV+ MSM | Gamma (3.4, 0.5) | 6.5 (1.5–15.0) | |||
| Black female | Gamma (3.4, 1.6) | 2.2 (0.5–5) | |||
| Hispanic female | Gamma (8.5, 7.5) | 1.1 (0.5–2.0) | |||
| Other female | Fixed | 1 | |||
| Relative risk of screening, by sexual activity group‡ | rr_ack | Assumption | |||
| Low sexual activity group | Fixed | 1 | |||
| High sexual activity group | 1 + Gamma (1.5, 5) | 1.3 (1.02–1.9) | |||
| Relative risk of screening, by age group | rr_agel | Assumption | |||
| Men aged 20–44 y | Fixed | 1 | |||
| Men aged 45–64 y | Gamma (3.4, 0.5) | 6.5 (1.5–15.0) | |||
| MSM aged 20–44 y | Fixed | 1 | |||
| MSM aged 45–64 y | Gamma (4.0, 4.4) | 0.9 (0.3–2.0) | |||
| Women aged 20–44 y | Fixed | 1 | |||
| Women aged 45–64 y | Gamma (8.5, 7.5) | 1.1 (0.5–2.0) | |||
| Probability case is reported | ω | Bezier curve | Assumption | ||
| Start (2010) | Beta (116.1, 12.1) | 0.91 (0.85–0.95) | |||
| End (2016) | Beta (109.7, 6.1) | 0.95 (0.90–0.98) | |||
| Relative risk case is reported, by method of identification | ηj | Assumption | |||
| Screening | Fixed | 1 | |||
| Seeking medical care, male | Beta (9.0, 27) | 0.77 (0.50–0.95) | |||
| Seeking medical care, female | Beta (9.0, 27) | 0.77 (0.50–0.95) | |||
| Annual increase in transmission probability (2010–2016) | MSM | crr | Beta (1.1, 36.9) | 0.03 (0.001–0.1) | Assumption |
*Subscripts i, j, k, and l indicate subpopulation, sex, sexual activity group, and age group, respectively.
†Gamma distributions are described by shape (α) and rate (β) parameters; beta distributions are described by shape parameters (α and β); normal distributions are described by mean (μ) and SD (σ).
‡Duration of immunity for treated early latent infection calculated as: 1/(ξps × rrimm).
§Annual screening rates (α) calculated as follows: ψjl × rr_popij × rr_ack × rr_agei.
Model Outputs and Analyses
We used the calibrated models to estimate the impact of different approaches to screening in the 2 epidemic contexts. We created a series of retrospective counterfactual scenarios with alternate approaches to screening implemented over the 2012 to 2016 period. Results were compared with the calibrated (base case) model, which represented our best estimates of screening in the 2 states over this period. This approach allowed us to quantify the difference in impact of alternate screening approaches, had they been implemented.
The scenarios included:
-
(i)
Screen MSM at levels recommended in US syphilis screening guidelines3: annual screening for all MSM, every 3 months for high sexual activity MSM, regardless of HIV status
-
(ii)
Screen all MSM every 3 months
-
(iii)
Screen all high sexual activity groups (heterosexual and MSM) every 3 months
-
(iv)
Screen individuals with prior diagnosed and treated syphilis infection (in the past 2 years) every 3 months
For all scenarios, female individuals aged 20 to 44 years continued to be screened at base case levels to capture screening in pregnant women. Although we did not include a separate state for pregnancy, our approach enabled us to define alternate screening scenarios that would be consistent with maintaining current levels of screening in pregnant women, as recommended by current guidelines. All other population groups not specifically identified for the intervention did not receive screening but were treated if they sought medical care. We also calculated the change in average number of screening tests performed for the different scenarios compared with the base case.
Model outcomes included incident infections, reported early syphilis infections, and prevalent early syphilis infections. We calculated median values and 95% credible intervals based on 1000 draws from parameter posterior distributions. For the counterfactual analysis, we calculated the percent change in outcome for the different scenarios compared with the base case within each of the 1000 parameter sets draws. The model was constructed, and all analyses were performed using R.29
RESULTS
Reported Syphilis in Louisiana and Massachusetts, 2012 to 2016
For the period 2012 to 2016, both Louisiana and Massachusetts experienced an increase in reported rates of early (primary, secondary, and early latent) syphilis in the population aged 20 to 64 years (Fig. 2). Rates of reported early syphilis were elevated in Louisiana compared with Massachusetts, and the difference was most apparent in female individuals of all ages.
Figure 2.
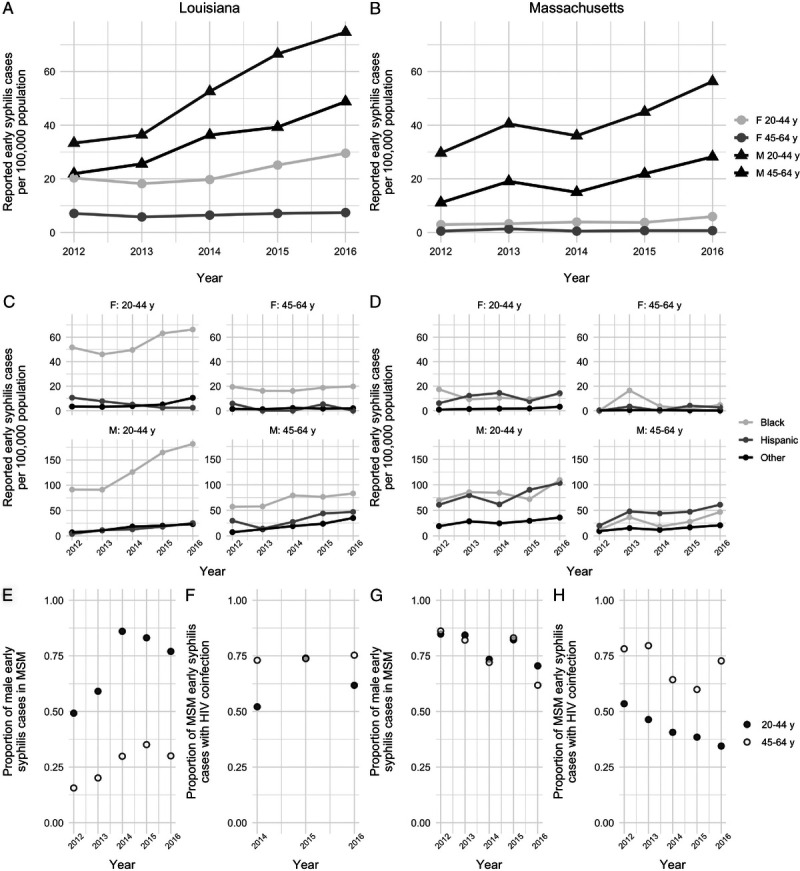
Reported early syphilis in Louisiana and Massachusetts, 2012 to 2016. Data are shown separately for Louisiana (A, C, E, F) and Massachusetts (B, D, G, H). A and B, Reported early syphilis cases per 100,000 population, by age group (20–44 and 45–64 years) and sex (female and male). C and D, Reported cases per 100,000 population by age, sex, and race/ethnicity. Note that the y axes are different for women and men. E and G, Proportion of early syphilis cases in men that are reported in MSM. F and H, Proportion of early syphilis cases in MSM occurring in men with HIV coinfection. Note that HIV coinfection data from Louisiana are only available for the years 2014 to 2016.
In Louisiana, the proportion of reported male cases identified as MSM increased in both age groups over this period: from 0.49 to 0.77 in men aged 20 to 44 years old and 0.16 to 0.30 in men 45 to 64 years (chi-squared test for tend in proportions, P < 0.001 for both age groups). There was a trend of an increasing proportion of MSM cases with HIV coinfection for the younger age group only (P = 0.16, data for 2014–2016). In the older age group, HIV coinfection was common in MSM cases (~75%) and did not change significantly between 2014 and 2016 (P = 0.75).
The proportion of reported cases occurring in MSM was high in Massachusetts in 2012 (0.85) and declined over time. In Massachusetts, the proportion of MSM cases with HIV coinfection declined over time (20–44 years: 0.54 in 2012 to 0.35 in 2016, P < 0.001; 45–64 years: 0.78 in 2012 to 0.73 in 2016, P = 0.024).
In Louisiana, reported diagnosis rates were elevated in the Black population relative to the non-Hispanic non-Black population (Fig. 2). In Massachusetts, there were higher rates of early syphilis diagnosis in Black and Hispanic populations relative to non-Hispanic non-Black populations for all age and sex groups.
Model Calibration
Through model calibration, we identified parameter sets that replicated the key features of the epidemics in Louisiana and Massachusetts described previously. Although the model captured overall trends in reported early syphilis (Fig. 3) and generally fit well to additional calibration targets (Supplementary Figure 1, http://links.lww.com/OLQ/A530), there were some exceptions. For Louisiana, the model underestimated the burden of reported cases in Black men aged 20 to 44 years and overestimated burden in Hispanic men aged 20 to 44 years. For Massachusetts, the model overestimated the proportion of male cases aged 45 to 64 years who were MSM.
Figure 3.
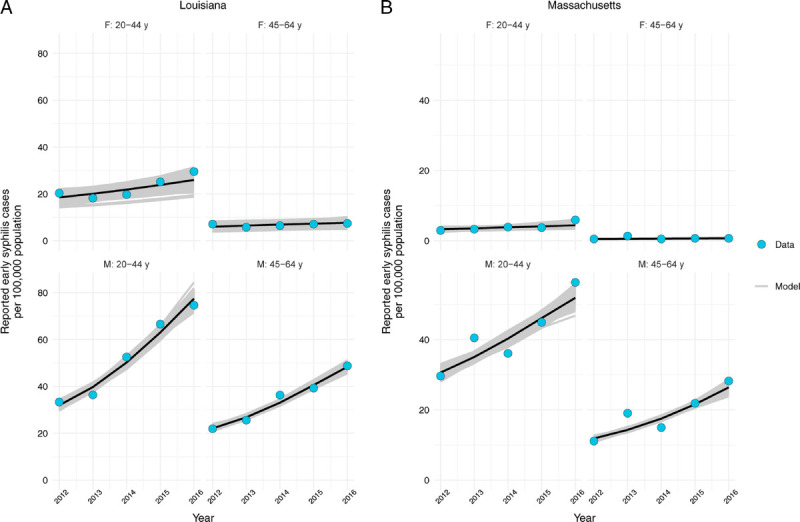
Comparison of model fits to reported early syphilis case data. Model-projected reported cases are shown for Louisiana (A) and Massachusetts (B) for the years 2012 to 2016. Modeled outputs are based on 1000 best-fit parameter sets. Median values are shown in black. Note that different y-axis ranges are used for the data from the 2 states. Early syphilis includes primary, secondary, and early latent syphilis cases.
The posterior values for the best-fitting parameter sets are presented in Supplementary Figures 2 and 3, http://links.lww.com/OLQ/A530. In Louisiana, screening rates were estimated to have increased over the calibration period in all population groups. By contrast, the best-fit estimates of screening rates in Massachusetts suggested a large increase in screening in MSM, with a trend of stable or decreasing screening rates in men who have sex with women and in women.
Comparison of Base Case With Perfect Adherence to Screening Recommendations in MSM
To investigate the importance of underlying differences in epidemic characteristics on the effectiveness of screening programs, we began by considering a hypothetical scenario of perfect adherence to screening recommendations in MSM between 2012 and 2016 (scenario i). Compared with the base case, we projected different outcomes for the populations of Louisiana and Massachusetts (Figs. 4 and 5). In Massachusetts, screening according to guidelines in MSM was projected to decrease early syphilis prevalence, while showing no change or small increases in incident and diagnosed cases. This trend was mainly driven by the male 45- to 64-year age group, in which syphilis incidence was projected to increase after an initial decline, when screening was implemented at levels recommended by guidelines.
Figure 4.
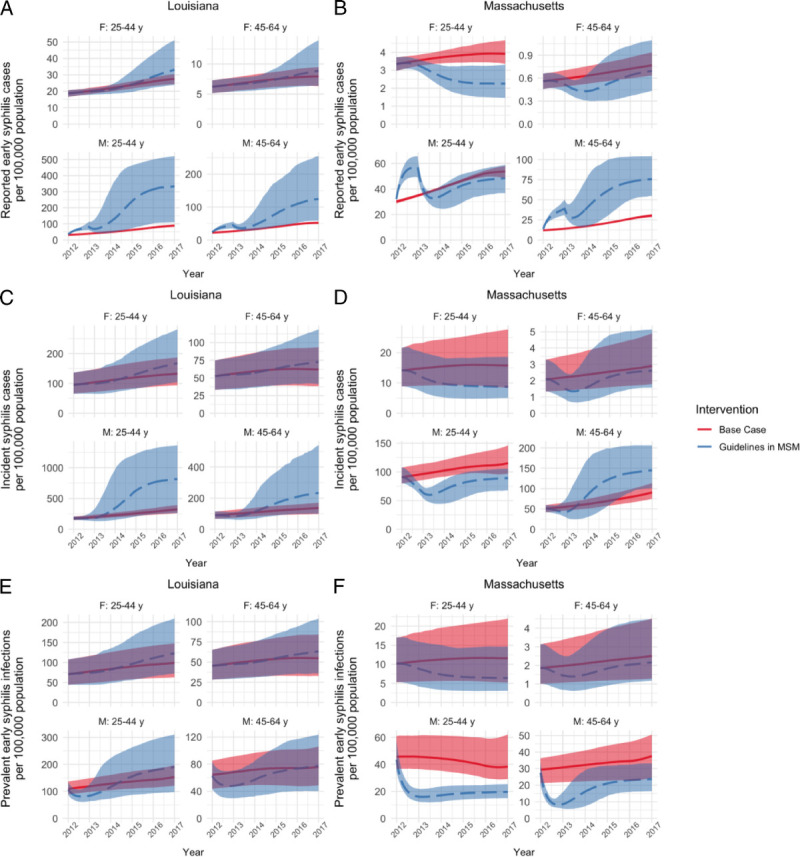
Comparison of base case (best-fit) model (solid line) to a counterfactual model with screening uptake in MSM at rates recommended by US syphilis screening guidelines (dashed line). Results are shown for different measures of syphilis burden in (A, C, and E) Louisiana and (B, D, and F) Massachusetts and are stratified by age group and sex. Reported early syphilis cases (A and B) represent primary, secondary, and early latent cases that are tested, treated, and reported to public health. Incident cases (C and D) include all new infections. Prevalent cases (E and F) include all cases with untreated primary, secondary, or early latent infection. Median and 95% credible intervals are shown for 1000 simulations for each intervention. Note that, because of large differences in outcome values, the y axes have different scales.
Figure 5.
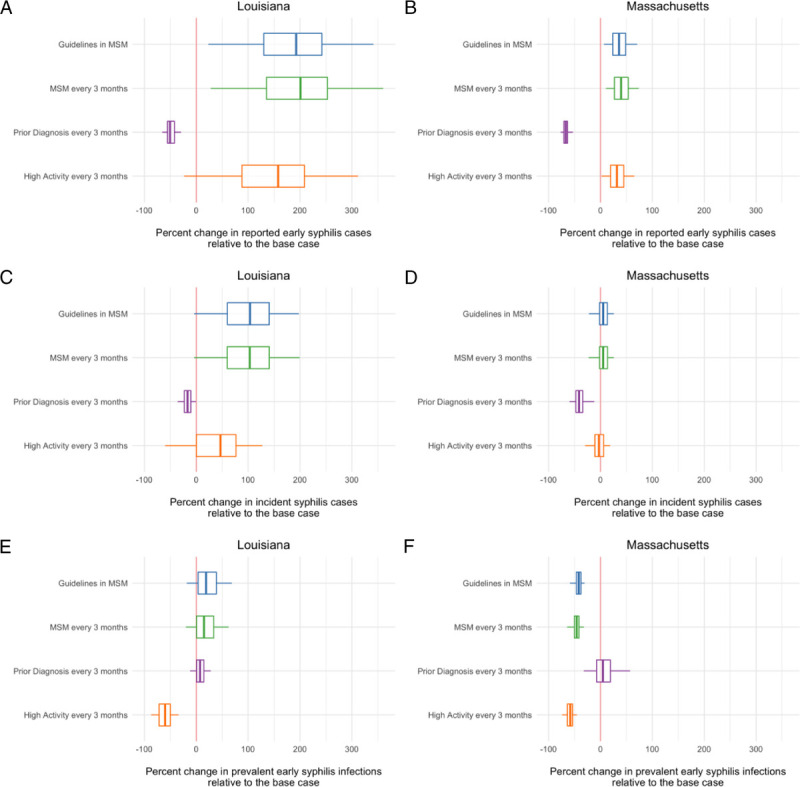
Comparison of impact of alternate screening approaches to best-fit model projections. The difference in total reported (A and B), incident (C and D), and prevalent (E and F) syphilis infections with each scenario and the base case model was calculated for the 5-year modeled period. Negative values indicate a reduction in the outcome compared with the base case, whereas positive values indicated an increase in the outcome with intervention, relative to the base case. The lower, middle, and upper hinges of the boxes correspond to the 25th, 50th, and 75th percentiles, with the whiskers extending to the largest and smallest values up to 1.5 times the interquartile range. Details of each scenario are provided in Methods.
This same scenario resulted in a large increase in diagnosed, incident, and, to a lesser extent, prevalent infections in the Louisiana population. In contrast to what was projected for Massachusetts, this effect was observed across all age and sex groups and was particularly striking for incident infections among men aged 20–44 years.
Alternate Screening Interventions
Given the differing effects of screening according to current guidelines on syphilis burden in the 2 epidemic contexts, we investigated alternate approaches to screening to determine if there were other plausible interventions that would reduce burden (Fig. 5 and Supplementary Figure 4, http://links.lww.com/OLQ/A530). The effectiveness of the different scenarios varied, depending on the outcome measure used and the modeled population (Supplementary Figure 4, http://links.lww.com/OLQ/A530).
In both Louisiana and Massachusetts, screening individuals with a prior diagnosed syphilis infection every 3 months was projected to have the most significant effect for reducing both diagnosed and incident cases in the population and was the only intervention evaluated that reduced diagnosed cases below what was estimated in our base case.
When considering early syphilis prevalence, screening high activity individuals was projected to be most effective in both epidemic contexts. Aside from screening individuals with a prior infection, all of the modeled interventions were projected to be an improvement over base case prevalence in Massachusetts. In Louisiana, only frequent screening of high activity individuals was projected to reduce prevalence below the base case. For both states, screening of individuals with a prior syphilis diagnosis and high-activity individuals were expected to require fewer screening tests than the number of tests required with the base case scenario (Table 3).
TABLE 3.
Average Change in Number of Screening Tests Performed for Each Counterfactual Scenario Compared With Base Case Screening Estimates
| Scenario | Percent Change in Number of Screening Tests (Relative to Base Case) | |
|---|---|---|
| Louisiana | Massachusetts | |
| Guidelines in MSM | 7.2 | 10.4 |
| MSM every 3 mo | 22.0 | 31.8 |
| Prior diagnosis every 3 mo | −52.0 | −63.5 |
| High activity every 3 mo | −54.9 | −64.3 |
DISCUSSION
Screening is a critical tool for the control of syphilis in populations. Using a mathematical model that was fit to describe trends in 2 US states experiencing large syphilis outbreaks, we show that screening may not always have the expected effect on curbing disease transmission in populations and thus may achieve less population-level disease reduction than might otherwise be anticipated. Specifically, we found that the effect of following US screening recommendations, which emphasize screening in MSM, a group disproportionately affected by the current outbreak, depended on the epidemic context. In Louisiana, where syphilis cases are more evenly distributed among MSM and heterosexual populations, MSM-focused approaches to syphilis screening might not be sufficient, compared with our best estimates of disease burden with current screening coverage. In Massachusetts, which has a more MSM-focused outbreak, screening according to guidelines was generally projected to be as or more effective than current screening coverage. The effects of screening were also observed to be heterogenous across population groups.
Notably, we found that our best-fit model estimates of screening coverage in the Louisiana population resulted in outcomes that were superior to any of the hypothetical screening interventions focused in the MSM population, suggesting that health care providers may already be successfully identifying and screening at-risk individuals. Our results for Massachusetts also suggested that MSM-focused screening may not be the most appropriate, depending on the policy goals the programs are expected to fulfill. The 2 MSM-focused scenarios we modeled were projected to reduce early syphilis prevalence but had negligible effects on incidence in Massachusetts.
Of the alternate screening interventions we considered, both screening of individuals with high numbers of partners and those with a history of syphilis infection were identified as potentially reducing syphilis incidence or prevalence. Interestingly, both of these approaches required fewer screening tests in the population than our base case estimates or MSM-focused scenarios. The use of prior infection as a marker for syphilis risk has been used previously,19 although the effectiveness of such an approach in practice remains to be evaluated.
A shift to less MSM-centric syphilis outbreaks has been reported in other high-income countries.3031s Possible reasons for these changing dynamics include bridging between MSM and heterosexual networks2, 32s or a link between drug use, particularly methamphetamine, and an associated increase in sexually transmitted infection acquisition due to coercive sexual behaviors and reduced access to health care.2,3s Given this observed shift, it is increasingly important to consider the broader population effects of screening programs that focus on specific population groups. For instance, in the Louisiana context, increased screening in MSM was projected to increase incidence in both men and women, which could have downstream effects on congenital syphilis occurrence.
Although, to our knowledge, ours is the first model to simultaneously model syphilis spread in MSM and heterosexual populations and the linkages between them, our findings that syphilis interventions can increase disease burden are not novel. This has previously been described both empirically15 and in mathematical models.10,12–1434s,35s The mechanism by which screening can increase incidence relates to syphilis natural history: in the absence of treatment, infected individuals transition to the latent stage, where they are no longer infectious to others. Screening returns previously removed individuals to the susceptible state, where they can be reinfected, and in turn, infect others. When screening reaches high-enough coverage and frequency in groups with high disease burden, this ongoing transmission cycle is disrupted. However, at suboptimal levels (and in the absence of reductions in risk behaviors), screening has the potential to contribute to syphilis persistence through the replenishment of susceptible individuals and possible cycles of reinfection in some individuals. We note that the utility of screening extends beyond reducing population transmission and has individual-level benefits, including prevention of progression to tertiary syphilis. However, if screening programs increase syphilis incidence, the individual-level benefits of case finding and treatment may be obscured by an overall increase in cases. The incorporation of the sequelae and costs associated with treated and untreated infections would provide a more complete quantification of the tradeoffs associated with different screening approaches.
Our modeling approach has limitations. We made simplifying assumptions about syphilis natural history and treatment, as well as population-level sexual behavior and mixing. For instance, we did not model syphilis test algorithms or test characteristics. Despite these simplifications, the model is complex, and because of issues of parameter identifiability, we used sets of parameters that best explained the available data. We relied on reported case data, which may misrepresent true disease burden if there is differential access to medical care and syphilis screening in the modeled population groups. We attempted to address this by including subpopulation-specific screening and treatment rates in the model. We did not model syphilis screening in pregnant women. Given that guidelines do recommend screening of this group, we used our estimates of screening in women aged 20 to 44 years to approximate these values. However, this may overestimate screening in pregnant women if high rates of screening in nonpregnant women are also occurring. Surveillance data have shown substantial gaps in syphilis screening and prenatal care in general among congenital syphilis case mothers, although adherence to screening guidelines is generally high, at least among women with health insurance,36s–38s The model framework we have developed could be extended to explicitly include pregnant women and congenital syphilis. Our model overestimated the proportion of older male cases in MSM in Massachusetts, suggesting that cases were more concentrated in this group than the data indicate. Although this could reflect underreporting of MSM status in the surveillance data, we are unable to confirm this possibility.
For the purposes of this analysis, we modeled hypothetical screening scenarios with perfect uptake in target groups and no opportunistic screening in nontarget groups, with the aim of qualitatively understanding how screening can alter syphilis transmission in different epidemic contexts. In practice, some of the screening approaches modeled might be difficult to implement (e.g., screening MSM every 3 months, which would require ongoing health care access and adherence). Consequently, the results should not be directly extrapolated to real-world populations and outbreaks, but rather used to raise awareness and guide discussion about the nuanced and potentially counterintuitive effects that syphilis screening can have in populations.
Our results suggest that MSM-focused approaches to screening are likely insufficient for control when there is significant transmission in heterosexual populations and may even have the unintended effect of worsening the outbreak by increasing the number of susceptible individuals in the highest-risk groups. Alternate inventions, such as screening individuals with a history of infection, may be more effective in these contexts. Given the challenges associated with syphilis test interpretation and the associated undesirability of unnecessary screening in low-risk groups, as well as the irreversible neurological or cardiovascular complications associated with untreated infections,39s ensuring that screening is occurring in the right populations at the right time is important.
Supplementary Material
Appendix
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A528.
Footnotes
A.R.T. and C.T. contributed equally to the study.
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest. This project was funded by the Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (No. 5U38PS004644)
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Code Availability: model code is available upon request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Christian Testa, Email: ctesta@hsph.harvard.edu.
Minttu Rönn, Email: mronn@hsph.harvard.edu.
Meghan Bellerose, Email: meghanebellerose@gmail.com.
Thomas Gift, Email: teg5@cdc.gov.
Jessica Fridge, Email: Jessica.Fridge@la.gov.
Lauren Molotnikov, Email: lauren.molotnikov@state.ma.us.
Catherine Desmarais, Email: ashleigh.tuite@utoronto.ca.
Andrés Berruti, Email: ilq9@cdc.gov.
Nicolas Menzies, Email: nmenzies@hsph.harvard.edu.
Yelena Malyuta, Email: ymalyuta24@gmail.com.
Katherine Hsu, Email: Katherine.hsu@state.ma.us.
Joshua A. Salomon, Email: salomon1@stanford.edu.
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2018. Available at: https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed December 23, 2019.
- 2.Torrone EA, Miller WC. Congenital and heterosexual syphilis: Still part of the problem. Sex Transm Dis 2018; 45(9S Suppl 1):S20–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Workowski KA Bolan GA, Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force (USPSTF), Bibbins-Domingo K Grossman DC Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA 2016; 315:2321–2327. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force, Curry SJ Krist AH Owens DK, et al. Screening for syphilis infection in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA 2018; 320:911–917. [DOI] [PubMed] [Google Scholar]
- 6.Centers for DIsease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, American Academy of HIV Medicine, Association of Nurses in AIDS Care, International Association of Providers of AIDS Care, et al. Recommendations for HIV prevention with adults and adolescents with HIV in the United States, 2014. Available at: https://stacks.cdc.gov/view/cdc/26062. Accessed December 23, 2019.
- 7.Breban R Supervie V Okano JT, et al. Is there any evidence that syphilis epidemics cycle? Lancet Infect Dis 2008; 8:577–581. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson A, Gelpi A, Tucker JD. The impact of penicillin on sexual healthcare delivery systems in mid-20th century Britain. Sex Transm Infect 2015; 91:70–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterman TA Su J Bernstein KT, et al. Syphilis in the United States: On the rise? Expert Rev Anti Infect Ther 2015; 13:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garnett GP Aral SO Hoyle DV, et al. The natural history of syphilis. Implications for the transmission dynamics and control of infection. Sex Transm Dis 1997; 24:185–200. [DOI] [PubMed] [Google Scholar]
- 11.Tuite AR, Burchell AN, Fisman DN. Cost-effectiveness of enhanced syphilis screening among HIV-positive men who have sex with men: A microsimulation model. PLoS One 2014; 9:e101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuite AR, Fisman DN, Mishra S. Screen more or screen more often? Using mathematical models to inform syphilis control strategies BMC Public Health 2013; 13:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RT Hoare A Prestage GP, et al. Frequent testing of highly sexually active gay men is required to control syphilis. Sex Transm Dis 2010; 37:298–305. [DOI] [PubMed] [Google Scholar]
- 14.Tuite A, Fisman D. Go big or go home: Impact of screening coverage on syphilis infection dynamics. Sex Transm Infect 2016; 92:49–54. [DOI] [PubMed] [Google Scholar]
- 15.Rekart ML Patrick DM Chakraborty B, et al. Targeted mass treatment for syphilis with oral azithromycin. Lancet 2003; 361:313–314. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson AL Scott N Tidhar T, et al. Estimating the syphilis epidemic among gay, bisexual and other men who have sex with men in Australia following changes in HIV care and prevention. Sex Health 2019. [DOI] [PubMed] [Google Scholar]
- 17.Juher D Saldana J Kohn R, et al. Network-centric interventions to contain the syphilis epidemic in San Francisco. Sci Rep 2017; 7:6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesson HW Kidd S Bernstein KT, et al. The cost-effectiveness of syphilis screening among men who have sex with men: An exploratory modeling analysis. Sex Transm Dis 2016; 43:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuite AR Shaw S Reimer JN, et al. Can enhanced screening of men with a history of prior syphilis infection stem the epidemic in men who have sex with men? A mathematical modelling study. Sex Transm Infect 2018; 94:105–110. [DOI] [PubMed] [Google Scholar]
- 20.Guy R El-Hayek C Fairley CK, et al. Opt-out and opt-in testing increases syphilis screening of HIV-positive men who have sex with men in Australia. PLoS One 2013; 8:e71436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callander D Baker D Chen M, et al. Including syphilis testing as part of standard HIV management checks and improved syphilis screening in primary care. Sex Transm Dis 2013; 40:338–340. [DOI] [PubMed] [Google Scholar]
- 22.Bissessor M Fairley CK Leslie D, et al. Frequent screening for syphilis as part of HIV monitoring increases the detection of early asymptomatic syphilis among HIV-positive homosexual men. J Acquir Immune Defic Syndr 2010; 55:211–216. [DOI] [PubMed] [Google Scholar]
- 23.Cohen CE Winston A Asboe D, et al. Increasing detection of asymptomatic syphilis in HIV patients. Sex Transm Infect 2005; 81:217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark EG, Danbolt N. The Oslo study of the natural history of untreated syphilis; an epidemiologic investigation based on a restudy of the Boeck-Bruusgaard material; a review and appraisal. J Chronic Dis 1955; 2:311–344. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson HJ Thomas EW Olansky S, et al. Inoculation syphilis in human volunteers. Medicine (Baltimore) 1956; 32:33–82. [DOI] [PubMed] [Google Scholar]
- 26.United States Census Bureau. Annual estimates of the resident population by sex, age, race, and Hispanic origin for the United States and States: April 1, 2010 to July 1, 2015. Available at: http://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html. Accessed May 17, 2017.
- 27.Grey JA Bernstein KT Sullivan PS, et al. Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health Surveill 2016; 2:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camacho A, Funk S. fitR: Tool box for fitting dynamic infectious disease models to time series. R package version 0.1. Available at: http://sbfnk.github.io/mfiidd/fitR.tar.gz 2016.
- 29.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Available at: https://www.R-project.org/, 2018. [Google Scholar]
- 30.Shaw SY Ross C Nowicki DL, et al. Infectious syphilis in women: What's old is new again? Int J STD AIDS 2017; 28:77–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


