Abstract
On the 15 November 2018, the Committee for Medicinal Products for Human Use adopted an extension to an existing indication for the use of nivolumab (Opdivo) in combination with ipilimumab (Yervoy) for the first-line treatment of adult patients with intermediate/poor-risk advanced renal cell carcinoma (RCC). The approval was based on results from the Pivotal CA209214 study, a randomised, open-label, phase III study, comparing nivolumab +ipilimumab with sunitinib in subjects≥18 years of age with previously untreated advanced RCC (not amenable for surgery or radiotherapy) or metastatic RCC, with a clear-cell component. A total of 1096 patients were randomised in the trial, of which 847 patients had intermediate/poor-risk RCC and received either nivolumab (n=425) in combination with ipilimumab administered every 3 weeks for 4 doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks or sunitinib (n=422) administered orally for 4 weeks followed by 2 weeks off, every cycle. A statistically significant difference in overall survival (OS) was observed in the nivolumab + ipilimumab group compared with the sunitinib group in intermediate/poor-risk subjects (HR 0.63 (99.8% CI 0.44 to 0.89); stratified log-rank 2-sided p-value<0.0001). The median OS was not reached for the nivolumab + ipilimumab group and was 25.95 months for the sunitinib group. The OS rates were 89.5% and 86.2% at 6 months, and 80.1% and 72.1% at 12 months in the nivolumab +ipilimumab and the sunitinib groups, respectively. K-M curves separated after approximately 3 months, favouring nivolumab + ipilimumab. This was not mirrored in the favourable-risk patients where no statistically significant difference was observed between nivolumab + ipilimumab and sunitinib in favourable-risk patients (HR 1.45 (descriptive 99.8% CI 0.51 to 4.12), p =0.2715).
Keywords: Committee for Medical Product for Human Use (CHMP), European Medicines Agency (EMA), renal cell carcinoma (RCC), metastatic RCC (mRCC), overall survival (OS)
Introduction
Recent advances in understanding of the importance of the role of checkpoint inhibitors programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) in immune surveillance have led to the development of groundbreaking therapies in oncology, and in some cases, have benefited patients in years of survival. The PD-1 receptor is expressed on activated T cells and downregulates T-cell effector functions when binding to its ligands, programme death ligand 1 (PD-L1) and PD-L2, on antigen-presenting cells. In the tumour microenvironment, PD-1 on tumour-infiltrating lymphocytes binding to PD-L1 on the tumour and other immune cells can downregulate antitumour activity. CTLA-4 surface expression on T cells is upregulated 24–48 hours following T cell activation and dampens CD28 costimulation of T cell signalling by increasing the threshold for activation of the immune response. By blocking CTLA-4 binding to their common ligands B7-1/B7-2 (CD80/CD86) on antigen presenting cells, the breaks holding T cell activation are released, thereby promoting an effective immune response. Therefore, blockade of the interaction between PD-1/PD-L1/2 and CTLA-4/B7-1/2, while restoring T cell activation, provides the basis for the mechanism of cancer immunotherapy.1
Nivolumab (Opdivo) is a human immunoglobulin G4 monoclonal antibody that acts by binding to PD-1 on T cells,2 while ipilimumab (Yervoy) is a fully human monoclonal antibody (IgG1κ) that blocks CTLA-4 mediated co-signalling.3
Renal cell carcinoma
Renal cell carcinoma (RCC) accounts for 2% of all adult malignancies. Worldwide, about 270 000 new cases are diagnosed and about 116 000 patients die each year. RCC is the most common cancer of the kidney and close to 90%–95% is of clear-cell histology.4 Approximately 30% of patients with RCC present with clinical manifestation of metastatic disease and in patients treated for localised tumour, recurrence develops in approximately 40% of patients.5–7 The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic score model was adopted for patient stratification of risk factors in the pivotal study CA209214.8 9 The IMDC model incorporates a scoring method (+1/0) for a series of variables that determine the overall risk level of patients with RCC based on pre-treatment measurements. These include treatment time (less than 1 year from time of diagnosis to systemic therapy), performance status <80% (based on Karnofsky Performance Status (KPS)), haemoglobin (<lower limit of normal (LLN)), calcium (>upper limit of normal (ULN)), neutrophil count (>ULN) and platelets count (ULN). The IMDC score identified three risk categories: favourable risk (0 factors), intermediate risk (1–2 factors) or poor-risk (3–6 factors).
The median overall survival (OS) is estimated to be around 7.8 months in the poor-risk group, 22.5 months in the intermediate risk group and 43.2 months in the favourable risk group.
Standard treatments for previously untreated advanced RCC
RCC has shown itself to be highly resistant to chemotherapy treatment. Before the introduction of tyrosine kinase inhibitors (TKIs), interleukin-2 or interferon alfa was previously used as the standard of care in first-line treatment of metastatic disease. Response rates were low (5% to 20%) with a median OS of approximately 12 months.10–14 Sunitinib malate, a small molecule TKI angiogenic inhibitor, was shown to have clinical activity in patients who had undergone previous cytokine therapy in two uncontrolled phase 2 trials.15 16 In a pooled analysis, the objective response rate was 42%,11 which well exceeded the rates reported for cytokine therapy as first-line treatment in metastatic disease.10 12 14 17
Currently, available targeted therapies for previously untreated advanced RCC can be divided into two classes, namely anti-angiogenic agents and mTOR (mammalian target of rapamycin) inhibitors. The anti-angiogenic agents are sorafenib, sunitinib, pazopanib, axitinib, tivozanib (VEGF (vascular endothelial growth factor)-binding tyrosine kinase inhibitors), cabozantinib and bevacizumab (VEGF-binding monoclonal antibody). Everolimus and temsirolimus target the mTOR pathway. More recently, pembrolizumab + axitinib and avelumab + axitinib have been approved for metastatic RCC (mRCC).
Nivolumab and ipilimumab in RCC
Nivolumab has been approved in RCC as monotherapy for advanced RCC after prior therapy in adults. In the phase 3 registration study CA209025, nivolumab monotherapy demonstrated statistically significant and superior OS compared with everolimus (HR 0.73 (98.52% CI 0.57 to 0.93); stratified log-rank test p-value=0.0018). Median OS was 25.00 months (95% CI 21.75 to NA) in the nivolumab group and 19.55 months (95% CI 17.64 to 23.06) in the everolimus group.18
Ipilimumab was investigated as monotherapy for mRCC in the phase 2 study MDX010-11. A total of 61 subjects received a single dose of ipilimumab 3 mg/kg followed by either 1 mg/kg (21 subjects; 3-to-1 mg/kg group) cohort A, or 3 mg/kg (40 subjects; 3-to-3 mg/kg group) cohort B. Only a few partial responses were observed in cohort A (1 subject (5%)) and cohort B (5 subjects (12.5%)). Although some partial responses were observed, patients had unexpectedly high incidences of adverse reactions to the treatment. It was shown that in cohort B, which received the highest dose of 3 mg/kg, 25 subjects (63%) had grade 3/4 adverse events (AEs), including 6 subjects (15%) with grade 4 AEs. Seventeen subjects (43%) had AEs that led to treatment discontinuation. There were four subjects that reported grade≥3 colitis leading to bowel perforation or colectomy, which ultimately resulted in death in two subjects. Based on these safety results, development of ipilimumab monotherapy for the treatment of advanced RCC was stopped.
Rationale for nivolumab + ipilimumab in RCC
The marketing authorisation holder applied for an extension of indication for nivolumab to include the first-line combination treatment with ipilimumab in adult patients with intermediate/poor-risk advanced RCC. This combination approach was based on observations that dual blockade of PD-1 and CTLA-4 in murine syngeneic tumour models resulted in synergistic anti-tumour activity. Furthermore, the combination of nivolumab+ipilimumab was approved for the treatment of unresectable or metastatic melanoma, where a favourable benefit–risk balance of the combination was established. Although the incidence of immune-related adverse reactions was increased in the combination therapy, there is now much more experience and knowledge on to how to manage the safety risks and concerns linked to immune-related adverse reactions. By following the recommended dose modifications and treatment with corticosteroid immunosuppressive therapy as described in the product information, these precautionary measures can help minimise the severity of the adverse reactions and promote better tolerability of the treatment in patients. Therefore, the rationale for the combination of nivolumab and ipilimumab in RCC was based on the prior knowledge on the efficacy and safety of nivolumab in other therapeutic indications and the preliminary efficacy data observed with ipilimumab in RCC.
Efficacy data
Two studies were submitted to support extending the indication for nivolumab: one phase III trial (CA209214) and one supportive phase I trial (CA209016).
Study CA209016
Study CA209016 was a phase 1 open-label study of nivolumab+sunitinib or pazopanib, or nivolumab+ipilimumab in subjects with mRCC. It was performed to explore various combination regimens with nivolumab + ipilimumab, namely nivolumab 1 mg/kg+ipilimumab 3 mg/kg (arm I-3), nivolumab 3 mg/kg+ipilimumab 1 mg/kg (arm I-1) and nivolumab 3 mg/kg+ipilimumab 3 mg/kg (arm IN-3) in subjects with advanced or mRCC. The purpose of the study was to determine the maximum tolerated dose and the recommended phase 2 dose of the combination regimens. Although the number of patients recruited was low in the IN-3 arm, treatment with a combination of 3 mg/kg nivolumab and 3 mg/kg ipilimumab, the doses approved for monotherapy, resulted in dose-limiting toxicities that exceeded the MTD. Therefore, the main focus was on the comparison between arm I-1 and arm I-3. Arm I-1 was selected as the dosing schedule to be used in the pivotal study based on the more favourable safety profile of arm I-1 compared with arm I-3 and the lack of difference in observable anti-tumour activity between I-1 and I-3. However, sample sizes were small in the dose–response study and imbalances in baseline characteristics hampered interpretation of the data for overall response rate (ORR), progression-free survival (PFS), OS and the safety results.
Study CA209214
Study CA209214 was a phase 3, randomised, open-label study of nivolumab 3 mg/kg combined with ipilimumab 1 mg/kg every 3 weeks for 4 doses followed by nivolumab 3 mg/kg every 2 weeks versus sunitinib monotherapy using the approved dose and schedule (50 mg orally one time a day for 4 weeks followed by 2 weeks off, every cycle) in adults (≥18 years) with previously untreated advanced RCC (either not amenable to curative surgery or radiation, or American Joint Committee on Cancer (AJCC) Stage IV) and were required to have histologically confirmed RCC with a clear-cell component.
CA209214 consisted of three phases: screening, treatment and follow-up. At the time of randomisation, subjects were stratified according to IMDC prognostic score. To be eligible for the intermediate/poor-risk cohort, at least one of the six following prognostic factors as per IMDC criteria had to be present: (1) KPS equal to 70%; (2) less than 1 year from diagnosis to randomisation; (3) haemoglobin <LLN; (4) corrected calcium concentration >10 mg/dL; (5) absolute neutrophil count >ULN; (6) platelet count >ULN. If none of the above factors were present, subjects were only eligible for the favourable-risk cohort. Subjects were also stratified by region. Subjects were assessed for response (Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1)19 by CT or MRI beginning 12 weeks (±1 week) from randomisation and continuing every 6 weeks (±1 week) for the first 13 months and then every 12 weeks until progression or treatment discontinuation, whichever occurred later. Subjects were allowed to continue study therapy after initial investigator-assessed RECIST v1.1-defined progression if the subject had an investigator-assessed clinical benefit and was tolerating therapy.
Outcomes and estimation
Efficacy in population of patients with intermediate/poor risk
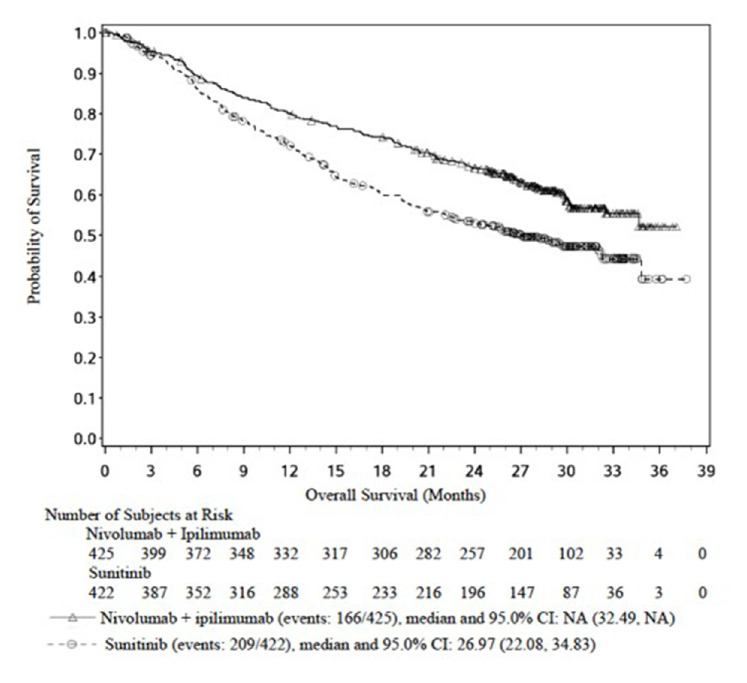
A statistically significant difference in OS was found between the nivolumab+ipilimumab group and the sunitinib group, favouring the nivolumab+ipilimumab group (HR 0.63 (99.8% CI 0.44 to 0.89), p<0.0001) (table 1). The median OS for the sunitinib arm was 25.95 months, whereas the median OS in the nivolumab+ipilimumab group was not reached. At approximately 3 months, the Kaplan-Meier (K-M) curves separated, favouring the nivolumab+ipilimumab group. The applicant provided an update of the OS data based on a database lock of 1 March 2018. No changes in K-M curves were observed in the updated OS curves for patients with intermediate and poor-risk RCC, and the OS benefit remained in favour of the combination arm ipilimumab+nivolumab. The OS rate at 12 months was 80.1% (95% CI 75.9% to 83.6%) for nivolumab+ipilimumab compared with 72.1% (95% CI 67.4% to 76.2%) for the sunitinib arm and the rate at 24 months was 66.5% (95% CI 61.8% to 70.9%) for nivolumab+ipilimumab and 52.9% (95% CI 47.9% to 57.7%) for sunitinib (figure 1).
Table 1.
Summary of efficacy results in CA209214
| Overall survival | Intermediate/poor-risk subjects | All randomised (any risk) subjects | ||
| Nivolumab+ipilimumab N=425 |
Sunitinib N=422 |
Nivolumab+ipilimumab N=550 |
Sunitinib N=546 |
|
| Co-primary objective | Secondary objective | |||
| N events (%) | 140 (32.9) | 188 (44.5) | 161 (29.3) | 204 (37.4) |
| Median OS (months)* | N.A. | 25.95 | N.A. | 32.92 |
| Exact 95% CI | (28.16 to N.A.) | (22.08 to N.A.) | – | (N.A. to N.A.) |
| HR (99.8% CI)† | 0.63 (0.44 to 0.89) | 0.68 (0.49 to 0.95) | ||
| p-value‡ | <0.0001 | 0.0003 | ||
| Overall survival (OS) by PD-L1 tumour expression (1% tumour cell membrane expression) | ||||
| Subjects with >1% PD-L1 expression, n/N | 28/100 | 57/114 | 30/113 | 60/127 |
| Median (months) | N.A. | 19.61 | N.A. | N.A. |
| 95% CI | (14.78 to N.A) | (15.47 to N.A.) | ||
| Subjects with <1% PD-L1 expression, n/N (%) | 93/284 | 114/278 | 108/386 | 126/376 |
| Median (months) | N.A. | N.A. | N.A. | 32.92 |
| 95% CI | (28.16 to N.A.) | (23.98 to N.A.) | (N.A. to N.A.) | |
| Subjects with non-quantifiable PD-L1 expression, n/N (%) | 19/41 | 17/30 | 23/51 | 18/43 |
| Median (months) | 24.34 | 15.7 | 24.34 | N.A. |
| 95% CI | (10.12 to N.A.) | (9.76 to N.A.) | (16.99 to N.A.) | (15.70 to N.A.) |
| IRRC-assessed progression-free survival | Co-primary objective | Secondary objective | ||
| N events (%) | 228 (53.6) | 228 (54.0) | 296 (53.8) | 271 (49.6) |
| Median PFS (months)* | 11.56 | 8.38 | 12.42 | 12.32 |
| Exact 95% CI | (8.71 to 15.51) | (7.03 to 10.81) | (9.89 to 16.53) | (9.79 to 15.24) |
| HR (99.1% CI)† | 0.82 (0.64, 1.05) | 0.98 (0.79, 1.23) | ||
| p-value‡ | 0.0331 | 0.8498 | ||
| IRRC-assessed objective response rate (CR+PR)§ | Co-primary objective | Secondary objective | ||
| N responders (%) | 177 (41.6) | 112 (26.5) | 213 (38.7) | 176 (32.2) |
| Exact 95% CI | 36.9 to 46.5 | 22.4 to 31.0 | 34.6 to 42.9 | 28.3 to 36.3 |
| Difference in ORR (95% CI)¶** | 16.0 (9.8 to 22.2) | 7.2 (1.8 to 12.7) | ||
| p-value†† | <0.0001 | 0.0191 | ||
| Best overall response | ||||
| Complete response (CR) (%) | 40 (9.4) | 5 (1.2) | 54 (9.8) | 12 (2.2) |
| Partial response (PR) (%) | 137 (32.2) | 107 (25.4) | 159 (28.9) | 164 (30.0) |
| Stable disease (%) | 133 (31.3) | 188 (44.5) | 199 (36.2) | 232 (42.5) |
| Progressive disease (%) | 83 (19.5) | 72 (17.1) | 99 (18.0) | 78 (14.3) |
| Unable to determine (%) | 31 (7.3) | 50 (11.8) | 38 (6.9) | 59 (10.8) |
*Median computed using the Kaplan-Meier method.
†Stratified Cox proportional hazard model. HR is nivolumab + ipilimumab over sunitinib.
‡Log-rank test stratified by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic risk score (0, 1–2, 3–6) and region (USA, Canada/W Europe/N Europe, ROW) as entered into the IVRS.
§CI based on the Clopper and Pearson method.
¶Strata adjusted difference in overall response rate (ORR; nivolumab + ipilimumab ‒ sunitinib) based on the DerSimonian and Laird method.
**Stratified by IMDC prognostic risk score (0, 1–2, 3–6) and region (USA, Canada/Western Europe/Northern Europe, Rest of World) as entered into the IVRS.
††Two-sided p value from DerSimonian and Laird Test.
IRRC, independent radiological review committee; N.A., not achieved; PD-L1, programmed death-ligand 1.
Figure 1.
Kaplan-Meier plot of overall survival in study CA209214 analysis (database lock 1 March 2018)—intermediate/poor-risk subjects
Unstratified HR for OS for nivolumab+ipilimumab versus sunitinib was 0.53 (95% CI 0.40 to 0.71) for patients aged <65 years, as compared with HR 0.86 (95% CI 0.58 to 1.27) and 0.97 (95% CI 0.48 to 1.95) for patients aged ≥65 to <75 years and patients aged ≥75 years, respectively. HR for OS for nivolumab+ipilimumab versus sunitinib was 0.55 (95% CI 0.41 to 0.73) for patients with KPS 90–100 compared with HR 0.86 (95% CI 0.61 to 1.20) for patients with KPS <90.
PFS per independent radiological review committee (IRRC)
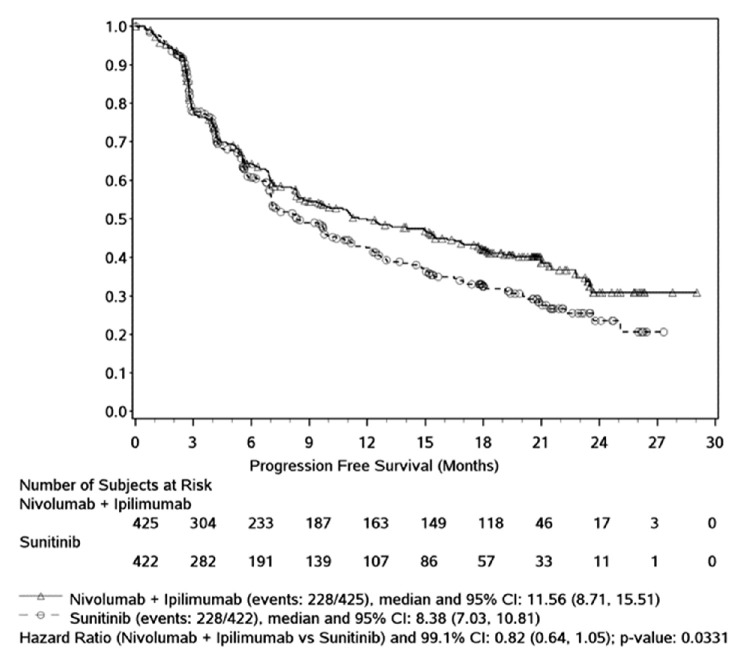
In intermediate/poor-risk subjects, the analysis of IRRC-assessed PFS (co-primary endpoint) using RECIST v1.1, and censoring for subsequent therapy (primary PFS definition) favoured nivolumab+ipilimumab versus sunitinib (HR=0.82 (99.1% CI 0.64 to 1.05), stratified 2-sided p=0.0331). This difference did not meet the stringent pre-specified α=0.009 for statistical significance.
The median PFS was 11.56 months (95% CI 8.71 to 15.51) in the nivolumab+ipilimumab group and 8.38 months (95% CI 7.03 to 10.81) in the sunitinib group, representing a difference in median PFS of 3.2 months. The 12 month PFS rate was 49.6% in the nivolumab+ipilimumab group and 42.6% in the sunitinib group. Rates at 24 months were not available due to censoring at this later time point. The K-M curves overlapped until approximately 6–7 months and then separated, favouring nivolumab+ipilimumab (figure 2).
Figure 2.
Progression-free survival per independent radiological review committee - primary analysis —all intermediate/poor-risk subjects
Efficacy population of patients with favourable risk
The results in the favourable risk group appear to show no clinical benefit from the combination treatment. A numerical difference in OS was observed, favouring sunitinib (HR 1.45 (descriptive 99.8% CI 0.51 to 4.12), p= 0.2715), as well as in PFS (HR 2.18 (descriptive 99.1% CI 1.29 to 3.68)) and ORR where the stratified difference in ORR was −23.0% (P=0.0002), favouring sunitinib. However, no firm conclusion can be drawn taking into account the exploratory nature of the analysis, the low number of events observed in these groups and the follow-up that was too short to definitively determine effects of the treatments on OS. These results have been clearly reported in the product information to better inform physicians.20
PD-L1 expression
In an analysis of the predictive relationship of PD-L1 tumour expression for OS, OS was similar in all PD-L1 evaluable subjects with PD-L1 tumour expression ≥1% compared with those with PD-L1 tumour expression <1% in the nivolumab+ipilimumab group (HR 0.93, 95% CI 0.62 to 1.39).
However, in the sunitinib group, OS was favoured in subjects with PD-L1 tumour expression <1% compared with those with PD-L1 tumour expression ≥1% (HR 1.64, 95% CI 1.20 to 2.23). Tumour PD-L1 expression seems to predict worse prognosis in patients with advanced RCC treated with anti-angiogenesis drugs, as patients with high PD-L1 expression had a worse OS outcome according to literature.21 For intermediate/poor-risk subjects, OS by baseline PD-L1 ≥1% expression favoured nivolumab+ipilimumab (HR 0.45 (95% CI 0.29 to 0.71)). This was also observed in subjects with PD-L1 <1% expression, but the HR was closer to 1 compared with PD-L1 ≥1% expression (HR 0.73 (95% CI 0.56 to 0.96)). In the favourable risk group, it can be seen that this increased effect in subjects with PD-L1 ≥1 expression is mostly caused by the steeper decrease in OS K-M curve in sunitinib for subjects with PD-L1 ≥1%. In intermediate/poor-risk subjects with PD-L1 <1% expression, no difference in PFS was observed between nivolumab+ipilimumab and sunitinib (HR 1.06 (95% CI 0.87 to 1.36)). For subjects with PD-L1 ≥1% expression, however, a strong PFS benefit was observed for nivolumab+ipilimumab (HR 0.47 (95% CI 0.34 to 0.64)). A benefit in ORR in the nivolumab+ipilimumab arm was observed regardless of PD-L1 expression in intermediate/poor-risk subjects (PD-L1≥1%, 58.0% (95% CI 47.7% to 67.8%); PD-L1 <1%, 37%.3 (95% CI 31.7% to 43.2%)). The ORR of sunitinib was lower in subjects with PD-L1 ≥1% (21.9%, 95% CI 14.7% to 30.6%) than in patients with PD-L1 <1% (28.4%, 95% CI 23.2% to 34.1%).
Quality of life
An exploratory objective of the study CA209214 was to evaluate health-related quality of life (HRQoL) as assessed by the Functional Assessment of Cancer Therapy-General (FACT-G), to assess disease-related symptoms in each arm based on the NCCN Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19) and to assess changes in global health status in each treatment arm based on EuroQol’s EQ-5D-3L. The results show that across all three patient-reported scales, the nivolumab+ipilimumab treated group reported numerically higher scores compared with the sunitinib group as well as to baseline scores (table 2). More recently, further results have been published on the QoL of study CA20921422.
Table 2.
Patient-reported outcomes results from study CA209214 using EQ-5D-3L questionnaire describing the proportion of patients reporting ‘no problem’ for mobility, self-care, activity, pain and anxiety, Functional Assessment of Cancer Therapy-General (FACT-G) mean total score and Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19) total score at baseline and at 52 weeks post-baseline.
| Baseline | 52 weeks post-baseline | |||
| Nivolumab+ipilimumab | Sunitinib | Nivolumab+ipilimumab | Sunitinib | |
| EQ-5D-3L | ||||
| Mobility | 75.7% | 76.3% | 83.5% | 71.8% |
| Self-care | 92.3% | 93.1% | 95.0% | 92.2% |
| Activity | 70.3% | 69.4% | 78.5% | 63.1% |
| Pain | 54.0% | 55.3% | 66.9% | 46.6% |
| Anxiety | 61.9% | 59.9% | 76.7% | 77.7% |
| FACT-G | ||||
| Mean | 23.9 | 23.7 | 25.1 | 23.3 |
| FKSI-19 | ||||
| Mean | 61.1 | 60.0 | 65.1 | 61.8 |
Clinical safety
The most frequently reported drug-related AEs in the nivolumab+ipilimumab group were fatigue (36.9%), pruritus (28.2%), diarrhoea (26.5%) and rash (21.6%). In the sunitinib group, the most frequently reported drug-related AEs were diarrhoea (52.0%), fatigue (49.3%), palmar-plantar erythrodysesthesia syndrome (43.2%), hypertension (40.4%), nausea (37.8%) and dysgeusia (33.5%). Grade 3–4 drug-related AEs were reported in 45.7% of subjects in the nivolumab+ipilimumab group and 62.6% of subjects in the sunitinib group (table 3).
Table 3.
Summary of drug-related adverse events (AEs) (equal of higher than 15% of any grade in either treatment group)—intermediate/poor-risk subjects and all treated subjects
| Intermediate/poor-risk subjects | All treated subjects | |||||||
| Nivolumab+ipilimumab (N =423) |
Sunitinib (N=416) |
Nivolumab+ipilimumab (N=547) |
Sunitinib (N=535) |
|||||
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Drug-related AEs, N (%) | 388 (91.7) | 190 (44.9) | 403 (96.9) | 254 (61.1) | 509 (93.1) | 250 (45.7) | 521 (97.4) | 335 (62.6) |
| Most frequent drug-related AEs (≥15% of any grade in either treatment group) | ||||||||
| Fatigue | 140 (33.1) | 16 (3.8) | 183 (44.0) | 34 (8.2) | 202 (36.9) | 23 (4.2) | 264 (49.3) | 49 (9.2) |
| Asthenia | 55 (13.0) | 6 (1.4) | 64 (15.4) | 10 (2.4) | 72 (13.2) | 8 (1.5) | 91 (17.0) | 12 (2.2) |
| Mucosal inflammation | 11 (2.6) | 0 | 113 (27.2) | 11 (2.6) | 13 (2.4) | 0 | 152 (28.4) | 14 (2.6) |
| Pruritus | 122 (28.8) | 3 (0.7) | 35 (8.4) | 0 | 154 (28.2) | 3 (0.5) | 49 (9.2) | 0 |
| Rash | 84 (19.9) | 8 (1.9) | 47 (11.3) | 0 | 118 (21.6) | 8 (1.5) | 67 (12.5) | 0 |
| Palmar-plantar | 2 (0.5) | 0 | 162 (38.9) | 32 (7.7) | 5 (0.9) | 0 | 231 (43.2) | 49 (9.2) |
| Erythrodysaesthesia syndrome | ||||||||
| Diarrhoea | 102 (24.1) | 15 (3.5) | 199 (47.8) | 19 (4.6) | 145 (26.5) | 21 (3.8) | 278 (52.0) | 28 (5.2) |
| Nausea | 78 (18.4) | 6 (1.4) | 142 (34.1) | 5 (1.2) | 109 (19.9) | 8 (1.5) | 202 (37.8) | 6 (1.1) |
| Vomiting | 42 (9.9) | 3 (0.7) | 88 (21.2) | 9 (2.2) | 59 (10.8) | 4 (0.7) | 110 (20.6) | 10 (1.9) |
| Stomatitis | 14 (3.3) | 0 | 100 (24.0) | 12 (2.9) | 23 (4.2) | 0 | 149 (27.9) | 14 (2.6) |
| Dyspepsia | 8 (1.9) | 0 | 66 (15.9) | 0 | 15 (2.7) | 0 | 96 (17.9) | 0 |
| Lipase increased | 67 (15.8) | 40 (9.5) | 43 (10.3) | 26 (6.3) | 90 (16.5) | 56 (10.2) | 58 (10.8) | 35 (6.5) |
| Decreased appetite | 55 (13.0) | 4 (0.9) | 102 (24.5) | 4 (1.0) | 75 (13.7) | 7 (1.3) | 133 (24.9) | 5 (0.9) |
| Hypothyroidism | 65 (15.4) | 2 (0.5) | 97 (23.3) | 1 (0.2) | 85 (15.5) | 2 (0.4) | 134 (25.0) | 1 (0.2) |
| Dysgeusia | 24 (5.7) | 0 | 128 (30.8) | 1 (0.2) | 31 (5.7) | 0 | 179 (33.5) | 1 (0.2) |
| Anaemia | 27 (6.4) | 2 (0.5) | 75 (18.0) | 22 (5.3) | 34 (6.2) | 2 (0.4) | 83 (15.5) | 24 (4.5) |
| Hypertension | 7 (1.7) | 1 (0.2) | 151 (36.3) | 60 (14.4) | 12 (2.2) | 4 (0.7) | 216 (40.4) | 85 (15.9) |
| Thrombocytopenia | 2 (0.5) | 0 | 69 (16.6) | 19 (4.6) | 2 (0.4) | 0 | 95 (17.8) | 25 (4.7) |
In the nivolumab+ipilimumab group, the most frequently reported grade 3–4 drug-related AEs were lipase increased (10.2%), amylase increased (5.7%), alanine aminotransferase increased (4.9%), fatigue (4.2%) and diarrhoea (3.8%). In the sunitinib group, the most frequently reported grade 3–4 drug-related AEs reported were hypertension (15.9%), fatigue (9.2%), palmar-plantar erythrodysesthesia syndrome (9.2%), platelet count decreased (6.7%), lipase increased (6.5%), neutropenia (6.0%) and diarrhoea (5.2%).
The safety profile of nivolumab+ipilimumab is characterised by a high frequency of immune-related AEs, that is, AEs observed during treatment with ipilimumab and/or nivolumab are consistent with an immune-related aetiology which is linked to the mechanism of action of nivolumab and ipilumumab. The most frequently reported any-grade drug-related immune-related AE categories were skin (48.8%), endocrine (32.5%) and gastrointestinal (GI; 28.2%). Most AEs related to endocrine, GI, hepatic, pulmonary, skin and hypersensitivity/infusion reaction were considered drug related by the investigator. A proportion of the immune-related AEs seen with nivolumab +ipilimumab did not resolve, for example, 102 of the 178 subjects with drug-related endocrine immune-related AEs did not have their AE resolved.
Thus, the safety profile of nivolumab+ipilimumab and the safety profile of sunitinib are very different. The frequency for grade 3–4 AEs in the combination treatment was less in the combination compared with sunitinib (45.7% vs 62.6%). However, drug-related SAEs were higher in the combination compared with sunitinib (29.6% vs 15.1%) in favour of sunitinib. However, overall the frequency of AEs regardless of causality was comparable between study arms. The poorer tolerability of the combination treatment is further illustrated by the relatively high frequency of treatment discontinuation. Drug-related AEs leading to discontinuation were reported in 21.6% of patients in the nivolumab +ipilimumab group and in 11.8% of patients in the sunitinib group, and grade 3–4 drug-related AEs leading to discontinuation were reported in 15.4% and 6.9% of the subjects, respectively.
Benefit–risk assessment
When the pivotal trial CA209214 was being conducted, the standard treatment option in previously untreated patients with RCC was sunitinib for favourable/intermediate-risk patients. For poor-risk patients, the standard treatment option was either sunitinib or temsirolimus. The median OS was less than 4 years for treatment-naive patients with the most favourable prognosis, and less than 1 year in patients with poor prognosis, indicating the need for more efficacious therapies. Since then, the combinations of nivolumab+ipilimumab, pembrolizumab +axitinib and avelumab +axitinib have been approved and are recommended for intermediate/poor-risk patients and cabozantinib, sunitinib and pazopanib have been approved as an option in treatment of naïve adults with intermediate or poor risk.
Nivolumab was initially granted an extension of the indication for second-line treatment of RCC, while ipilimumab had no prior approved indication in RCC. Hence, the primary analysis population (intermediate/poor risk) in study CA209214 represented a population with a high unmet medical need (median OS for favourable risk patients is 43 months but for intermediate risk it is 23 months and poor risk, 8 months). While agents used for treatment of first-line advanced RCC demonstrated statistically significant benefits in terms of PFS, so far no agent in this population had been approved based on OS benefit. In addition, no agent demonstrated superiority to sunitinib based on randomised, controlled phase 3 studies over the past 10 years. In the phase 3 CA209214 study, the nivolumab 3 mg/kg and ipilimumab 1 mg/kg combination regimen demonstrated statistically significant improvement in OS compared with the standard of care sunitinib, in previously untreated, intermediate or poor-risk advanced RCC, reducing the risk of death by 37%. The median OS for nivolumab+ipilimumab was not reached whereas for sunitinib was 25.95 months. A longer follow-up of the study has yielded consistent results.23 OS was favoured with nivolumab+ipilimumab versus sunitinib across all predefined subgroups. This improvement in OS was statistically significant and accompanied by a clinically meaningful 16% improvement in ORR ((95% CI 9.8 to 22.2), p<0.0001, but no test prespecified so formally not significant), including complete responses in 9.4% of participants versus 1.2% in the sunitinib arm, as well as a 3.2 months improvement in median PFS (not statistically significant). The observed OS benefit was considered clinically relevant and unprecedented in this therapeutic context. The PFS results from the primary analysis further support the observed OS benefit in addition to the convincing difference in ORR and a high proportion of CR in the nivolumab+ipilimumab arm compared with sunitinib arm. Overall, the depth of response has been shown to correlate with improved survival outcomes, emphasising the clinical significance of the complete responses observed in supporting the clinically highly relevant benefit in OS.
Clinical benefit for OS for nivolumab+ipilimumab was seen regardless of tumour PD-L1 expression. Although the magnitude of benefit for nivolumab+ipilimumab compared with sunitinib was greater among PD-L1 positive (>1% tumour expression) subjects (HR=0.45). However, even among PD-L1 negative (<1% tumour expression) subjects in CA209214, Kaplan-Meier curves showed improved OS for nivolumab+ipilimumab compared with sunitinib (HR=0.73). Only in evaluation of PFS was significant benefit of nivolumab+ipilimumab compared with sunitinib restricted to PD-L1 positive subjects while PFS was similar between treatment groups among PD-L1 negative subjects.
The totality of available data supported favourable benefit–risk for the combination of nivolumab and ipilimumab compared with sunitinib.
The results of the exploratory analysis on HRQoL showed numerically higher scores between baseline and at week 52 for nivolumab+ipilimumab compared with sunitib for FACT-G, FKSI-19 and EQ-5D. These are considered small differences in patient-related outcome (PRO) and it was not clear which difference in outcome could be considered a clinically relevant difference. Since the number of subjects included in the PRO assessment was decreased after 1 year, and the number of completed assessments was low, together with the open-label design of the trial, the relevance of the PRO results remained uncertain. Therefore, no statement on the PRO assessment was included in the SmPC.22
The safety profile showed that the combination of nivolumab+ipilimumab had distinct toxicities from sunitinib owing to different mechanisms of action, but overall toxicities were acceptable. The majority occurred within the initial few weeks of initiating treatment, when the combination was used, and were well managed with established treatment algorithms, which resulted in resolution in most cases. In contrast, sunitinib toxicity commonly affects skin, GI and vascular systems, with longer times to resolution and often requiring chronic management for as long as sunitinib dosing continued. Although there was a higher discontinuation rate with nivolumab+ipilimumab arm than sunitinib, this was partly due to the ability for dose reduction in the sunitinib arm at the expense of efficacy and the majority of patients who discontinued due to toxicity in nivolumab+ipilimumab arm continued to derive efficacy benefit. Approximately 20% of subjects discontinued nivolumab+ipilimumab due to toxicity. The OS benefit observed, together with the manageable safety profile relative to sunitinib, was therefore considered to outweigh the added toxicity of ipilimumab to the combination.
The main uncertainty remained in the contribution of ipilimumab to the efficacy of the combination therapy nivolumab+ipilimumab. The pivotal study did not compare efficacy of the combination therapy with either nivolumab monotherapy or ipilimumab monotherapy and the dose–response relationship of 1 mg/kg ipilimumab in RCC was poorly characterised. The lack of demonstration of the contribution of ipilimumab to efficacy of the combination treatment was considered an important issue, especially because it was evident that addition of ipilimumab led to substantial additional toxicity. The combination of two or more drugs is often an adequate way to achieve or improve efficacy and/or improve safety compared with using single agents. The establishment of adequate combinations and doses is crucial, as outlined in the guideline on the evaluation of anticancer medicinal products in man (EMA/CHMP/205/95 Rev.5). The Committee for Medicinal Products for Human Use (CHMP) considered that based on general methodological principles, in order to establish the efficacy and safety of each product in this combination, a three-arm phase III design (a randomised study of nivolumab+ipilimumab vs nivolumab vs reference treatment) would have been appropriate. Ipilimumab’s activity and contribution to efficacy in the proposed dose and clinical setting are lacking and in order to further elucidate the contribution of ipilimumab to the efficacy and toxicity of the combination regimen of nivolumab and ipilimumab, the CHMP requested the MAH to submit the results of a randomised study comparing the efficacy and safety of the combination of nivolumab and ipilimumab to nivolumab monotherapy in previously untreated adult patients with intermediate/poor-risk advanced RCC and with an appropriate spectrum of PD-L1 expression. The MAH was also recommended to provide additional biomarker data that could help identify patients more likely to benefit from the combination compared with standard of care.
Conclusion
The CHMP considered that the benefit/risk of nivolumab (Opdivo) in combination with ipilimumab (Yervoy) for the first-line treatment of adult patients with intermediate/poor-risk advanced RCC was positive. However, further investigation is still required to try to elucidate the contribution of ipilimumab to the efficacy and toxicity of the combination regimen. As a result of this uncertainty, the CHMP imposed a post-authorisation efficacy measure and requested the company to submit the results of a randomised study comparing the efficacy and safety of the combination to nivolumab monotherapy, in previously untreated adult patients with intermediate/poor-risk advanced RCC and with an appropriate spectrum of PD-L1 expression.
Acknowledgments
The scientific assessment summarised in this report is based on important contributions from the rapporteur and co-rapporteur assessment teams, CHMP members and additional SAG assessment following the application for a marketing authorisation. The authors thank Luca Moscetti for the critical review of the manuscript.
Footnotes
Contributors: Data analysis and interpretation: JC, PH, BB, MSG, CS, BOS, MO, FJ and BK-S. Manuscript writing: SA, JC, PH, BB, MSG, CS, BOS, MO, FJ, BK-S, NZ, EP, SdRD and FP. Final approval of manuscript: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: This publication summarises, but is not limited to, the European Public Assessment Report (EPAR), the summary of product characteristics and other published product information. The EPAR is published on the EMA website (www.ema.europa.eu). For the most current information on this marketing authorisation, refer to the EMA website. The authors of this paper remain solely responsible for the opinions expressed in this publication.
Competing interests: SA reports speaking fees, consulting fees and honoraria for symposiums by Novartis, BMS, Pfizer, Jazz Pharmaceuticals. BB declared an executive role in Nordic Nanovector ASA; JB declared strategic advisory role for Merck, investigator role in studies sponsored by Roche, Boehringer-Ingelheim, Pfizer, Merck, AstraZeneca and has received grants/funding from Sanofi Aventis, Amgen, Merck, Roche, Pfizer, Bayer and AstraZeneca. JC, PH, MSG, CS, BOS, MO, FJ, BK-S, NZ, EP, SdRD and FP declare no competing interests.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Wang C, Thudium KB, et al. In vitro characterization of the anti-PD-1 antibody nivolumab. Cancer Immunol Res 2014;2:846–56. [DOI] [PubMed] [Google Scholar]
- 2.Lim JSJ, Soo RA. Nivolumab in the treatment of metastatic squamous non-small cell lung cancer: a review of the evidence. Ther Adv Respir Dis 2016;10:444–54. 10.1177/1753465816661091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan DV, Gibson HM, Aufiero BM, et al. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun 2014;15:25–32. 10.1038/gene.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. 10.3322/canjclin.55.2.74 [DOI] [PubMed] [Google Scholar]
- 5.Lam JS, Leppert JT, Belldegrun AS, et al. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol 2005;23:202–12. 10.1007/s00345-004-0466-0 [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Bander NH, Nanus DM. Renal-Cell carcinoma. N Engl J Med 1996;335:865–75. 10.1056/NEJM199609193351207 [DOI] [PubMed] [Google Scholar]
- 7.Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843–52. 10.1016/S0094-0143(03)00056-9 [DOI] [PubMed] [Google Scholar]
- 8.Noe A, de Bruijn RE, Blank C, et al. Comparison of pre-treatment MSKCC and IMDC prognostic risk models in patients with synchronous metastatic renal cell carcinoma treated in the era of targeted therapy. World J Urol 2016;34:1067–72. 10.1007/s00345-016-1769-7 [DOI] [PubMed] [Google Scholar]
- 9.Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International metastatic renal-cell carcinoma database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141–8. 10.1016/S1470-2045(12)70559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher RI, Rosenberg SA, Fyfe G. Long-Term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am 2000;6:S55–7. [PubMed] [Google Scholar]
- 11.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005;23:133–41. 10.1200/JCO.2005.03.206 [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Murphy BA, Bacik J, et al. Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. JCO 2000;18:2972–80. 10.1200/JCO.2000.18.16.2972 [DOI] [PubMed] [Google Scholar]
- 13.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N Engl J Med 1998;338:1272–8. 10.1056/NEJM199804303381805 [DOI] [PubMed] [Google Scholar]
- 14.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. JCO 2003;21:3127–32. 10.1200/JCO.2003.02.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. JCO 2006;24:16–24. 10.1200/JCO.2005.02.2574 [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006;295:2516–24. 10.1001/jama.295.21.2516 [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Tannir NM, McDermott DF. For the CheckMate 214 Investigators nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015;21:1071–7. 10.1158/1078-0432.CCR-14-1993 [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Grünwald V, Escudier B, et al. Patient-Reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol 2019;20:297–310. 10.1016/S1470-2045(18)30778-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. 10.1016/S1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]