Abstract
Objective
Small bowel adenocarcinoma (SBA) is a rare malignancy with limited evidence regarding outcomes after curative resection of localised disease. We aimed to evaluate presentation and prognostic factors affecting overall survival (OS), relapse-free survival (RFS) and recurrence of SBA.
Methods
Consecutive patients with completely resected localised SBA (1979–2019) were retrospectively reviewed for presentation, patient and tumour characteristics, perioperative treatment, recurrence, outcomes, and prognostic factors.
Results
Among 257 total patients, median age was 58 years. Primary location was in the duodenum, jejunum and ileum in 52%, 29%, and 19% of patients, respectively. Median OS was 57.5 months and median follow-up was 40 months. In multivariate analysis, lymph node involvement, lymphovascular invasion, histologic grade and race were independent predictors of RFS, while race, stage and histologic grade were independent predictors of OS. No significant difference in OS or RFS was seen when evaluating the role of perioperative treatment. Median time to diagnosis from first medical evaluation was 31 days and did not change over time. Overall recurrence rate was 56%. Recurrence rate was higher in ileal (77%), than duodenal (54%) and jejunal (65%) SBA (p=0.01). Recurrence presented most commonly as distant metastasis (71%). Proficient mismatch repair was associated with decreased risk of locoregional recurrence (LR) but increased risk of distant recurrence (DR) when compared with deficient mismatch repair (dMMR) in univariate analysis.
Conclusions
Despite advances in diagnostic modalities, this study did not show any improvement in earlier diagnosis of SBA over the course of the past three decades. The predominant pattern of disease recurrence was distant across all SBA locations, but dMMR status demonstrated a robust predilection for LR as opposed to DR. Perioperative treatment did not improve outcomes; however, a lower stage disease was seen in patients that received neoadjuvant therapy, suggesting further exploration of this approach.
Keywords: small intestine adenocarcinoma, small bowel adenocarcinoma, prognosis, localized, small intestine, mismatch repair recurrence
Key questions.
What is already known about this subject?
Primary adenocarcinoma of the small bowel is a rare malignancy that challenges physicians in diagnosis and treatment.
Surgical resection is the only potentially curative treatment for patients with small bowel adenocarcinoma.
Delayed diagnosis is common usually due to non-specific signs and symptoms associated with difficulty in performing small bowel examination.
What does this study add?
Added refinement of prognostic data beyond large national databases such as the Surveillance, Epidemiology, and End Results Programme and the National Cancer Data Base.
Novel information regarding recurrence patterns across small bowel subsites.
Updated information regarding delays in diagnosis for this rare cancer.
The importance of mismatch repair with regard to the type of recurrence is also explored.
How might this impact on clinical practice?
This study demonstrates the importance of detailed pathological assessment, including perineural invasion, lymphovascular invasion and mismatch repair testing, along with other routinely assessed histological factors. This study suggests the need for further improvement of diagnostic testing within the small bowel, and exploration of the use of neoadjuvant therapy in this disease type.
Introduction
Primary adenocarcinoma of the small bowel is a rare malignancy that challenges physicians in both diagnosis and treatment. The incidence of all small bowel cancers in the USA has been increasing over time, with an estimated 10 590 new cases in 2019, of which adenocarcinoma histology occurs in approximately 30%.1 2
Historically, a significant delay from onset of symptoms to diagnosis has been described for small bowel adenocarcinoma (SBA), related to the vague and non-specific symptoms and the challenges of evaluating the small intestine.3 4 The impact of recent advances in enteroscopy, capsule endoscopy and cross-sectional imaging techniques on diagnosis are not known.
Prognostic factors have been established via retrospective evaluation of large population databases such as the Surveillance, Epidemiology, and End Results (SEER) Programme and the National Cancer Data Base (NCDB) and retrospective datasets generally examining all stages of disease.5–11 For localised SBA, the American Joint Committee on Cancer (AJCC) staging (eighth edition) stages patients based on T stage and N stage.12 In lymph node, sampling has been shown to impact overall survival, likely related to incomplete clearance of occult nodal disease in lymph node negative patients.7 8 In addition, primary site appears to be prognostic with duodenal adenocarcinomas having been reported to have a worse outcome.5 9 10 13
The impact of additional relevant clinicopathological factors such as predisposing factors of inflammatory bowel disease, Lynch syndrome or coeliac disease has been investigated in limited small retrospective series.13–17 A recent report of 66 stage II SBA patients from the Small Bowel Italian Consortium found both deficient mismatch repair (dMMR) and coeliac disease to be predictors of improved cancer-specific survival.17 In addition, the impact of two common factors used in localised colorectal cancer, lymphovascular invasion and perineural invasion has not been well studied in SBA. Within this context, the aim of this study was to report the outcomes and prognostic factors for relapse and overall survival of one of the largest single-institution cohorts for resected SBA.
Methods
Patient selection
This is a retrospective single-institution cohort study that analysed patients who underwent macroscopically and microscopically margin negative resected (R1/R0) stages I–III SBA between March 1978 and August 2019 who were seen at the University of Texas MD Anderson Cancer Center (UTMDACC).
In total, 675 patients with SBA were identified, and 257 met inclusion criteria. Reasons for exclusion included stage IV disease (309 patients), unresectable primary (52 patients), periampullary/ampullary primary (37 patients), R2 resection (10 patients), missing stage (6 patients) and unknown small bowel primary site (4 patients).
The data extracted included patient demographics, inflammatory bowel disease, coeliac disease, primary tumour site, tumour morphology and histologic grade, lymphovascular and perineural invasion, tumour stage, lymph node metastasis, margin status, dMMR as determined by either standard immunohistochemical staining or PCR for determination of microsatellite instability status, treatment, recurrence and survival. Tumour stage was defined according to AJCC Cancer Staging Manual, eighth edition. As pretreatment staging for neoadjuvant treated patients was highly variable and inconsistent, pathological staging was used for preoperatively treated patients. Predefined categories for total number of lymph nodes (TLNs) assessed were established as <7 lymph nodes and ≥7 lymph nodes for stage II patients.4 In a predefined exploratory analysis of lymph node ratio, stage III patients were subclassified as N1 (1–2 lymph nodes) into N1 >10% positive lymph nodes (PLNs), N1 ≤10% PLNs and N2 (≥3 lymph nodes) into N2 >30% PLNs and N2 ≤30% PLNs. Treatment modalities were defined as neoadjuvant if the patient received systemic chemotherapy, chemoradiation or both, before resection (regardless of the type of therapy after resection), and adjuvant therapy if systemic chemotherapy or chemoradiation was given after resection. Patients who received both neoadjuvant and adjuvant treatment were included only in the neoadjuvant group. This research was conducted under a UTMDACC’s institutional review board approved protocol.
Statistical analysis
Relapse-free survival (RFS) and overall survival (OS) were defined as the time from surgical resection or initial tissue diagnosis, whichever was earlier, until disease relapse or death. Patients lost to follow-up were censored at the time of last known disease status. Patient demographic and clinical characteristics were summarised by their frequency (%) across the three anatomic tumour locations. We compared these frequencies using the non-parametric Fisher’s exact test. When data were missing in a category, we excluded those subjects with missing data and did not perform any imputation.
The Kaplan-Meier method was used to visualise and estimate survival curves for time-to-event outcomes, including OS and RFS. The log-rank test was used to compare survival times between groups. Cox proportional hazards regression models were applied to assess the association between patient characteristics and time-to-event outcomes while controlling for covariates. We also calculated restricted mean survival times to summarise survival outcomes in a non-parametric manner. P values less than 0.05 were considered statistically significant.
We used a backward elimination technique to build multivariate models for RFS and OS. In this procedure, we first performed univariate regression of clinical factors one at a time against the outcome. The factors that showed univariate associations at p<0.1 were passed to a second stage for multivariate modelling. In the second stage, we placed all variables in the model and remove the least significant factors until only factors with p<0.05 were left in the model. The same backward elimination technique was used in logistic regression to build models for locoregional recurrence and distant recurrence. Analyses were performed in GraphPad Prism V.8.0.0 and R V.3.5.1.
Results
Clinicopathologic characteristics
Baseline characteristics of these patients are listed in table 1. Median age was 58 years (range=21–84 years) with 52% of patients having a duodenal primary. The median number of TLNs assessed was 11 (range=0–55), and was significantly different between the three subsites (duodenum=15 TLNs, jejunum=8 TLNs and ileum=11 TLNs, p<0.01). This may reflect the high rate of pancreaticoduodenectomies, 65%, for duodenal primaries. Median TLNs assessed increased over time with resections before 2008 demonstrating 9.5 TLNs (median) and resections after 2007 demonstrating 14 TLNs, p=0.01. Among the patients with stage II disease, TLNs assessed were <7 in 46% and ≥7 in 54%.
Table 1.
Patient characteristics by tumour location
| Total (N=257) | Duodenum (N=133) | Jejunum (N=74) | Ileum (N=50) |
P value | |
| Age, median (minimum, maximum) (in years) | 58 (21–84) | 62 (29–84) | 53 (27–77) | 53 (21–80) | <0.001 |
| Gender, number (%) | |||||
| Female | 101 (39) | 49 (37) | 33 (45) | 19 (38) | 0.55 |
| Male | 156 (61) | 84 (63) | 41 (55) | 31 (62) | |
| Race, number (%) | |||||
| White | 198 (77) | 103 (77) | 55 (74) | 40 (80) | 0.71 |
| Black | 23 (9) | 12 (9) | 8 (11) | 3 (6) | |
| Hispanic | 24 (9) | 14 (11) | 7 (10) | 3 (6) | |
| Other | 12 (5) | 4 (3) | 4 (5) | 4 (8) | |
| Histologic grade, number (%) | |||||
| Grade 1 | 6 (2) | 3 (2) | 3 (4) | 0 (0) | 0.76 |
| Grade 2 | 133 (52) | 72 (54) | 36 (49) | 25 (50) | |
| Grade 3 | 84 (33) | 44 (33) | 23 (31) | 17 (34) | |
| Unknown | 34 (13) | 14 (11) | 12 (16) | 8 (16) | |
| T stage, number (%) | |||||
| T1/T2 | 30 (12) | 27 (20) | 3 (4) | 0 (0) | <0.001 |
| T3 | 133 (52) | 55 (41) | 46 (62) | 33 (66) | |
| T4 | 92 (36) | 51 (38) | 25 (34) | 16 (32) | |
| Unknown | 1 (0) | 0 (0) | 0 (0) | 1 (2) | |
| Pathologic stage, number (%) | |||||
| 1 | 14 (5) | 12 (9) | 2 (3) | 0 (0) | 0.02 |
| 2 | 115 (45) | 49 (37) | 41 (55) | 25 (50) | |
| 3A (N1) | 71 (28) | 40 (30) | 20 (27) | 11 (22) | |
| 3B (N2) | 57 (22) | 32 (24) | 11 (15) | 14 (28) | |
| Margin status, number (%) | |||||
| R0 | 250 (83) | 129 (85) | 72 (82) | 49 (78) | >0.99 |
| R1 | 7 (3) | 4 (3) | 2 (3) | 1 (2) | |
| Perineural invasion, number (%) | |||||
| No | 200 (78) | 103 (77) | 57 (77) | 40 (80) | 0.96 |
| Yes | 57 (22) | 30 (23) | 17 (23) | 10 (20) | |
| Lymphovascular invasion, number (%) | |||||
| No | 152 (59) | 82 (62) | 42 (57) | 28 (56) | 0.69 |
| Yes | 105 (41) | 51 (38) | 32 (43) | 22 (44) | |
| IBD,1 number (%) | |||||
| No | 238 (93) | 133 (100) | 70 (95) | 35 (70) | <0.001 |
| Yes | 19 (7) | 0 (0) | 4 (5) | 15 (30) | |
| Coeliac disease, number (%) | |||||
| No | 246 (96) | 129 (97) | 67 (91) | 50 (100) | 0.02 |
| Yes | 11 (4) | 4 (3) | 7 (9) | 0 (0) | |
| Mismatch repair status, number (%) | |||||
| dMMR | 35 (14) | 17 (13) | 10 (14) | 8 (16) | 0.96 |
| pMMR | 68 (26) | 34 (25) | 20 (27) | 14 (28) | |
| Unknown | 154 (60) | 82 (62) | 44 (59) | 28 (56) | |
| Perioperative treatment, number (%) | |||||
| Adjuvant | 137 (53) | 59 (44) | 45 (61) | 33 (66) | 0.01 |
| Neoadjuvant±adjuvant | 22 (9) | 18 (14) | 3 (4) | 1 (2) | |
| No perioperative treatment | 76 (29) | 45 (34) | 20 (27) | 11 (22) | |
| Unknown | 22 (9) | 11 (8) | 6 (8) | 5 (10) | |
| Radiation therapy, number (%) | |||||
| No | 199 (77) | 90 (68) | 65 (88) | 44 (88) | <0.001 |
| Yes | 36 (14) | 32 (24) | 3 (4) | 1 (2) | |
| Unknown | 22 (9) | 11 (8) | 6 (8) | 5 (10) |
dMMR, deficient mismatch repair; IBD, inflammatory bowel disease; pMMR, proficient mismatch repair.
Prognostic factors
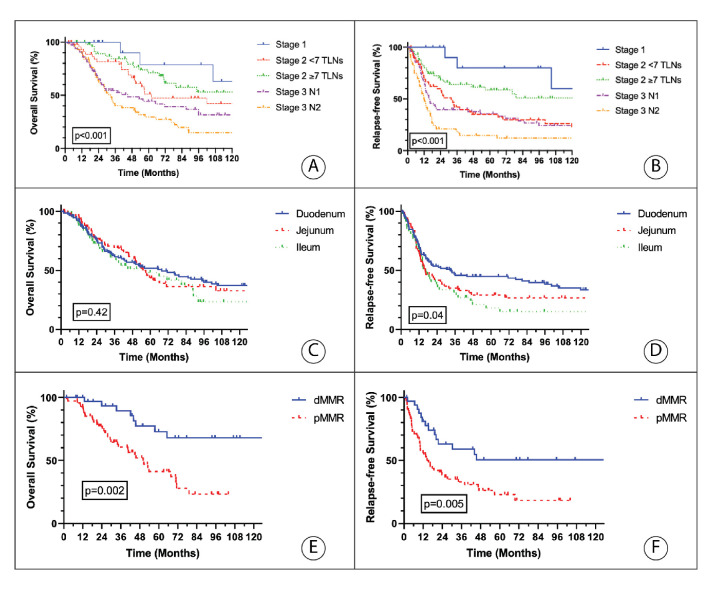
Median follow-up was 40 months (range: 2–392 months), and median OS was 57.5 months. Both OS and RFS differed by stage and number of assessed lymph nodes (figure 1A, B). The 5-year Kaplan-Meier estimates for OS of patients with pathologic stage I, II and III were 79%, 58% and 38%, respectively (p<0.001) (online supplemental figure 1A, B), and their RFS were 80%, 40% and 26%, respectively (p<0.001). RFS and OS differed when stratified by TLNs assessed for stage II disease, <7 TLNs assessed versus ≥7 TLNs (RFS=31 months vs 203 months, respectively, p=0.02; OS=61 months vs 132 months, p=0.38). There was no significant difference of OS between primary tumour subsites (figure 1C); however, ileal tumours had decreased RFS when compared with duodenal tumours (figure 1D). For duodenal adenocarcinomas, the comparison of pancreaticoduodenectomy versus segmental resection demonstrated an improved RFS (39 months vs 13 months, p<0.01) but no difference in OS (74 months vs 46 months, p=0.28), respectively.
Figure 1.
Kaplan-Meier estimates of 10-year OS and 10-year RFS according to pathological stage (A and B), primary tumour location (C and D) and mismatch repair status (E and F). dMMR=deficient mismatch repair; OS: overall survival; pMMR=proficient mismatch repair; RFS: relapse-free survival; TLNs=total number of lymph nodes.
esmoopen-2020-000960supp001.pdf (739.1KB, pdf)
We considered seven possible covariates in building a multivariate model for RFS. Lymph node involvement (stage III disease), lymphovascular invasion, histologic grade and race were independent predictors of RFS. We also considered a different set of 10 covariates in building a multivariate model for OS (table 2). Race, stage and histological grade were independent predictors of OS. Instead of lymphovascular invasion, perineural invasion was the fourth factor included in the OS model.
Table 2.
Univariate and multivariate analysis for OS and RFS
| Category | Predictor | RFS | OS | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate | ||||||
| HR (95% CI for HR) |
P value | HR (95% CI for HR) |
P value | HR (95% CI for HR) |
P value | HR (95% CI for HR) |
P value | ||
| Age | ≥58 years | 1 | 1 | ||||||
| <58 years | 0.96 (0.7 to 1.31) | 0.80 | 1.14 (0.81 to 1.59) | 0.45 | |||||
| Gender | Male | 1 | 1 | ||||||
| Female | 0.94 (0.69 to 1.3) | 0.72 | 0.96 (0.68 to 1.34) | 0.80 | |||||
| Race | White | 1 | 1 | 1 | 1 | ||||
| Black | 0.44 (0.23 to 0.85) | 0.01 | 0.45 (0.23 to 0.89) | 0.02 | 0.34 (0.16 to 0.73) | 0.01 | 0.36 (0.16 to 0.83) | 0.02 | |
| Hispanic | 0.87 (0.5 to 1.51) | 0.61 | 0.87 (0.47 to 1.59) | 0.65 | 0.9 (0.51 to 1.61) | 0.73 | 1.1 (0.6 to 2.02) | 0.76 | |
| Other | 0.8 (0.37 to 1.72) | 0.57 | 0.6 (0.26 to 1.38) | 0.23 | 0.89 (0.39 to 2.03) | 0.79 | 0.65 (0.24 to 1.78) | 0.40 | |
| Primary tumour location | Duodenum | 1 | 1 | ||||||
| Jejunum | 1.25 (0.87 to 1.81) | 0.23 | 1 (0.67 to 1.49) | 0.99 | |||||
| Ileum | 1.63 (1.1 to 2.41) | 0.01 | 1.31 (0.86 to 2) | 0.21 | |||||
| Mismatch repair status | dMMR | 1 | 1 | ||||||
| pMMR | 2.36 (1.3 to 4.3) | 0.005 | 3.39 (1.56 to 7.37) | 0.002 | |||||
| IBD | No | 1 | 1 | ||||||
| Yes | 1.88 (1.13 to 3.11) | 0.01 | 1.81 (1.02 to 3.23) | 0.04 | |||||
| Pathological stage | Stage 1–2 | 1 | 1 | 1 | 1 | ||||
| Stage 3 | 1.76 (1.29 to 2.41) | <0.001 | 1.62 (1.14–2.29) | 0.01 | 1.94 (1.38 to 2.71) | <0.001 | 1.88 (1.29 to 2.74) | 0.001 | |
| T stage | T1/T2 | 1 | 1 | ||||||
| T3 | 2.28 (1.21 to 4.27) | 0.01 | 2.02 (1.04 to 3.92) | 0.04 | |||||
| T4 | 2.88 (1.52 to 5.45) | 0.001 | 2.36 (1.21 to 4.63) | 0.01 | |||||
| Margin status | R0 | 1 | 1 | ||||||
| R1 | 1.66 (0.73 to 3.76) | 0.23 | 2.1 (0.85 to 5.16) | 0.11 | |||||
| Histologic grade | 1–2 | 1 | 1 | 1 | |||||
| 3 | 1.81 (1.29 to 2.54) | <0.001 | 1.62 (1.14 to 2.31) | 0.01 | 1.88 (1.3 to 2.71) | <0.001 | 1.69 (1.16 to 2.45) | 0.01 | |
| Perineural invasion | No | 1 | 1 | 1 | |||||
| Yes | 1.63 (1.13 to 2.34) | 0.01 | 1.92 (1.3 to 2.83) | <0.001 | 1.82 (1.21 to 2.75) | 0.004 | |||
| Lymphovascular invasion | No | 1 | 1 | 1 | |||||
| Yes | 1.75 (1.28 to 2.4) | <0.001 | 1.47 (1.03 to 2.1) | 0.03 | 1.47 (1.05 to 2.06) | 0.02 | |||
| Perioperative therapy | No perioperative treatment | 1 | 1 | ||||||
| Adjuvant | 0.81 (0.57 to 1.14) | 0.22 | 0.86 (0.59 to 1.25) | 0.44 | |||||
| Neoadjuvant (±adjuvant) |
0.66 (0.35 to 1.23) | 0.19 | 0.95 (0.5 to 1.79) | 0.87 | |||||
| Radiation therapy | No | 1 | 1 | ||||||
| Yes | 0.58 (0.35 to 0.96) | 0.04 | 0.58 (0.33 to 1.03) | 0.06 | |||||
dMMR, deficient mismatch repair; IBD, Inflammatory Bowel Disease; OS, overall survival; pMMR, proficient mismatch repair; RFS, relapse-free survival.
Lymphovascular invasion and perineural invasion were both statistically significant in univariate analysis for both RFS and OS but showed a very high degree of correlation with each other, p<0.001, with nearly all tumours demonstrating perineural invasion also having lymphovascular invasion (55 out of 57) (online supplemental figure 2A–D). However, when evaluating stage II patients only, neither perineural nor lymphovascular invasion were statistically correlated with RFS or OS (online supplemental figure 2E–H). Mismatch repair status was known in only 103 patients (40%) and was not included in multivariate modelling; however, in univariate analysis, proficient mismatch repair (pMMR) was associated with decreased OS (HR=3.39; 95% CI: 1.56–7.37; p=0.002) and RFS (HR=2.36; 95% CI: 1.3–4.3; p=0.005) when compared with dMMR (figure 1E, F). When stratified by stages I and II (HR=3.49; p=0.05) and stage III (HR=3.69, p=0.01), dMMR compared with pMMR remained prognostic for OS and for stage III (HR=2.4, p=0.04) RFS but not stages I and II (HR=2.14, p=0.09) RFS.
esmoopen-2020-000960supp002.pdf (1.1MB, pdf)
Given the known importance of the number of lymph nodes accessed in stage II disease, we evaluated predefined lymph node ratios in stage III disease with N1 stratified by <10% PLNs and ≥10% PLNs, and N2 stratified by <30% PLNs and ≥30% PLNs (online supplemental figure 3A, B). For N1 disease, the median RFS was 85.3 months for N1 <10% PLNs versus 14.4 months for N1 ≥10% PLNs (p=0.03) and median OS was 135.1 months for N1 <10% PLNs versus 28.5 months for N1 ≥10% PLNs (p=0.04). No significant difference was seen when comparing RFS and OS in the two subgroups of N2 disease (N2 <30% PLNs vs N2 ≥30% PLNs, p>0.05).
esmoopen-2020-000960supp003.pdf (809.8KB, pdf)
Neoadjuvant and adjuvant treatment
The role of perioperative therapy was evaluated in 235 patients, of whom 9% (n=22) received neoadjuvant treatment (±adjuvant), 58% (n=137) received adjuvant treatment and 32% (n=76) underwent surgery alone (online supplemental tables 1,2). We found no significant difference in OS nor RFS between these three groups (online supplemental figure 4A, B). When comparing pathological staging between neoadjuvant-treated and adjuvant-treated patients, there was numerically lower stage disease in patients receiving neoadjuvant therapy with T3/T4 disease in 86% compared with 94% (p=0.18) and N2 disease in 18% compared with 29% (p=0.44), respectively. When comparing all neoadjuvant-treated patients with stage III adjuvant-treated patients, similar RFS and OS are seen (online supplemental figure 5A, B). Radiation therapy was administered to 33 patients (14%) of 235 patients and in univariate analysis was associated with improved RFS (HR=0.58; 95% CI: 0.35–0.96; p=0.04). The association of radiation therapy with OS was not statistically significant (HR=0.58; CI: 0.33–1.03; p=0.06).
esmoopen-2020-000960supp004.pdf (267.3KB, pdf)
esmoopen-2020-000960supp005.pdf (106.3KB, pdf)
esmoopen-2020-000960supp006.pdf (840KB, pdf)
Diagnostic testing and time to diagnosis
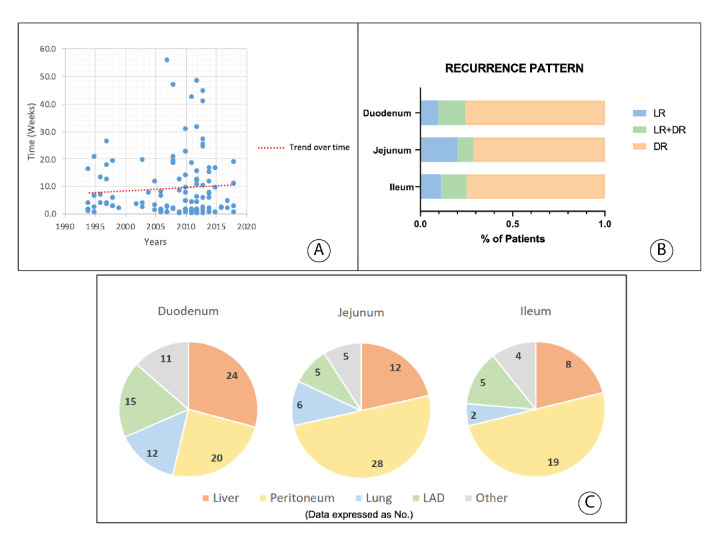
The data about the specific procedure used for diagnosis were available in 197 patients. Tissue diagnosis was made by endoscopy in 38%, surgery in 35%, radiographic imaging in 21% and capsule endoscopy in 6%. The data on the approximate time from initial medical evaluation to diagnosis were present in 115 patients; the median time to diagnosis was 31 days (range=1–390). The time to diagnosis did not change over time (figure 2A).
Figure 2.
Time from first medical evaluation until diagnosis (A), relapse pattern (B) and distant metastatic organ prevalence per primary tumour site (C). DR, distant recurrence; LR, locoregional recurrence; LR+DR, locoregional recurrence synchronously with distant recurrence. LAD, distant lymphadenopathy.
Recurrence pattern
At the time of analysis, a total of 145 patients (56%) developed recurrence: 7% in stage I, 59% in stage II and 71% in stage III (p<0.001). Recurrence differed by small bowel subsite with more recurrences seen in ileal (77%), compared with jejunal (65%) and duodenal (54%, p=0.01) SBA (figure 1D). Among patients who recurred, median time to recurrence was 14.7 months for stage II (range=2–119 months) and 10.8 months (range=1–80 months) for stage III (p<0.001), and 11.8 months (range=1–119 months), 11.5 months (range=1–70 months) and 14.4 months (range=1–66 months) for duodenal, jejunal and ileal SBA, respectively (p=0.6).
Information on recurrence location was available in 142 patients (figure 2B). Recurrence as distant metastases was identified in 101 patients (71%), isolated locoregional recurrence (LR) was detected in 23 patients (16%) and LR occurred synchronously with distant recurrence (DR) in 18 patients (13%). Among patients who recurred, median time to DR, LR and LR with DR was 11 months (range=1–119 months), 15 months (range=6–47 months) and 11 months (range=4–57 months), respectively (p=0.16). A higher rate of peritoneal recurrence was seen for jejunal and ileal primaries compared with duodenal primaries (figure 2C).
Predictors of LR and DR are listed in online supplemental table 3. In univariate analysis, MMR status was strongly associated with recurrence pattern with pMMR demonstrating a reduced risk of LR (OR=0.12, 95% CI: 0.03–0.5, p=0.004) but an increased risk of DR (OR=5.72, 95% CI: 1.42–22.98, p=0.01).
Discussion
In this large retrospective cohort study over four decades, we summarise the prognostic factors associated with resected localised SBA. Interestingly, we find that despite advances in diagnostic modalities, the time to diagnosis from first medical evaluation has remained unchanged over time. The primary recurrence pattern was DR with peritoneal recurrence more common for jejunal and ileal primary sites. Neoadjuvant therapy was used in 9% of cases. Although comparisons did not reach statistical significance, the similar outcomes to adjuvantly treated stage III patients suggest that further exploration of neoadjuvant therapy in patients with SBA should continue.
Multivariate analysis suggests that lymph node involvement, poor histological differentiation, non-black race, perineural invasion (OS only) and lymphovascular invasion (RFS only) are independent predictors for poor survival in SBA. The importance of both perineural and lymphovascular invasion has not been well delineated in prior literature and strongly demonstrate the importance of detailed pathological assessment in these patients. In contrast to other reports, the improved outcome for black race has not been seen and the significance of this finding in this report given the small sample size is uncertain. Though only tested in 40% of SBA in this report, dMMR status was correlated with improvements in both RFS and OS, and demonstrate a robust predilection for LR as opposed to DR. This locoregional predilection for dMMR cancers has been previously seen in colorectal cancer and likely reflects the unique biology of dMMR cancers.18 In addition, in univariate analysis, the predisposing condition of inflammatory bowel disease (IBD) was associated with worse RFS and OS.
The effect that the anatomic location of the SBA primary site has on patient outcome has been controversial.9–11 Although many studies have identified a duodenal location as a negative predictor of survival, this has often been ascribed to a lower percentage of those patients undergoing cancer-directed surgery.19–21 Other results suggest that the impact of small bowel anatomic site on outcome decreases as the number of TLNs assessed increases.7 We did not identify prognostic value of the primary anatomic site in multivariate models for OS or RFS.11 22 Interestingly, however, median RFS was lower for ileal primaries in comparison to duodenal primaries (16.5 months vs 32.9 months, p=0.01), which may reflect the high rate, 30%, of IBD in the ileal subsite. In addition, we found a high rate of TLNs in duodenal primaries, which may reflect the high rate of pancreaticoduodenectomies performed (65% of duodenal surgeries in this dataset).
The AJCC guidelines (eighth edition) states that the minimum number of regional lymph nodes needed for accurate staging and effective treatment of SBA is 6, although different studies have argued in favour of revising this threshold to a higher one.12 23 24 In patients with SBA, low number of assessed lymph nodes and high lymph–node ratio (LNR) (>50%–75%) have been associated with decreased survival.7 11 20 Within this report, we found >7 TLNs assessed to be associated with improved RFS but not OS, which may reflect the small size, N=115, of our stage II population. In addition, the low 5-year OS of 58% for stage II patients may reflect the high rate of inadequate lymph node sampling with 46% of stage II patients having <7 TLNs assessed. Though limited by small numbers with regard to substratification of stage III patients, we explored a pre-planned assessment of per cent lymph node involvement in N1 and N2 diseases. This analysis demonstrated a statistical difference between N1 <10% PLNs vs N1 >10% for OS (135.1 months vs 28.5 months, respectively, p=0.04) and RFS (85.3 months vs 14.4 months, respectively, p=0.03), but not for stage III N2 disease (<30% PLNs vs ≥30 PLNs). Though preliminary, this suggests that for patients with low lymph node involvement, the LNR is an important factor that should be incorporated into prognostication.
Diagnosing localised SBA has been challenging because of the anatomic location and non-specific symptoms. Similar to what has been reported in other studies, most of the diagnoses in our patient sample were made by endoscopy (38%; n=74) and by surgery (35%; n=69). Accurate preoperative diagnosis has been reported only in 30%–72% of cases.11 20 25–27 Overall, the median time from first medical evaluation to diagnosis in our cohort was 31 days. This is similar to other recent studies.25–27 This is in contrast to older investigations which demonstrated much longer time to diagnosis of 6–8 months.3 4 Though diagnostic methods for small bowel tumours have evolved substantially with improved cross-sectional imaging and endoscopic approaches such as capsule endoscopy, the current study did not identify any trend towards earlier diagnosis over the course of the three decades (1993–2009). Though the reasons for this are not known, it may reflect the fundamental challenges with non-specific symptoms and suggests a need for greater disease awareness.
Prior retrospective investigations have identified mixed results with regards to the benefit of adjuvant treatment for SBA, though its use has been increasing.6 Most have not found significant survival benefit with the use of adjuvant therapy28–30; and in those who have found a benefit, it has been limited to patients with high risk of relapse (eg, stage III disease).31–34 Neither adjuvant nor neoadjuvant therapy were associated with OS or RFS, which may reflect the limitations of both sample size and selection bias towards providing such therapy to patients at higher risk of recurrence. However, it is interesting to note that we did find similar outcomes for stage III adjuvant-treated and neoadjuvant-treated patients and a suggestion of pathological downstaging with neoadjuvant therapy. Though the use of neoadjuvant therapy is often limited in SBA, as the diagnosis if often made at the time of surgery, this data does support further exploration of the use of neoadjuvant therapy in this disease type. At present, the ongoing randomised phase III BALLAD study (NCT04257461) is evaluating the role of adjuvant chemotherapy in SBA.
Potential study limitations include its retrospective design and inherent selection biases. However, the single-institution dataset allowed for more granular data collection that would be impossible in a national dataset. Furthermore, given the limited size of subgroup analyses, these findings should be considered exploratory.
In conclusion, this report demonstrates the need for continued focus on diagnostic awareness to help improve the time to diagnosis for patients with SBA, the distant recurrence predilection, the importance for thorough pathological analysis and the importance of mismatch repair testing. Though the role or perioperative therapy remains uncertain, this report suggests that continued exploration of neoadjuvant approaches are warranted.
Footnotes
Contributors: AC, HH, RS, KR and MJO: conception/design and collection and/or assembly of data. AC, HH, HW, MHGK, RS, JEL, JT, C-WT, RAW, KR and MJO: manuscript writing and final approval of manuscript.
Funding: Funding provided by the Kavanagh Family Foundation (MDACC) and Kevin T Doner Memorial Fund (MDACC) and by a National Institute of Health Cancer Centre Support Grant (P30CA016672).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Deidentified participant data.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.National Cancer Institute National Cancer Institute’s SEER database. Available: http://seer.cancer.gov/
- 2.Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol 2009;19:58–69. 10.1016/j.annepidem.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zollinger RM, Sternfeld WC, Schreiber H. Primary neoplasms of the small intestine. Am J Surg 1986;151:654–8. 10.1016/0002-9610(86)90035-8 [DOI] [PubMed] [Google Scholar]
- 4.Wilson JM, Melvin DB, Gray GF, et al. Primary malignancies of the small bowel: a report of 96 cases and review of the literature. Ann Surg 1974;180:175–9. 10.1097/00000658-197408000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63–71. 10.1097/SLA.0b013e31818e4641 [DOI] [PubMed] [Google Scholar]
- 6.Legué LM, Bernards N, Gerritse SL, et al. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in the Netherlands. Acta Oncol 2016;55:1183–9. 10.1080/0284186X.2016.1182211 [DOI] [PubMed] [Google Scholar]
- 7.Overman MJ, Hu C-Y, Wolff RA, et al. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer 2010;116:5374–82. 10.1002/cncr.25324 [DOI] [PubMed] [Google Scholar]
- 8.Thiessen M, Tang PA, Lee-Ying R, et al. Impact of the number of nodes examined on survival in node negative small bowel adenocarcinoma: a seer database analysis. Ann Oncol 2018;29:viii263–4. 10.1093/annonc/mdy282.156 [DOI] [Google Scholar]
- 9.Falcone R, Romiti A, Filetti M, et al. Impact of tumor site on the prognosis of small bowel adenocarcinoma. Tumori 2019;105:524–8. 10.1177/0300891619839297 [DOI] [PubMed] [Google Scholar]
- 10.Lee TC, Wima K, Morris MC, et al. Small bowel adenocarcinomas: impact of location on survival. J Surg Res 2020;252:116–24. 10.1016/j.jss.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 11.Halfdanarson TR, McWilliams RR, Donohue JH, et al. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg 2010;199:797–803. 10.1016/j.amjsurg.2009.05.037 [DOI] [PubMed] [Google Scholar]
- 12.Coit DG, Kelsen D, Tang LH, et al. Small Intestine : AJCC Cancere staging manual. 8 edn Chicago: AJCC, 2017. [Google Scholar]
- 13.Zhang S, Yuan W, Zhang J, et al. Clinicopathological features, surgical treatments, and survival outcomes of patients with small bowel adenocarcinoma. Medicine 2017;96:e7713. 10.1097/MD.0000000000007713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aparicio T, Henriques J, Manfredi S, et al. Small bowel adenocarcinoma: results from a nationwide prospective ARCAD-NADEGE cohort study of 347 patients. Int J Cancer 2020;147:967–77. 10.1002/ijc.32860 [DOI] [PubMed] [Google Scholar]
- 15.Wieghard N, Mongoue-Tchokote S, Young JI, et al. Prognosis of small bowel adenocarcinoma in Crohn's disease compares favourably with de novo small bowel adenocarcinoma. Colorectal Dis 2017;19:446–55. 10.1111/codi.13531 [DOI] [PubMed] [Google Scholar]
- 16.Vanoli A, Di Sabatino A, Furlan D, et al. Small bowel carcinomas in coeliac or Crohn's disease: clinico-pathological, molecular, and prognostic features. A study from the small bowel cancer Italian Consortium. J Crohns Colitis 2017;11:942–53. 10.1093/ecco-jcc/jjx031 [DOI] [PubMed] [Google Scholar]
- 17.Vanoli A, Grillo F, Guerini C, et al. Prognostic role of mismatch repair status, Histotype and high-risk pathologic features in stage II small bowel adenocarcinomas. Ann Surg Oncol 2020;10. 10.1245/s10434-020-08926-4. [Epub ahead of print: 05 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CG, Ahn JB, Jung M, et al. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br J Cancer 2016;115:25–33. 10.1038/bjc.2016.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe JR, Karnell LH, Menck HR, et al. The American College of surgeons Commission on cancer and the American cancer Society. adenocarcinoma of the small bowel: review of the National cancer data base, 1985-1995. Cancer 1999;86:2693–706. [DOI] [PubMed] [Google Scholar]
- 20.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518‐–26. 10.1002/cncr.20404 [DOI] [PubMed] [Google Scholar]
- 21.Wu T-J, Yeh C-N, Chao T-C, et al. Prognostic factors of primary small bowel adenocarcinoma: univariate and multivariate analysis. World J Surg 2006;30:391–8. 10.1007/s00268-005-7898-6 [DOI] [PubMed] [Google Scholar]
- 22.Aparicio T, Svrcek M, Zaanan A, et al. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br J Cancer 2013;109:3057–66. 10.1038/bjc.2013.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholl MB, Ahuja V, Conway WC, et al. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol 2010;17:2728–32. 10.1245/s10434-010-1109-x [DOI] [PubMed] [Google Scholar]
- 24.Sarela AI, Brennan MF, Karpeh MS, et al. Adenocarcinoma of the duodenum: importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann Surg Oncol 2004;11:380–6. 10.1245/ASO.2004.05.021 [DOI] [PubMed] [Google Scholar]
- 25.Moon YW, Rha SY, Shin SJ, et al. Adenocarcinoma of the small bowel at a single Korean Institute: management and prognosticators. J Cancer Res Clin Oncol 2010;136:387–94. 10.1007/s00432-009-0668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaiyasate K, Jain AK, Cheung LY, et al. Prognostic factors in primary adenocarcinoma of the small intestine: 13-year single institution experience. World J Surg Oncol 2008;6:12. 10.1186/1477-7819-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal S, McCarron EC, Gibbs JF, et al. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol 2007;14:2263–9. 10.1245/s10434-007-9428-2 [DOI] [PubMed] [Google Scholar]
- 28.Ye X, Zhang G, Chen H, et al. Meta-analysis of postoperative adjuvant therapy for small bowel adenocarcinoma. PLoS One 2018;13:e0200204. 10.1371/journal.pone.0200204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijer LL, Alberga AJ, de Bakker JK, et al. Outcomes and treatment options for duodenal adenocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol 2018;25:2681–92. 10.1245/s10434-018-6567-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydin D, Sendur MA, Kefeli U, et al. Evaluation of prognostic factors and adjuvant chemotherapy in patients with small bowel adenocarcinoma who underwent curative resection. Clin Colorectal Cancer 2017;16:220–7. 10.1016/j.clcc.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 31.Ecker BL, McMillan MT, Datta J, et al. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: a propensity score-matched analysis. Cancer 2016;122:693–701. 10.1002/cncr.29840 [DOI] [PubMed] [Google Scholar]
- 32.Huffman BM, Jin Z, Yadav S, et al. Novel prognostic factors in resected small bowel adenocarcinoma. Clin Colorectal Cancer 2019;18:218–25. 10.1016/j.clcc.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 33.Swartz MJ, Hughes MA, Frassica DA, et al. Adjuvant concurrent chemoradiation for node-positive adenocarcinoma of the duodenum. Arch Surg 2007;142:285–8. 10.1001/archsurg.142.3.285 [DOI] [PubMed] [Google Scholar]
- 34.Overman MJ, Kopetz S, Lin E, et al. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol 2010;49:474–9. 10.3109/02841860903490051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000960supp001.pdf (739.1KB, pdf)
esmoopen-2020-000960supp002.pdf (1.1MB, pdf)
esmoopen-2020-000960supp003.pdf (809.8KB, pdf)
esmoopen-2020-000960supp004.pdf (267.3KB, pdf)
esmoopen-2020-000960supp005.pdf (106.3KB, pdf)
esmoopen-2020-000960supp006.pdf (840KB, pdf)