Abstract
Objective
The present study was designed to demonstrate the relationships among shift work, hair cortisol concentration (HCC) and sleep disorders.
Design
A cross-sectional study.
Setting
Three petroleum administrations in Karamay city of Xinjiang, China.
Participants
435 individuals including 164 males and 271 females participated in the research.
Outcome measures
Information on shift work was collected by a self-administered questionnaire. HCC was determined using an automatic radioimmunoassay instrument. Sleep quality was measured on the Pittsburgh Sleep Quality Index scale.
Results
Shiftwork was associated with an increased prevalence of sleep disorders compared with the fixed day shift (two shifts: OR 3.11, 95% CI 1.57 to 6.19; three shifts: OR 2.87, 95% CI 1.38 to 5.98; four shifts: OR 2.22, 95% CI 1.17 to 4.18; others: OR 3.88, 95% CI= 1.36 to 11.08). Workers with different shift patterns had higher HCC levels than day workers ((fixed day shift: geometric mean±geometric SD=2.33±1.65; two shifts: 3.76±1.47; three shifts: 3.15±1.64; four shifts: 3.81±1.55; others: 3.60±1.33) ng/g hair, η2=0.174) and high HCC was associated with the higher prevalence of sleep disorders (OR 4.46, 95% CI 2.70 to 7.35). The mediating effect of HCC on the relationship between shift work and sleep disorders was 0.25 (95% CI 0.09 to 0.41).
Conclusion
We found that, when compared with the fixed day shift, shiftwork was associated with both the higher HCC, and also with an increased risk of sleep disorders. High HCC was associated with the occurrence of sleep disorders. In addition, HCC had mediating effect in shift work and sleep disorders. Thus, HCC can be considered as an early marker of shiftwork circadian disruption to early detection and management of sleep disorders.
Keywords: sleep medicine, occupational & industrial medicine, epidemiology
Strengths and limitations of this study.
As we know, this is the first study researching the mediating effect of hair cortisol concentration (HCC) between shift work and sleep disorders.
We used HCC reflecting the long-term cortisol exposure which is important in the aetiology of chronic disease related to hypothalamic–pituitary–adrenal axis activation.
The relatively small sample size and recall bias may influence the results of the study.
Cross-sectional study design does not allow establishing causality among shiftwork, HCC and sleep disorders.
Some important confounding factors, such as depression and stress, were not taken into account.
Introduction
Sleep disorders are very common in the general population. A study of 150 000 residents of 36 US states found that the prevalence of sleep disorders for men ranged from 13.7% to 18.1%, and for women the prevalence ranged from 17.7% to 25.1%.1 Shift work is an indispensable part of the lifestyle of a large proportion of the population. In Europe, about 20% of the working population, on average.2 The percentage in Asia has been estimated to be at least 30%.3 Shift work has been shown to be one of the causes of sleep disorders. The prevalence of sleep disorders in shift workers (39.0%) was significantly higher than that in day workers (24.6%) in the study by Kerkhof.4 A statistically significant increase in risk of sleep disorders (OR=8.8) was observed in a study of 403 females employed in shift work and 205 females employed in administrative units of the same enterprise.5 There are few studies on the relationship between different shift patterns and sleep disorders, although the findings may allow enterprises to choose shift patterns reasonably. Therefore, it is important to study this question.
Cortisol is a glucocorticoid hormone that is released by the adrenal cortex through stimulation of the hypothalamic–pituitary–adrenal axis.6 It is commonly known that cortisol is a stress hormone as it is released in larger amounts under pressure.7 Present studies suggest that sleep disorders are associated with cortisol concentrations. A study testing the association between sleep and neuroendocrine activity in children proposed that the lower the children’s sleep sufficiency, the higher the overall level of salivary cortisol and the smaller the decrease salivary cortisol during the day.8 Insomniacs with a high degree of objective sleep disorders, when compared with individuals with a low degree of sleep disturbance, secreted a higher level of cortisol.9 The secretion of cortisol fluctuates according to the circadian rhythm, and the concentration of cortisol in blood, urine and saliva is easily affected by external factors so that it does not reflect the cortisol exposure of individuals accurately.10 Hair cortisol can reflect the cumulative secretion and the long-term cortisol exposure of subjects. The hair cortisol concentration (HCC) is more stable than cortisol concentrations in blood, urine and saliva.10 Therefore, HCC was adopted in this study in order to obtain more accurate results. Manenschijn et al found that shift workers had higher hair cortisol levels than day workers: 48.53 pg/mg hair vs 26.42 pg/mg hair (p<0.001) in the group under 40. But in the group over 40, there was no significant difference in HCC between shift workers and day workers.11 Janssens et al found a significantly lower mean HCC in shift workers compared with day workers, adjusted for age,12 which is inconsistent with the proposed mechanism and most similar studies. The reasons are worth exploring.
Based on previous researches, we believe that there may be a correlation between shift work, HCC and sleep disorders, and that different shift patterns may have different effects on HCC and sleep disorders, as different shift patterns may lead to different shift frequency, working hours and rest hours. Therefore, we studied different shift patterns separately and put forward the following hypotheses: (1) shift work is associated with sleep disorders; (2) employees with sleep disorders have a higher level of HCC and (3) HCC is a mediating variable between shift work and sleep disorders. It is also worth investigating whether the difference in HCC levels between shift workers and day workers is age- related.
Methods
Population
A total of 460 workers of three petroleum administrations in Karamay city of Xinjiang were randomly selected as a target group for the study. The sampling method was as follows: There were five factories in the three petroleum administrations, each with 4 teams and a total of 20 teams. These 20 teams were numbered, and randomly selected 10 teams according to random number table method. A total of 460 petroleum workers from 10 teams were used as research objects. The inclusion criteria were as follows: (1) participants aged 20–60; (2) those who had worked for more than 1 year. The exclusion criteria were as follows: (1) participants with incomplete questionnaire data (n=15); (2) participants with previous depression, schizophrenia and other diseases that may lead to sleep disorders (n=2) and (3) participants with hair shorter than 3 cm (n=8). Finally, 435 individuals, 164 men and 271 women, participated in this research and their hair samples were collected.
Shift work
Information on the shift work was obtained using a questionnaire. We defined the normal day shift employees as having a fixed day shift, and the others as undertaking shift work. Shift work was divided into ‘two shifts,’ ‘three shifts,’ ‘four shifts’ and ‘others’ according to the shift mode. ‘Two shifts’ included a day shift and a night shift, 12 hours per shift. Considering the biological rhythm of employee, two shifts were rotated once for one or 2 weeks, which means that an employee would work around the clock at least twice a month. This shift pattern may lead to longer working hours and make workers more tired. ‘Three shifts’ meant that employees were divided into three groups. There were two groups of employees worked every day (divided into day shift and night shift, 12 hours per shift) and one group rest. They worked in turn according to the arrangement order. ‘Four shifts’ meant that employees were divided into four groups, three groups worked (divided into day shift, mid shift and night shift, 8 hours per shift) and one rest. ‘Others’ referred to all shift patterns except the modes mentioned above.
Sleep disorders
The Pittsburgh Sleep Quality Index scale was used to assess the sleep quality of the participants over the past month. It consists of 19 self-evaluation items generating seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction, and five items evaluated by family members that were not counted in the total score. Each of the seven components was scored 0–3, and the total score range was 0–21. Higher scores indicated worse sleep quality. It was considered as sleep disorder if the cumulative score was more than 7.
Hair cortisol concentration
Hair samples of 2–3 cm and 20–30 mg were collected from the hair roots of the participants. The pretreatment process for hair sampling followed the experimental procedures described in the patent ‘a pretreatment method for detecting cortisol content in hair.’ The hair samples were washed and exfoliated with 2–3 mL isopropanol for 5 min; the exfoliated hair samples were frozen with liquid nitrogen for more than 4 hours and then crushed; the crushed hair samples were placed in centrifuge tubes, 5 mL methanol solution and 3 mL ether solution (methanol: ether volume ratio 5:3) added, and placed in a water bath at 50.8°C for 16 hours for extraction and incubation. The analytical process involved mixing the hair debris by inversion several times and centrifugation at low speed (3500 rpm) for 15 min. The supernatant was removed and put it into a 4 mL Eppendorf tube; a nitrogen blower was used to dry the mixture after extraction; 2 mL phosphate buffer solution was added, and the sample placed in a refrigerator at −4°C for cold storage until the test day. A radioimmunoassay kit for detecting iodine [125I] cortisol and an automatic radioimmunoassay instrument were used to determine HCC.
Covariates
In this study, we collected covariates, including gender, age, ethnicity, education, marital status, monthly income (yuan), physical exercise, smoking status, alcohol consumption, length of service (years) and type of work, using a self-administered questionnaire. Age was divided into ‘<30,’ ‘30–,’ ‘40–’ years; ethnicity was divided into ‘Han,’ ‘Uighur’ and ‘others’; education was divided into ‘high school and below,’ ‘junior college’ and ‘bachelor degree and above’; marital status was divided into ‘single,’ ‘married’ and ‘others (divorced, widowed and remarried)’; monthly income (yuan) was divided into ‘<3000,’ ‘3000–5000’ and ‘≥5000’; physical exercise was divided into ‘never,’ ‘<3 times/week,’ ‘≥3 times/week’ and ‘irregular’ according to the frequency; smoking status was divided into ‘frequently’ (≥1 cigarette/day), ‘occasionally’ (<1 cigarette/day), ‘quitted’ and ‘never’; alcohol consumption was quantified in ‘g’ for beer, rice wine, red wine, white wine and divided into ‘frequently’ (≥8 g/day), ‘occasionally,’ (<8 g/day) ‘quitted’ and ‘never’; length of service (years) was divided into ‘<20’ and ‘≥20’; type of work was divided into ‘oil transportation,’ ‘oil extraction,’ ‘refinery’ and ‘others.’
Statistical analysis
HCCs were log transformed to obtain a normal distribution and were presented in table 1 as the median (first quartile (Q1) to third quartile (Q3)) and geometric mean±geometric SD (GM±GSD) of the original HCC. Binary logistic regression was used to calculate descriptive statistics for demographic variables, compared between those with and without sleep disorders. Variance analysis and the student t-test were used to estimate the relationships between demographic variables and transformed HCC. Cohen’s d and η2 were used to describe the effect sizes of t-tests and variance analyses.
Table 1.
The relationship between shift work and HCC
| Shift work | N (%) | Original HCC Median (Q1–Q3) (ng/g hair) |
Original HCC GM±GSD (ng/g hair) |
||
| Total | <40 | ≥40 | |||
| Fixed day shift | 127 (29.20) | 2.15 (1.55–3.21) | 2.33±1.65 | 2.32±1.67 | 2.34±1.65 |
| Two shifts | 87 (20.00) | 3.61 (2.94–5.25) | 3.76±1.47* | 3.59±1.52† | 3.99±1.39‡ |
| Three shifts | 64 (14.71) | 3.24 (2.01–4.59) | 3.15±1.64* | 3.18±1.66† | 3.12±1.63‡ |
| Four shifts | 135 (31.03) | 3.84 (2.63–5.20) | 3.81±1.55* | 3.64±1.52† | 4.01±1.58‡ |
| Others | 22 (5.06) | 3.38 (2.16–5.81) | 3.60±1.33* | 3.45±1.66† | 3.24±1.86‡ |
| η2 | 0.174 | 0.145 | 0.211 | ||
η2: the effect size of the variance analysis.
*†‡: there was no significant difference between groups marked with the same letter.
GM±GSD, geometric mean±geometric SD; HCC, hair cortisol concentration.
Logistic regression was used to estimate the difference in the prevalence of sleep disorders between shift workers and day workers. ‘Shift work’ was divided into five groups according to the shift patterns and entered into the logistic model as an independent variable, and the referent level was ‘fixed day shift’. As previous studies showed that high HCC level may related to sleep disorders, while the normal reference range of HCC has not been confirmed. When we analysed the relationship between HCC and sleep disorders, HCCs were divided into ‘low and intermediate HCC’ (referent level) and ‘high HCC’ at the Q3(4.71 ng/g hair) threshold as an independent variable. Model 1 analysed the relationship between shift work or HCC and sleep disorders without adjusting for any covariables; model 2 adjusted for gender, age, ethnicity, education, marital status, monthly income, type of work, and length of service. Model 3 adjusted for smoking status, alcohol consumption and physical exercise with the covariates in model 2.
Mediation analysis is conducted to understand the mechanisms through which one variable influence another. The coefficient between shift work and sleep disorders is the total effect. The direct effect is the coefficient between shift work and sleep disorders with HCC as a mediator. The mediating effect, that is, indirect effect can be obtained by subtracting the direct effect from the total effect.13 The Karlson, Holm and Breen (KHB) method14 is a general decomposition method that is unaffected by the rescaling or attenuation bias that arises in cross-model comparisons in nonlinear models. We used the method to verify the significance of mediating effect of HCC by decomposing the direct effect and indirect effect (online supplemental material). HCC fully mediated the relationship between shift work and sleep disorders when the total effect and the indirect effect are significant while the direct effect is not,15 but it acts as a part mediator when the effect are all significant.16 In this study, shift work was divided into two categories named ‘day workers’ (referent level) and ‘shift workers’ as an independent variable. HCCs were divided into ‘low and intermediate HCC’ (referent level) and ‘high HCC’ at the Q3 threshold as a mediator. Percentage of the total effect that is mediated by HCC will be obtained by the method. All analyses were carried out in Stata V.13.0. α=0.05 (two tailed).
bmjopen-2020-038786supp001.pdf (43.1KB, pdf)
All the participants signed an informed consent form after learning about research-related information.
Patient and public involvement
No patient involved.
Results
A total of 435 employees were included in this research, of whom 127 (29.20%) were fixed day workers, 87 (20%) were two-shift workers, 64 (14.71%) were three-shift workers, 135 (31.03%) were four-shift workers and 22 (5.06%) were other workers. The age range was 20–58 years, with an average age of 38.32±7.42 years. A total of 124 participants had sleep disorders in this study, of whom 21 were fixed day workers, 40 were two-shift workers, 22 were three-shift workers, 32 were four shift workers and 9 were other workers. The original HCC range was 1.07–9.67 ng/g hair and the median was 3.20 (2.15–4.71) ng/g hair. To investigate whether the demographic characteristics were associated with sleep disorders or with HCC level, we analysed these factors separately but we didn’t find significant differences (online supplemental table 1).
bmjopen-2020-038786supp002.pdf (93.6KB, pdf)
In the present study, HCC levels of workers with different shift patterns were significantly higher than that of fixed day shift workers ((fixed day shift: GM±GSD=2.33±1.65; two shifts: 3.76±1.47; three shifts: 3.15±1.64; four shifts: 3.81±1.55; others: 3.60±1.33) ng/g hair, η2=0.174). But we did not find the relationship among workers with two shifts, three shifts, four shifts and others in HCC. As previous study showed that shift workers had higher HCC than day workers in the group under 40 and the difference was not statistically significant in the group over 40, we divided the workers into two groups at the age of 4011, and analysed the differences in HCC among workers with different shift patterns. The results were consistent with those without grouping (table 1).
The prevalence rates of sleep disorders of different shift patterns was significantly higher than which of fixed day shift (two shifts: OR 2.94, 95% CI 1.55 to 5.57; three shifts: OR 2.64, 95% CI 1.32 to 5.31; four shifts: OR 2.12, 95% CI 1.17 to 3.86; others: OR 3.50, 95% CI 1.32 to 9.22). In order to control the influence of demographic characteristics on sleep disorders, we set up logistic regression models to adjust for covariates, and the results remained statistically significant. However, there was no significant difference in the prevalence of sleep disorders among workers with two shifts, three shifts, four shifts and others (table 2).
Table 2.
ORs of sleep disorder by shift type
| Shift work | Sleep disorders | Model 1 | Model 2 | Model 3 | ||||
| N/N | % | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Fixed day shift | 21/127 | 16.54 | 1.00 | 1.00 | 1.00 | |||
| Two shifts | 32/87 | 36.78* | 2.94 (1.55 to 5.57) | 0.001 | 3.10 (1.58 to 6.08) | 0.001 | 3.11 (1.57 to 6.19) | 0.001 |
| Three shifts | 22/64 | 34.38* | 2.65 (1.32 to 5.31) | 0.006 | 2.86 (1.39 to 5.89) | 0.004 | 2.87 (1.38 to 5.98) | 0.005 |
| Four shifts | 40/135 | 29.63* | 2.13 (1.176 to 3.86) | 0.013 | 2.23 (1.19 to 4.18) | 0.012 | 2.22 (1.17 to 4.18) | 0.014 |
| Others | 9/22 | 40.91* | 3.50 (1.32 to 9.22) | 0.011 | 3.74 (1.33 to 10.51) | 0.012 | 3.88 (1.36 to 11.08) | 0.011 |
Model 1: unadjusted.
Model 2: adjusted for gender, age, ethnicity, education, marital status, monthly income, type of work, and length of service.
Model 3: additionally adjusted for smoking status, alcohol consumption and physical exercise with the covariates in model 2.
*There was no significant difference between groups marked with the same letter.
HCCs were dichotomised at Q3(4.71 ng/g hair) threshold. Compared with workers in low and intermediate HCC, the prevalence of sleep disorders of high HCC was significantly higher (OR 4.34, 95% CI 2.73 to 6.90). The results remained significant after controlling the covariates (table 3).
Table 3.
ORs of sleep disorders by HCC
| HCC | Sleep disorders | Model 1 | Model 2 | Model 3 | ||||
| N/N | % | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Low and intermediated HCC | 67/327 | 20.49 | 1.00 | 1.00 | 1.00 | |||
| High HCC | 57/108 | 52.78 | 4.34 (2.73 to 6.90) | <0.001 | 4.51 (2.75 to 7.40) | <0.001 | 4.46 (2.70 to 7.35) | <0.001 |
Low and intermediated HCC: HCC <Q3(4.71 ng/g hair); High HCC: HCC ≥Q3.
Model 1: unadjusted.
Model 2: adjusted for gender, age, ethnicity, education, marital status, monthly income, type of work, length of service and shift work.
Model 3: additionally adjusted for smoking status, alcohol consumption and physical exercise with the covariates in model 2.
HCC, hair cortisol concentration.
We used KHB method to assess the mediating effect of HCC between shift work and sleep disorders. We did not find difference among different shift patterns in the prevalence of sleep disorders or in HCC level, so we dichotomised shift work and HCC. The mediating effect analysis showed that the regression coefficient between shift work and sleep disorders showed was statistically significant (B (95% CI)=1.04 (0.46 to 1.61); OR (95% CI)=2.82 (1.58 to 5.02)). The coefficients between shift work and HCC (B (95% CI)=1.07 (0.47– to 1.66); OR (95% CI)=2.91 (1.61 to 5.25)), HCC and sleep disorders (B(95% CI)=1.61 (1.12 to 2.11); OR (95% CI)=5.02 (3.06 to 8.26)) were both significant. The coefficient was also significant when HCC was added as a mediator (B(95% CI)=0.78 (0.20 to 1.36); OR (95% CI)=2.19 (1.23 to 3.91)) and the mediating effect of HCC was 0.25 ((95% CI 0.09 to 0.41; OR (95% CI)=1.29 (1.10 to 1.51)) (figure 1). This means that mediating effect of HCC between shift work and sleep disorders was significant and it acts as a part mediator in the relationship. The mediating effect accounted for 24.38% of the total effect. All the results were obtained after controlling the covariates (table 4).
Figure 1.
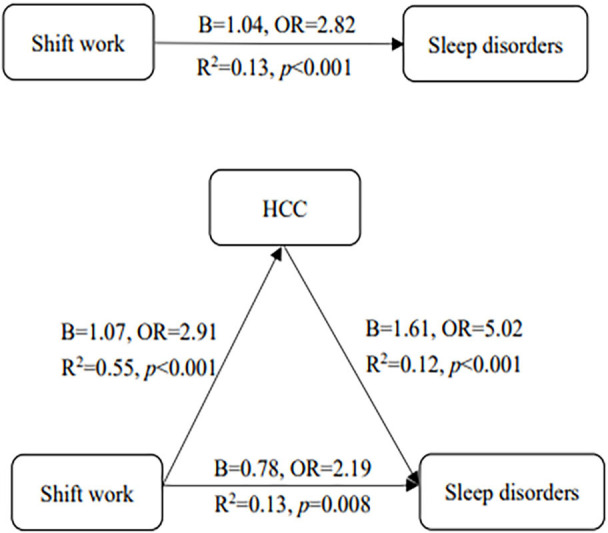
The mediating effect of HCC between shift work and sleep disorders. HCC, hair cortisol concentration.
Table 4.
The mediating effect of HCC between shift work and sleep disorders
| Sleep disorders | Β (95% CI) | SE(β) | Z | OR (95% CI) | P value | (%) | |
| Shift work | Total effect | 1.04 (0.46 to 1.61) | 0.30 | 3.51 | 2.82 (1.58 to 5.02) | <0.001 | 24.38 |
| Direct effect | 0.78 (0.20 to 1.36) | 0.30 | 2.65 | 2.19 (1.23 to 3.91) | 0.008 | ||
| Indirect effect | 0.25 (0.09 to 0.41) | 0.08 | 3.10 | 1.29 (1.10 to 1.51) | 0.002 |
*Percentage of mediating effect in total effect.
HCC, hair cortisol concentration.
Discussion
This study mainly found that shift work was related to a higher prevalence of sleep disorders, and that the HCC levels of shift workers were higher than that of day workers. we also found that workers in high HCC had higher prevalence of sleep disorders than that of workers in low and intermediated HCC. In addition, HCC acts as a part mediator in the relationship between shift work and sleep disorders.
In this study, the prevalence of sleep disorders in workers performing shift work was significantly higher than that of fixed day shift workers, which is consistent with many previous studies. Drake et al found that the incidence of insomnia in night workers and shift workers was 18.5% and 15.7%, respectively, far higher than that in day shift workers (8.6%).17 A prospective study of 1908 individuals showed that entering a shift increased the risk of difficulty falling asleep, while leaving the shift reduced the risk.18 A 5-year cohort study of 2351 Danish employees found that, compared with daytime work, the risk of sleep disorders in those with irregular working hours is higher.19 Another study analysed different shift patterns separately and showed that workers on two shifts and three shifts exhibited higher risks of sleep disorders than fixed day shift workers, and that the influence of three shifts was stronger than that of two shifts.20 Workers on two shifts have more fixed working hours than other shift patterns and can extend sleep time during their days off.21 However, we found no difference among workers on two shifts, three shifts, four shifts and others. While two shifts can prolong working time and make more tired, other shift patterns may increase shift frequency, which may lead to irregular sleep and sleep disorders.
Previous study in Korean firefighters indicated that the serum cortisol response was positively related to night-shift work and serum cortisol levels were different by shift schedule.22 In our study, shift workers had higher HCC level compared with day workers, and the differences between different shift patterns were not statistically significant. The reason for the inconsistent results may be that serum cortisol fluctuates according to the circadian rhythm and is easily disturbed by external factors, while HCC is stable and can reflect the long-term cortisol exposure of body. Another reason may be that working hours, shift patterns and recovery hours of the participants were different in the two studies. In another study, researchers observed elevated mean HCC in participants who had a rotating shift schedule compared with those who worked only during the day at young age, but the difference was not statistically significant among participants over 40. The study inferred that it may be related with healthy work effect and selection bias.11 The present finding indicates that HCC has no relation with age and the differences at each age group were consistent with those without age stratification. It is consistent with the results obtained from The Whitehall Ⅱ occupational cohort study, in which the difference by age group was not statistically significant.23 Additionally, researchers did not find the evidence for suggestion of more shift work problems with increasing age in a review.24 But our finding of the relationship between shift work and HCC is inconsistent with a study on the relationship of occupational stress and HCC among Belgian workers, including 102 participants, which showed that employees working a fixed day shift have significantly higher HCC levels than those undertaking shift work.12 A possible reason may be that the population studied was relatively small, which may have led to poor reliability of the result. Another reason may be that the sample consisted of all those workers who were not working in a regular daytime work regime.
We found that workers with high HCC had higher prevalence of sleep disorders compared with workers with low and intermediated HCC. Although there was no report on the relationship between HCC and sleep disorders, this result was consistent with the previous studies on the relationship between salivary cortisol level and sleep disorders. In the Whitehall Ⅱ Study, participants who reported short sleep twice showed a steeper morning rise in cortisol compared with those who never reported sleep problems.25 Additionally, we found the mediating effect of HCC between shift work and sleep disorders. Because of the results in previous studies of which shift work can cause the disruption of circadian rhythm, which affects the release of cortisol and can lead to increased prevalence of sleep disorders, we suggested that HCC can be taken as a biomarker for sleep disorders caused by shift work. The findings underscore the importance of monitoring HCC among shift workers. There is an urgent need for development and implementation of multidisciplinary interventions focusing on the improvement of sleep quality in affected shift workers
In this study, shift work, HCC and sleep disorders were analysed together for the first time. We studied the different shift patterns separately and found their respective associations with sleep disorders and HCC. We also found the mediating effect of HCC between shift work and sleep disorders. However, some limitations were inevitable. First, a total sample of 435 cannot meet the minimum sample requirement (n=476) for this study based on sample size calculation and it might lead to bias on the results. Second, a cross-sectional study can only describe the relationships among shift work, sleep disorders and HCC, but cannot explain the temporality and casual relationships among the three. Third, demographic information and sleep quality were obtained through a self-reported questionnaire which may lead to recall bias. Finally, some important confounding factors, such as depression and stress, were not considered in this study. A cohort study with more participants and factors should be performed to overcome these limitations. Studies aiming to research other potential mediators between shift work and sleep disorders should also be conducted to improve sleep quality of shift workers.
Conclusion
In this study, we found that shift work may increase the risk of sleep disorders compared with fixed day shift work. Shift workers had higher HCC levels than day workers, and higher HCC was associated with the occurrence of sleep disorders. The mediating effect of HCC between shift work and sleep disorders was found too. Considering the significant relationship between HCC and diabetes and hypertension,26 27 HCC can be considered as an early marker of negative effect of shift work.
Supplementary Material
Acknowledgments
We appreciated for the help from Xinjiang Karamay CDC and Karamay Hospital's occupational diseases department.
Footnotes
Contributors: YL, YZ, JS, ZZ, LS, XZ, MC, TT and JX participated in study conception and design. All authors participated in acquiring data. YL and YZ participated in drafting of manuscript. YZ, JS, ZZ and LS contributed to analysis and interpretation of data. All authors participated in the critical revision and have approved the article.
Funding: This work was supported by the Natural Science Foundation of Jiangsu Province, China (Grant Number: BK20171256); Qinglan Project of Jiangsu Province of China (Jiangsu Provincial Education Department, http://jyt.jiangsu.gov.cn/).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Ethics Committees of Nantong University (2013-L073).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementa information. Extra data are available by emailing YL.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Grandner MA, Martin JL, Patel NP, et al. Age and sleep disturbances among American men and women: data from the U.S. behavioral risk factor surveillance system. Sleep 2012;35:395–406. 10.5665/sleep.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hicklin D, Schwander J. [Shift Work and Sleep]. Praxis 2019;108:119–24. 10.1024/1661-8157/a003163 [DOI] [PubMed] [Google Scholar]
- 3.Mcmenamin TM. A time to work: recent trends in shift work and flexible schedules. Monthly Lab Rev 2007;130:3–15. [Google Scholar]
- 4.Kerkhof GA. Shift work and sleep disorder comorbidity tend to go hand in hand. Chronobiol Int 2018;35:219–28. 10.1080/07420528.2017.1392552 [DOI] [PubMed] [Google Scholar]
- 5.Kukhtina EG, Solionova LG, Fedichkina TP, et al. [Night shift work and health disorder risk in female workers]. Gig Sanit 2015;94:86–91. [PubMed] [Google Scholar]
- 6.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep 2017;9:151–61. 10.2147/NSS.S134864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 2001;22:502–48. 10.1210/edrv.22.4.0436 [DOI] [PubMed] [Google Scholar]
- 8.Räikkönen K, Matthews KA, Pesonen A-K, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab 2010;95:2254–61. 10.1210/jc.2009-0943 [DOI] [PubMed] [Google Scholar]
- 9.Chrousos G, Vgontzas AN, Kritikou I. Hpa axis and sleep. Endotext, 2016. Available: https://www.ncbi.nlm.nih.gov/books/NBK279071/#hpa-axis-sleep.abstract
- 10.Gow R, Thomson S, Rieder M, et al. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int 2010;196:32–7. 10.1016/j.forsciint.2009.12.040 [DOI] [PubMed] [Google Scholar]
- 11.Manenschijn L, van Kruysbergen RGPM, de Jong FH, et al. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab 2011;96:E1862–5. 10.1210/jc.2011-1551 [DOI] [PubMed] [Google Scholar]
- 12.Janssens H, Clays E, Fiers T, et al. Hair cortisol in relation to job stress and depressive symptoms. Occup Med 2017;67:114–20. 10.1093/occmed/kqw114 [DOI] [PubMed] [Google Scholar]
- 13.Miočević M, O'Rourke HP, MacKinnon DP, et al. Statistical properties of four effect-size measures for mediation models. Behav Res Methods 2018;50:285–301. 10.3758/s13428-017-0870-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler U, Karlson KB, Holm A. Comparing coefficients of nested nonlinear probability models. Stata J 2011;11:420–38. 10.1177/1536867X1101100306 [DOI] [Google Scholar]
- 15.Judd CM, Kenny DA. Process analysis estimating mediation in treatment evaluations. Eval Rev 1981;5:602–19. [Google Scholar]
- 16.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 17.Drake CL, Roehrs T, Richardson G, et al. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep 2004;27:1453–62. 10.1093/sleep/27.8.1453 [DOI] [PubMed] [Google Scholar]
- 18.Akerstedt T, Nordin M, Alfredsson L, et al. Sleep and sleepiness: impact of entering or leaving shiftwork--a prospective study. Chronobiol Int 2010;27:987–96. 10.3109/07420528.2010.489423 [DOI] [PubMed] [Google Scholar]
- 19.Rugulies R, Norborg M, Sørensen TS, et al. Effort-reward imbalance at work and risk of sleep disturbances. cross-sectional and prospective results from the Danish work environment cohort study. J Psychosom Res 2009;66:75–83. 10.1016/j.jpsychores.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Wei F, Nie G, et al. Relationship between shift work schedule and self-reported sleep quality in Chinese employees. Chronobiol Int 2018;35:261–9. 10.1080/07420528.2017.1399902 [DOI] [PubMed] [Google Scholar]
- 21.Karhula K, Härmä M, Ropponen A, et al. Sleep and satisfaction in 8- and 12-h forward-rotating shift systems: industrial employees prefer 12-h shifts. Chronobiol Int 2016;33:768–75. 10.3109/07420528.2016.1167726 [DOI] [PubMed] [Google Scholar]
- 22.Lim G-Y, Jang T-W, Sim C-S, et al. Comparison of cortisol level by shift cycle in Korean firefighters. Int J Environ Res Public Health 2020;17:4760. 10.3390/ijerph17134760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abell JG, Stalder T, Ferrie JE, et al. Assessing cortisol from hair samples in a large observational cohort: the Whitehall II study. Psychoneuroendocrinology 2016;73:148–56. 10.1016/j.psyneuen.2016.07.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blok MM, de Looze MP. What is the evidence for less shift work tolerance in older workers? Ergonomics 2011;54:221–32. 10.1080/00140139.2010.548876 [DOI] [PubMed] [Google Scholar]
- 25.Abell JG, Shipley MJ, Ferrie JE, et al. Recurrent short sleep, chronic insomnia symptoms and salivary cortisol: a 10-year follow-up in the Whitehall II study. Psychoneuroendocrinology 2016;68:91–9. 10.1016/j.psyneuen.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazgelytė E, Karčiauskaitė D, Linkevičiūtė A, et al. Association of hair cortisol concentration with prevalence of major cardiovascular risk factors and allostatic load. Med Sci Monit 2019;25:3573–82. 10.12659/MSM.913532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iob E, Steptoe A, lob E, Disease C. Cardiovascular disease and hair cortisol: a novel biomarker of chronic stress. Curr Cardiol Rep 2019;21:116. 10.1007/s11886-019-1208-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038786supp001.pdf (43.1KB, pdf)
bmjopen-2020-038786supp002.pdf (93.6KB, pdf)


